Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeffrey Scott Cross | -- | 4850 | 2023-02-23 23:46:26 | | | |
| 2 | Rita Xu | + 50 word(s) | 4900 | 2023-02-24 03:47:24 | | | | |
| 3 | Rita Xu | -27 word(s) | 4873 | 2023-02-24 03:48:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moklis, M.H.; Cheng, S.; Cross, J.S. Crude Glycerol Upgrading. Encyclopedia. Available online: https://encyclopedia.pub/entry/41603 (accessed on 11 January 2026).
Moklis MH, Cheng S, Cross JS. Crude Glycerol Upgrading. Encyclopedia. Available at: https://encyclopedia.pub/entry/41603. Accessed January 11, 2026.
Moklis, Muhammad Harussani, Shou Cheng, Jeffrey S. Cross. "Crude Glycerol Upgrading" Encyclopedia, https://encyclopedia.pub/entry/41603 (accessed January 11, 2026).
Moklis, M.H., Cheng, S., & Cross, J.S. (2023, February 23). Crude Glycerol Upgrading. In Encyclopedia. https://encyclopedia.pub/entry/41603
Moklis, Muhammad Harussani, et al. "Crude Glycerol Upgrading." Encyclopedia. Web. 23 February, 2023.
Copy Citation
Crude glycerol is the main byproduct of biodiesel manufacturing from oleaginous crops and other biomass-derived oils. Approximately 10% crude glycerol is produced with every batch of biodiesel. Worldwide, there is a glut of glycerol and the price of it has decreased considerably. There are real opportunities for valorizing crude glycerol into higher value-added chemicals which can improve the economic viability of biodiesel production as an alternative fuel. Exploring new potential applications of glycerol in various sectors is needed such as in pharmaceuticals, food and beverages, cosmetics, and as a transportation fuel.
crude glycerol
biodiesel
thermo-, electro-, and biochemical glycerol upgrading
1. Introduction
Renewable energy is a rapidly developing industry in order to reduce the dependence on fossil fuels, oil, and natural gas as main resources for energy generation. In addition to energy savings, the shift to a civilization powered by renewable energy sources including waste, solar, wind, biomass, tidal, wave, and geothermal energy becomes an even more significant option in global use of energy. Based on the International Energy Agency (IEA) [1] report at the COP26 Climate Change Conference, it is anticipated that between 2021 and 2026, there will be a 50% increase in renewable capacity compared to the period between 2015 and 2020 which is due to aggressive support from government policies towards clean energy goals and the replacement of non-renewable fossil fuels. Renewable energy sources are anticipated to increase at the quickest rate of all energy sources according to the World Energy Outlook [2]. Furthermore, this type of energy is also expected to dominate two-thirds of the total global primary energy supply in 2050 based on the REmap Case, held in Abu Dhabi [3]. Municipal solid waste (MSW), including biomass and plastic waste, is the only energy source in this spectrum that is based on carbon. Figure 1 illustrates a breakdown of renewables in the energy sector, where the key role of bioenergy (30%) [3] comprised of biomass industry, biomass buildings, liquid biofuels and biogas as well as biomass power, which is mainly sourced from biomass and waste. Hence, there is much potential for further analysis for green energy development.
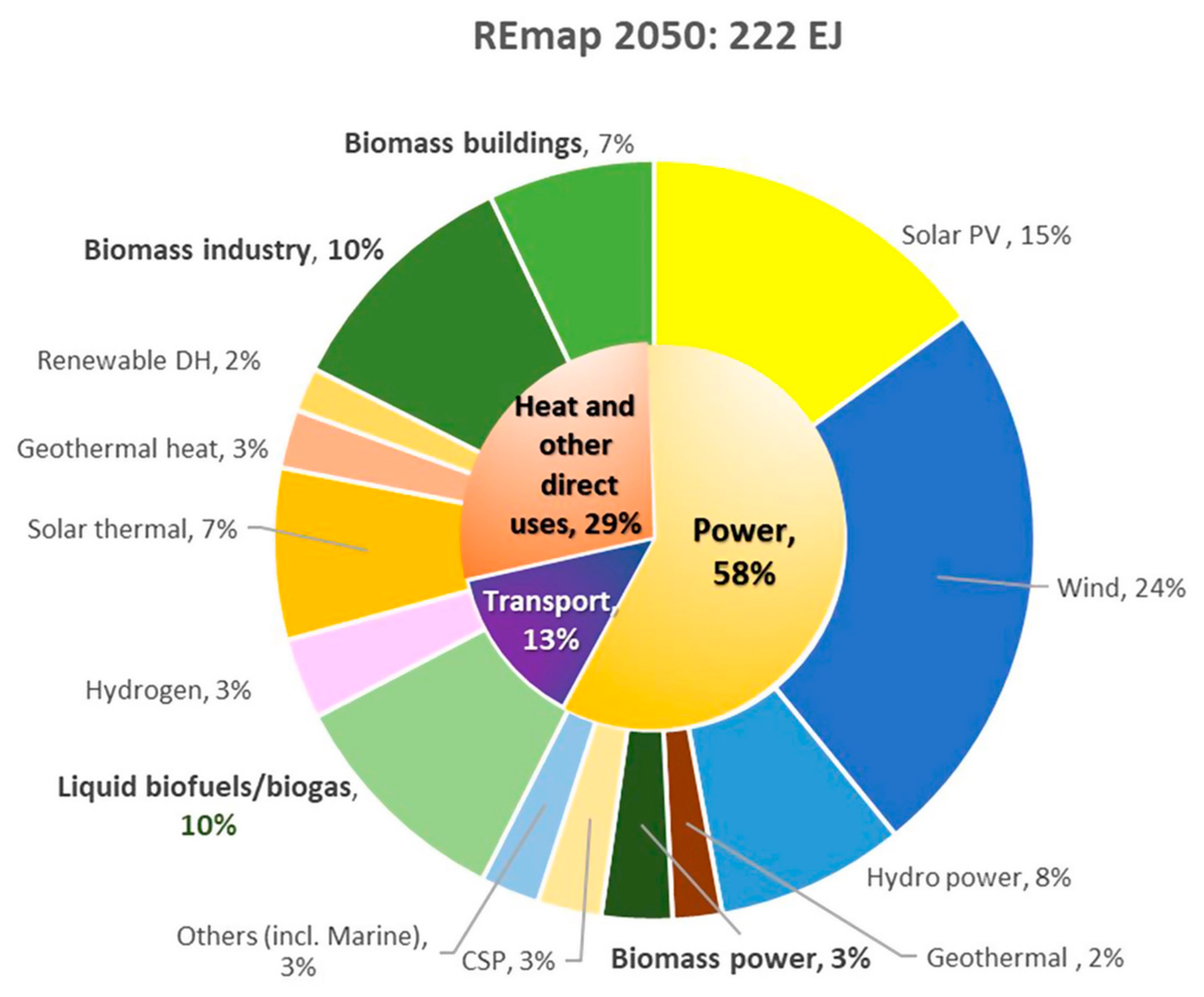
Figure 1. Percentage of total final energy consumption by renewables, according to the IRENA’s Remap CASE 2050 (%). Note: Excludes non-energy use. DH refers to district heat. CSP refers to concentrated solar power.
