Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Robert Zymliński | -- | 2941 | 2023-02-24 07:40:55 | | | |
| 2 | Rita Xu | -1 word(s) | 2940 | 2023-02-24 07:54:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Guzik, M.; Urban, S.; Iwanek, G.; Biegus, J.; Ponikowski, P.; Zymliński, R. Novel Therapeutic Devices in Heart Failure. Encyclopedia. Available online: https://encyclopedia.pub/entry/41613 (accessed on 08 February 2026).
Guzik M, Urban S, Iwanek G, Biegus J, Ponikowski P, Zymliński R. Novel Therapeutic Devices in Heart Failure. Encyclopedia. Available at: https://encyclopedia.pub/entry/41613. Accessed February 08, 2026.
Guzik, Mateusz, Szymon Urban, Gracjan Iwanek, Jan Biegus, Piotr Ponikowski, Robert Zymliński. "Novel Therapeutic Devices in Heart Failure" Encyclopedia, https://encyclopedia.pub/entry/41613 (accessed February 08, 2026).
Guzik, M., Urban, S., Iwanek, G., Biegus, J., Ponikowski, P., & Zymliński, R. (2023, February 24). Novel Therapeutic Devices in Heart Failure. In Encyclopedia. https://encyclopedia.pub/entry/41613
Guzik, Mateusz, et al. "Novel Therapeutic Devices in Heart Failure." Encyclopedia. Web. 24 February, 2023.
Copy Citation
Heart failure (HF) constitutes a significant clinical problem and is associated with a sizeable burden for the healthcare system. Numerous novel techniques, including device interventions, are investigated to improve clinical outcome. Interventions regarding autonomic nervous system imbalance, i.e., baroreflex activation therapy; vagus, splanchnic and cardiopulmonary nerves modulation; respiratory disturbances, i.e., phrenic nerve stimulation and synchronized diaphragmatic therapy; decongestion management, i.e., the Reprieve system, transcatheter renal venous decongestion system, Doraya, preCardia, WhiteSwell and Aquapass, are presented.
heart failure
cardiorenal syndrome
autonomic dysregulation
1. Introduction
Heart failure (HF) is a clinical syndrome resulting from structural and/or functional abnormality of the heart, leading to elevated intracardiac pressures and/or insufficient cardiac output. Increased cardiac filling pressures and neuro-hormonal disturbances resulting in fluid retention and redistribution are major factors responsible for congestion development and acute decompensation in heart failure [1].
As the HF pathophysiology is multidimensional, device interventions allow direct or indirect targeting of biological HF pathways, e.g. methods to manipulate sympathetic nervous system (SNS) imbalance, respiratory dysregulation or volume overload have been developed (Table 1). To preserve the research's coherence and compactness, researchers decided not to describe all promising techniques, but they focused on selected pathophysiological processes crucial in HF (Figure 1).
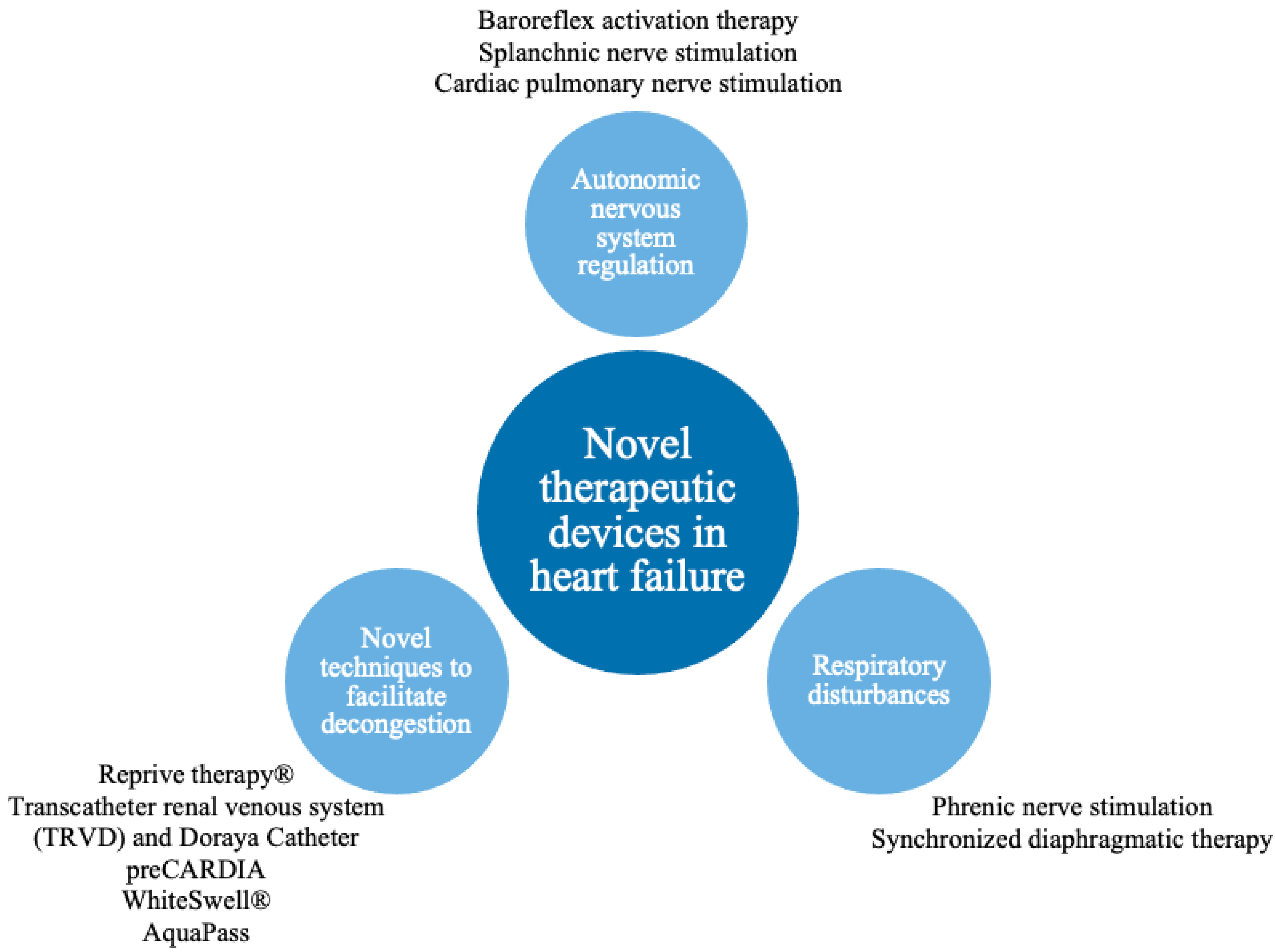
Figure 1. Pathophysiological pathways addressed by novel therapeutic devices.
Table 1. Summary of the proposed novel methods.
| Method | Pathophysiological Mechanism | Solution | Trial Design and Size | Primary Outcomes | Evidence | Adverse Events |
|---|---|---|---|---|---|---|
| Baroreflex activation therapy | Overactivity of SNS (increased heart rate, arterial pressure, RAAS activity and negative cardiac remodeling). | Stimulation of carotid bodies to restore autonomic system balance. | Multicenter, prospective, controlled trial n = 408 |
Rate of cardiovascular and HF morbidity, MANCE, Change in: NT-proBNP, 6 MHW, MLWHF QOL |
BeAT-HF showed improvements of quality of life, exercise capacity, functional status and decrease of NT-proBNP [2] | MANCE event-free rate: 97%. A system or procedure-related serious adverse event occurred in seven patients. |
| Single-center, open-label n = 11 |
Not reported | Dell’Oro et al. demonstrated significant improvement of EF and reduction in hospitalization [3] | No adverse effects were reported. | |||
| Vagus nerve stimulation | Overactivity of SNS (increased heart rate, arterial pressure, RAAS activity and negative cardiac remodeling). | Increase of PNS activity. | Multicenter, prospective, randomized, controlled trial n = 95 |
Change in LVESD, Percentage of surviving patients. |
NECTAR-HF presented significant improvement in quality of life, NYHA class and functional status [4] | There were no significant differences in serious adverse events between control and therapy groups. The overall rate of implantation-related infections was 7.4% |
| Multicenter, open-label, uncontrolled trial n = 60 |
Change in: LVESV EF, Adverse events. |
ANTHEM-HF showed positive, durable improvement of cardiac function [5] | Serious adverse events occurred in 16 patients. There was one death related to system implantation due to an embolic stroke that occurred 3 days after surgery. |
|||
| Splanchnic nerve stimulation | Excessive cardiac filling pressure due to overactivity of SNS resulting in visceral vasoconstriction and rapid volume shift from visceral to central compartment during exercise. | GSN modulation preventing exercise provoked visceral vasoconstriction and subsequent fluid shift from the visceral compartment to the central venous system. | Single-center, prospective, open-label, uncontrolled trials n = 11, n = 15 |
Change in CVPPAMP PCWP |
Splanchnic-HF 1, and Splanchnic-HF 2 showed a reduction in PCPW and improvement of the cardiac index during exercise [6][7] | No adverse events were reported. |
| Multicenter, prospective, uncontrolled, pilot study |
Change in: mean PCPW at rest and exercise (20 W). Adverse events. |
REBALANCE-HF confirmed the reduction in exercise PCPW in HFpEF and NYHA class improvement [8] | There were three non-serious device-related adverse events reported in this study: HF decompensation due to periprocedural fluid overload, transient hypertension and back pain following ablation. | |||
| Cardiopulmonary nerve stimulation | Impaired LV contractility and relaxation. | Stimulation of the autonomic system area responsible for LV contractility resulting in positive lusitropic and inotropic effects. | Single-center, first-in-human, proof-of-concept study n = 15 |
Adverse events. | A proof-of-concept study showed improvement of LV contractility and an increase in mean arterial pressure without affecting the heart rate [9] | No device-related serious adverse events were reported. |
| Phrenic nerve stimulation | Central apnea due to periodic drop in CO2 partial pressure to below the threshold for triggering the action potential in the respiratory center caused by greater sensitivity to carbon dioxide leading to potent stimulus of rhythmic breathing. | Transvenous stimulation of phrenic nerve during apneas. | Multicenter, randomized, open-label study n = 151 |
Reduction in AHI and freedom from serious adverse events | The remedē System Pivotal Trial showed significant reduction in AHI, arousal index, desaturation and apnea episodes. It also revealed improvement in quality of life, sleep structure and EF [10][11] | Cumulatively, 21 (14%) serious adverse events were observed in 5-year follow-ups (15; (10%) in the first 12 months). It predominantly included electrode dysfunction, electrode dislocation and infection of the implantation site [10] |
| Asymptomatic diaphragmatic stimulation | High left ventricle pre-load and after-load pressures increase remodeling and HF progression. | Stimulation of diaphragm muscle fibers synchronized with cardiac cycle to decrease intrathoracic pressures. | Single-center, randomized, open-label study n = 33 | LVEF improvement | EPIPHRENIC II Study showed significant improvement of LVEF, maximal power on effort, reduction in NYHA class, without differences in 6-min walking test or BNP concentration [12][13] | Three patients were excluded due to dysfunctional diaphragmatic electrode. No adverse events were observed [12] |
| Multicenter, non-randomized, open-label study n = 15 |
Freedom from serious adverse events during procedural recovery or acute therapy | VisONE study showed improvement in LVEF and life quality (evaluated in SF-36); extended walking distance during the 6 MWT was observed at a 1-year follow-up. [13] | No adverse events were observed during procedural recovery, acute therapy (primary outcome) and in 12month follow-up (secondary outcome) [13] | |||
| Reprieve system | Problems with controlling decongestive therapy to avoid too rapid diuretic response and hypovolemia and, on the other hand, providing too much fluid, which worsens volume overload. | Sustaining the accurate fluid balance by measuring the urine output and providing the exact amount of replacement solution to achieve preset fluid balance. | Non-randomized, single-center, prospective, open-label, studies, both n = 19 |
Device and procedure-related adverse events and decongestive efficacy | Higher urine output and decrease in CVP in comparison to the baseline. Actual fluid loss did not exceed target fluid loss at the end of therapy in every patient [14] | No serious adverse events were observed. One case of hypokalemia occurred. |
| Transcatheter renal venous decongestion system | Congestion in renal veins. | Transfemoral inserted flow pump, which reduces renal vein pressure to the desired level. | No results have been published so far. | Device and procedure-related adverse events, technical and procedural feasibility | The trial to evaluate TRVD was terminated prematurely, no results have been published so far. | No results have been published so far. |
| Doraya Catheter | Congestion in renal veins. | Partial obstruction of the flow in the inferior vena cava below the level of the renal veins reduces renal vein pressure | First in-human, single-arm, open-label study n = 9 |
Serious adverse events. | The catheter was successfully deployed in all patients. Clinical symptoms, as well as diuresis and natriuresis, improved [15] | No device-related or embolic events were reported. One serious procedure-related adverse event: bleeding hematoma from the injection site, resolved without sequelae. |
| preCARDIA | Increased right ventricle preload. | Obstruction of the superior vena cava leading to an intermittent decrease in preload. | Multicenter, prospective, single-arm exploratory safety and feasibility, open-label, trial n = 30 |
Freedom from device or procedure-related serious adverse events | Successful decrease in right atrial pressure and PCWP, increase in net fluid balance and urine output [16] | No device or procedure-related serious adverse events were observed. |
| WhiteSwell | Increased preload causes lymphatic congestion, which impairs interstitial drainage and exacerbates oedema. | Reduction in the pressure in the area of lymphatic duct outflow into venous vessels. | The animal model study, n = 7 sheep, used in 1 human, n = 1 |
Serious adverse events. | Examined in a ovine model. Trend toward improved oxygenation an diuresis was noticed [17] | No adverse events were reported in in-human application. |
| AquaPass | Insufficient urine volume removal. | Enhancing the sweat rate to remove fluid directly from interstitial space. | Feasibility and short-term performance, single-arm, open-label study, n = 16 |
Serious adverse events, treatment tolerance, ability to control skin temperature between 33 and 38 Celsius degrees). | The procedure was safe in HF patients, successful weight loss was observed. Increased skin temperature without elevating core temperature above average was achieved in each patients [18] | No adverse event occurred. |
HF remains a major medical problem and is associated with a high occurrence of rehospitalization and deaths, which constitute a huge problem for patients as well as healthcare systems worldwide [19]. Given that, numerous methods to improve outcome in HF have arisen, some including device-based treatment techniques.
2. Targeting Autonomic Nervous System Regulation
2.1. Potential Pathophysiological Target
Physiologically, the autonomic nervous system (ANS) may be described as a highly dynamic structure, driven by uncountable neurohormonal reactions to maintain homeostasis. The imbalance of the ANS plays a crucial role in the pathogenesis of HF as the SNS exceeds the buffer capabilities of the parasympathetic nervous system (PNS). The ANS is responsible for modulation of the heart rate, systemic vascular resistance, arterial blood pressure and cardiac afterload, whereby constant overactivity of SNS leads to undesired maladaptations and cardiovascular remodeling. This phenomenon is reflected in the treatment of HF. From the clinical point of view, there are several possible targets for ANS modulation. Modulation of selected subtypes of receptors (e.g., baroreflex activation therapy) allows for interaction with specific ANS branches (sympathetic or parasympathetic). Via the afferent nerves, stimuli are transmitted from receptors to the central nervous system (CNS). On this level, impulses are analyzed and transferred to the effector pathways. The efferent nerves transmit impulses from the CNS to the neurochemical synapses. Modulation of this process directly influences PNS (Vagus nerve stimulation) or SNS (Splanchnic nerve modulation). In the end, impulses reach the presynaptic membrane resulting in the secretion of neurochemical transmitters (e.g., epinephrine, norepinephrine and acetylcholine), which react with receptors localized in the effector tissue. Crucial for HF is the overactivity of SNS mediated by adrenergic receptors [20]. Numerous studies of beta-adrenergic receptor blockers have proven their impact on survival in HFpEF patients [21][22]. Additionally, the SNS is directly connected with the Renin-Angiotensin-Aldosterone system (RAAS), responsible for increased sodium and water reabsorption with subsequent fluid accumulation, which elevates cardiac filling pressure and promotes congestion development, the indisputable targets of HF therapy [1]. Although the role of the SNS in HF is certain, the knowledge about its mechanisms responsible for HF is still unclear, and the ANS is an area for ongoing research in HF therapies especially using novel biomedical technologies.
2.2. Baroreflex Activation Therapy
Baroreflex activation therapy (BAT) uses a physiological reflex pathway to rebalance the activity of the ANS. Electrical stimulation of the carotid bodies sends afferent nerve impulses to the CNS that reacts by increasing PNS firing and decreasing SNS outflow [23]. The cardiovascular system response is acute and results in the decrease of heart rate and systemic vascular resistance with subsequent reduction in both systolic and diastolic blood pressure [23].
2.2.1. Existing Evidence
Several clinical studies have evaluated the effectiveness and safety of BAT. A multicenter, prospective, randomized, controlled trial–Baroreflex Activation Therapy for Heart Failure (BeAT-HF, NCT02627196)–showed that in the group of 264 patients with the FDA-approved enrolment criteria for BAT (EF ≤ 35%, NT-proBNP < 1.600 pg/mL, NYHA functional class III and without Class I indication for CRT), BAT is a safe procedure that significantly improves quality of life, exercise capacity and functional status, while it decreases NT-proBNP and reduces the number of HF hospitalizations per year. The study reported that the overall major adverse neurological and cardiovascular event-free rate was 97.2%, while the system and procedure-related complication event-free rate was 85.9% [2]. Cardiovascular mortality and HF morbidity rates are still under investigation (1200 participants, 5 years of observation, NCT02627196) Dell’Oro et al. demonstrated that in the group of seven patients who completed follow-up, BAT significantly improved EF (from 32.3 ± 2 to 36.7 ± 3% in 43 months, p < 0.05) and reduced heart failure-related hospitalization rate. There were no side effects reported in this study [3]. Apart from HF, BAT is also widely investigated as a potential drug-resistant arterial hypertension treatment [23].
2.2.2. Weaknesses or Unexplained Issues
Despite positive early results, there is a need for further, well-powered clinical trials before BAT can be incorporated into HF clinical practice. BAT needs at least larger-scale research that includes longer follow-up, a higher number of patients and clarified outcomes with mortality risks [24]. The study performed by Dell’Oro et al. was not registered as a clinical trial.
2.3. Vagus Nerve Stimulation
Vagus nerve stimulation (VNS) is an autonomic system modulation that aims to level autonomic system imbalance by increasing PNS activity. Electrostimulation of the easily accessed right cervical vagus nerve induces neurohormonal reactions that buffer the overactivity of SNS [25].
2.3.1. Existing Evidence
The Neural Cardiac Therapy for Heart Failure (NECTAR-HF, NCT01385176, 95 participants, 63 randomized to therapy) trial was the first study that evaluated the usefulness of VNS in HFrEF. It showed improvements in quality of life, NYHA class and exercise capacity without changes in echocardiographic measures (primary endpoint defined as the change in left ventricle end-systolic diameter) in the VNS treated patients. There were no significant differences in the serious adverse event (SAE) rates between the control and therapy groups. The overall rate of implantation-related infections was 7.4% [4]. The Autonomic Regulation Therapy for the Improvement of Left Ventricular Function and Heart Failure Symptoms (ANTHEM-HF, NCT01823887, 60 participants) uncontrolled design study delivered information about the safety of this procedure, and it showed positive, durable improvements in cardiac function and echocardiography parameters after 6 months of treatment. Additionally, this study confirmed significant improvement in NYHA functional class and exercise tolerance. One death related to the device implantation procedure caused by an embolic stroke that occurred 3 days after surgery in a patient suffering from extensive atherosclerosis of the carotid arteries was reported [5]. The promising application of VNS may be heart rate-dependent stimulation, which, apart from balancing the autonomic system, restores physiological relations [26].
2.3.2. Weakness or Unexplained Issues
Although VNS has a significant positive impact on a patient’s functional status, it does not impact the prognosis [27]. The ANTHEM-HF study was conducted without a control group, which is a significant limitation. To exclude the placebo effect and assess the safety of the procedure, there is a need for a randomized, controlled clinical trial [5]. Moreover, positive echocardiographic changes are not reported by any studies [27]. Interestingly, positive functional changes observed during VNS therapy are not accompanied by NT-proBNP serum level decrease.
2.4. Splanchnic Nerve Modulation
The splanchnic nerves are responsible for autonomic innervation of the upper abdominal viscera (e.g., liver) and are highly connected with splanchnic vascular volume management, primarily caused by visceral vasoconstriction during exercise. The visceral vascular bed is a natural reservoir of blood volume that can be quickly relocated for an urgent need (like hypovolemia, hemorrhage, or exercise). Redistribution of blood volume from the extra-thoracic compartments into the central circulation is believed to be a significant contributor to elevated filling pressures in HF patients, including HF with preserved ejection fraction (HFpEF) [8]. Modulation (blockage or partial blockage) of the splanchnic nerves (SNM) decreases sympathetic tone. It thereby prevents the rapid shift of blood from the splanchnic bed to the central circulation during physical exercise.
SNM may protect the central venous system from acute volume redistribution and subsequent cardiac filling pressure increase [28]. SNM is reached by uni- or bilateral chemical, electrical or surgical greater splanchnic nerve blockage.
2.4.1. Existing Evidence
The splanchnic-HF 1 (NCT02669407) and 2 (NCT03453151) trials reported promising effects of SNM therapy in both acute decompensated (ADHF) and chronic heart failure (CHF). Eleven ADHF patients with advanced HFrEF underwent bilateral temporary percutaneous splanchnic nerve block with lidocaine. In this group, significant reduction in pulmonary capillary wedge pressure (from 30 ± 7 mmHg at baseline to 22 ± 7 mmHg at 30 min, p < 0.001) and an increase in cardiac index (from 2.17 ± 0.74 L/min/m2 at baseline to 2.59 ± 0.65 L/min/m2 at 30 min p = 0.007) were reported [6]. Similar findings were provided by a study of 18 CHF patients who underwent the same procedure [7]. In HFpEF, permanent ablation of the right greater splanchnic nerve resulted in the reduction of intracardiac filling pressures during exercise, as early as 24 h after the procedure [29]. Moreover, a European two-center study investigated the feasibility of permanent surgical right-sided SNM for the treatment of HFpEF (Surgical Resection of the Greater Splanchnic Nerve in Subjects Having Heart Failure with Preserved Ejection Fraction, NCT03715543) demonstrated a significant reduction of PCPW at a 3-month follow-up and significant improvement in NYHA class and quality of life at 12 months after the procedure [28]. The early results of the REBALANCE-HF study (NCT04592445, the ongoing multicenter evaluation of splanchnic ablation for volume management in HFpEF) delivered auspicious results. In the group of 18 enrolled patients, the 20 W exercise PCWP and peak exercise PCWP decreased significantly 1 month after the procedure. At least one NYHA class improvement was experienced by 39% of patients at 1 month and 50% at 3 months after the SNM procedure. This study reported three non-serious device-related adverse events (AE): HF decompensation due to periprocedural fluid overload, transient hypertension and back pain following ablation [8].
2.4.2. Weakness or Unexplained Issues
Safety and efficacy of SNM in the treatment of HF needs to be further investigated. Current scientific reports are based on small patient populations and very limited follow-ups. Notably, the abovementioned studies were proof-of-concept clinical trials without a control group. Additionally, a unified procedure for HF SNM application must be established [28].
2.5. Cardiac Pulmonary Nerve Stimulation
This method uses anatomical relations between pulmonary arteries and the cardiac autonomic system elements. An endovascular delivered electrode placed in pulmonary arteries stimulates the surrounding autonomic nerves resulting in positive lusitropic (increasing relaxation of the myocardium during diastole) and positive inotropic (increasing myocardial contractility) effects without an influence on heart rate. Thus, this percutaneous device has at least theoretical potential to improve cardiac function and systemic perfusion and facilitate decongestion in ADHF [9].
2.5.1. Existing Evidence
The first in-human, proof-of-concept, uncontrolled study (NCT04814134) revealed promising cardiac pulmonary nerve stimulation (CPNS) effects. CPNS in HF resulted in LV contractility improvement and an increase in mean arterial pressure without affecting the heart rate. Moreover, the CPNS 2 Feasibility Study demonstrated short-term safety (no SAE reported) and feasibility in chronic HF patients undergoing a catheterization procedure or implantable cardioverter-defibrillator/cardiac resynchronization therapy implantation [9].
2.5.2. Weakness or Unexplained Issues
CPNS is a concept that needs further investigation. Well organized clinical trials are required to provide information about CPNS effectiveness, safety and impact on outcomes.
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726.
- Zile, M.R.; Lindenfeld, J.; Weaver, F.A.; Zannad, F.; Galle, E.; Rogers, T.; Abraham, W.T. Baroreflex Activation Therapy in Patients With Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2020, 76, 1–13.
- Dell’Oro, R.; Gronda, E.; Seravalle, G.; Costantino, G.; Alberti, L.P.; Baronio, B.; Staine, T.; Vanoli, E.; Mancia, G.; Grassi, G. Restoration of normal sympathetic neural function in heart failure following baroreflex activation therapy: Final 43-month study report. J. Hypertens. 2017, 35, 2532–2536.
- Zannad, F.; De Ferrari, G.M.; Tuinenburg, A.E.; Wright, D.; Brugada, J.; Butter, C.; Klein, H.; Stolen, C.; Meyer, S.; Stein, K.M.; et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: Results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur. Heart J. 2015, 36, 425–433.
- Premchand, R.K.; Sharma, K.; Mittal, S.; Monteiro, R.; Dixit, S.; Libbus, I.; Dicarlo, L.A.; Ardell, J.L.; Rector, T.S.; Amurthur, B.; et al. Autonomic Regulation Therapy via Left or Right Cervical Vagus Nerve Stimulation in Patients With Chronic Heart Failure: Results of the ANTHEM-HF Trial. J. Card. Fail. 2014, 20, 808–816.
- Fudim, M.; Ganesh, A.; Green, C.; Jones, W.S.; Blazing, M.A.; Devore, A.D.; Felker, G.M.; Kiefer, T.L.; Kong, D.F.; Boortz-Marx, R.L.; et al. Splanchnic nerve block for decompensated chronic heart failure: Splanchnic-HF. Eur. Heart J. 2018, 39, 4255–4256.
- Fudim, M.; Jones, W.S.; Boortz-Marx, R.L.; Ganesh, A.; Green, C.L.; Hernandez, A.F.; Patel, M.R. Splanchnic Nerve Block for Acute Heart Failure. Circulation 2018, 138, 951–953.
- Fudim, M.; Fail, P.S.; Litwin, S.E.; Shaburishvili, T.; Goyal, P.; Hummel, S.L.; Borlaug, B.A.; Mohan, R.C.; Patel, R.B.; Mitter, S.S.; et al. Endovascular ablation of the right greater splanchnic nerve in heart failure with preserved ejection fraction: Early results of the REBALANCE-HF trial roll-in cohort. Eur. J. Heart Fail. 2022.
- Goedeke, S.; Emani, S.; Abraham, W.T.; Brandt, M.M.; Schaefer, J.A. Cardiac Pulmonary Nerve Stimulation (CPNSTM)): A Novel Treatment for Acute Decompensated Heart Failure. JACC Basic Transl. Sci. 2022, 7, 324–325.
- Costanzo, M.R.; Ponikowski, P.; Coats, A.; Javaheri, S.; Augostini, R.; Goldberg, L.R.; Holcomb, R.; Kao, A.; Khayat, R.N.; Oldenburg, O.; et al. Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur. J. Heart Fail. 2018, 20, 1746–1754.
- Costanzo, M.R.; Javaheri, S.; Ponikowski, P.; Oldenburg, O.; Augostini, R.; Goldberg, L.R.; Stellbrink, C.; Fox, H.; Schwartz, A.R.; Gupta, S.; et al. Transvenous Phrenic Nerve Stimulation for Treatment of Central Sleep Apnea: Five-Year Safety and Efficacy Outcomes. Nat. Sci. Sleep 2021, 13, 515–526.
- Beeler, R.; Schoenenberger, A.W.; Bauerfeind, P.G.K.-H.; Kobza, R.; Bergner, M.; Mueller, X.; Schlaepfer, R.; Zuberbühler, M.; Erne, S.; Erne, P. Improvement of cardiac function with device-based diaphragmatic stimulation in chronic heart failure patients: The randomized, open-label, crossover Epiphrenic II Pilot Trial. Eur. J. Heart Fail. 2014, 16, 342–349.
- Cleland, J.G.; Young, R.; Jorbenadze, A.; Shaburishvili, T.; Demyanchuk, V.; Buriak, R.; Todurov, B.; Rudenko, K.; Zuber, M.; Stämpfli, S.F.; et al. A First in Human Multi-center, Open Label, Prospective Study to Evaluate Safety, Usability and Performance of the VisONE System for Heart Failure with a Reduced Left Ventricular Ejection Fraction. J. Card. Fail. 2020, 26, S64.
- Biegus, J.; Zymlinski, R.; Siwolowski, P.; Testani, J.; Szachniewicz, J.; Tycińska, A.; Banasiak, W.; Halpert, A.; Levin, H.; Ponikowski, P. Controlled decongestion by Reprieve therapy in acute heart failure: Results of the TARGET-1 and TARGET-2 studies. Eur. J. Heart Fail. 2019, 21, 1079–1087.
- Zymliński, R.; Dierckx, R.; Biegus, J.; Vanderheyden, M.; Bartunek, J.; Ponikowski, P. Novel IVC Doraya Catheter Provides Congestion Relief in Patients With Acute Heart Failure. JACC Basic Transl. Sci. 2022, 7, 326–327.
- Kapur, N.K.; Kiernan, M.S.; Gorgoshvili, I.; Yousefzai, R.; Vorovich, E.E.; Tedford, R.J.; Sauer, A.J.; Abraham, J.; Resor, C.D.; Kimmelstiel, C.D.; et al. Intermittent Occlusion of the Superior Vena Cava to Improve Hemodynamics in Patients With Acutely Decompensated Heart Failure: The VENUS-HF Early Feasibility Study. Circ. Heart Fail. 2022, 15, e008934.
- Abraham, W.T.; Jonas, M.; Dongaonkar, R.M.; Geist, B.; Ueyama, Y.; Render, K.; Youngblood, B.; Muir, W.; Hamlin, R.; del Rio, C.L. Direct Interstitial Decongestion in an Animal Model of Acute-on-Chronic Ischemic Heart Failure. JACC Basic Transl. Sci. 2021, 6, 872–881.
- Aronson, D.; Nitzan, Y.; Petcherski, S.; Bravo, E.; Habib, M.; Burkhoff, D.; Abraham, W.T. Enhancing sweat rate using a novel device for the treatment of congestion in heart failure. Eur. Heart J. 2021, 42, ehab724.1056.
- Lesyuk, W.; Kriza, C.; Kolominsky-Rabas, P. Cost-of-illness studies in heart failure: A systematic review 2004–2016. BMC Cardiovasc. Disord. 2018, 18, 74.
- McCorry, L.K. Physiology of the Autonomic Nervous System. Am. J. Pharm. Educ. 2007, 71, 78.
- Florea, V.G.; Cohn, J.N. The Autonomic Nervous System and Heart Failure. Circ. Res. 2014, 114, 1815–1826.
- Kishi, T. Heart failure as an autonomic nervous system dysfunction. J. Cardiol. 2012, 59, 117–122.
- Victor, R.G. Carotid baroreflex activation therapy for resistant hypertension. Nat. Rev. Cardiol. 2015, 12, 451–463.
- Babar, N.; Giedrimiene, D. Updates on Baroreflex Activation Therapy and Vagus Nerve Stimulation for Treatment of Heart Failure With Reduced Ejection Fraction. Cardiol. Res. 2022, 13, 11–17.
- De Ferrari, G.M.; Crijns, H.J.; Borggrefe, M.; Milasinovic, G.; Smid, J.; Zabel, M.; Gavazzi, A.; Sanzo, A.; Dennert, R.; Kuschyk, J.; et al. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 2011, 32, 847–855.
- Schwartz, P.J.; De Ferrari, G.M.; Sanzo, A.; Landolina, M.E.; Rordorf, R.; Raineri, C.; Campana, C.; Revera, M.; Ajmone-Marsan, N.; Tavazzi, L.; et al. Long term vagal stimulation in patients with advanced heart failure First experience in man. Eur. J. Heart Fail. 2008, 10, 884–891.
- Gold, M.R.; Van Veldhuisen, D.J.; Hauptman, P.J.; Borggrefe, M.; Kubo, S.H.; Lieberman, R.A.; Milasinovic, G.; Berman, B.J.; Djordjevic, S.; Neelagaru, S.; et al. Vagus Nerve Stimulation for the Treatment of Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 149–158.
- Fudim, M.; Ponikowski, P.P.; Burkhoff, D.; Dunlap, M.E.; Sobotka, P.A.; Molinger, J.; Patel, M.R.; Felker, G.M.; Hernandez, A.F.; Litwin, S.E.; et al. Splanchnic nerve modulation in heart failure: Mechanistic overview, initial clinical experience, and safety considerations. Eur. J. Heart Fail. 2021, 23, 1076–1084.
- Gajewski, P.; Fudim, M.; Kittipibul, V.; Engelman, Z.J.; Biegus, J.; Zymliński, R.; Ponikowski, P. Early Hemodynamic Changes following Surgical Ablation of the Right Greater Splanchnic Nerve for the Treatment of Heart Failure with Preserved Ejection Fraction. J. Clin. Med. 2022, 11, 1063.
More
Information
Subjects:
Primary Health Care
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
546
Revisions:
2 times
(View History)
Update Date:
24 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