Crude glycerol, which is widely produced as a byproduct of biodiesel production, has become of particular interest as a potential feedstock to convert into value-added biofuels, e.g., bio ethanol [4][5], green methanol [6], bio propane [7], and hydrogen [8][9]. Several publications report that glycerol yield is between 10 and 20% of the overall amount of biodiesel synthesized. In other words, for every 100 kg of biodiesel produced, roughly 10 kg of glycerol is also generated [10]. According to the most recent figures available, European Union (EU) countries recorded annual biodiesel production increased from 3.7 million tons of oil in 2006 to approximately 11 million tons of oil in 2015 and forecasted to increase slightly by 2050 [11]. This level of production will yield more than 1.2 million tons of crude glycerol as the main co-product. Given that crude glycerol exhibits poor characteristics including low heating value (16–22 MJ/kg), high oxygen content (52 wt.%), and poor combustion performance, the expanding biodiesel production in the future will undoubtedly result in an increasing supply and disposal problem. When disposed without proper treatment, there will be social and environmental problems, as along with glycerol, biodiesel washing wastewaters, methanol, and solid byproducts are also generated [12]. According to Nitayavardhana and Khanal [13], large scale biodiesel producers mainly valorized the solid bio-residues as compost or animal food whereas the crude glycerol was refined into pure form and utilized for commercial uses. However, the refinement process of crude glycerol is expensive and economically unviable [13]; as a result, many studies have been reported on crude glycerol upgrading into various valuable byproducts. Thus, researchers and industrialists believe that this approach will improve the economics of biodiesel by reducing its manufacturing costs.
Thus, there is a growing number of research papers to examine alternative technologies to improve crude glycerol into higher quality fuels with lower capital costs that are environmentally benign. Interest in this subject has been growing rapidly by the frequency of publications from 2010 to 2022 as shown in Figure 2. The number of publications of upgrading crude glycerol via biological approaches is the highest, more than 1152 studies. From previous studies, it is mentioned that this kind of technology is favored because of cheap start-up and running costs as well as mild operating conditions. However, the longer reaction time, high solvent usage, and complexity in handling microorganisms which act as biocatalysts are the main drawbacks. Moreover, the number of studies using physicochemical methods was reported as more than 479 published articles. Works relating to esterification, transesterification, and etherification of crude glycerol were prominent techniques to emulsify blended fuels from crude glycerol which can be directly used as transportation fuels. However, several hindrances do affect their development including the challenges to scale up the methodologies as batch processes to an industrial scale. Additionally, due to the high oxygen concentration of feedstock and the time-consuming process [14][15], esterification and transesterification are still insufficient as a single process for upgrading crude glycerol.
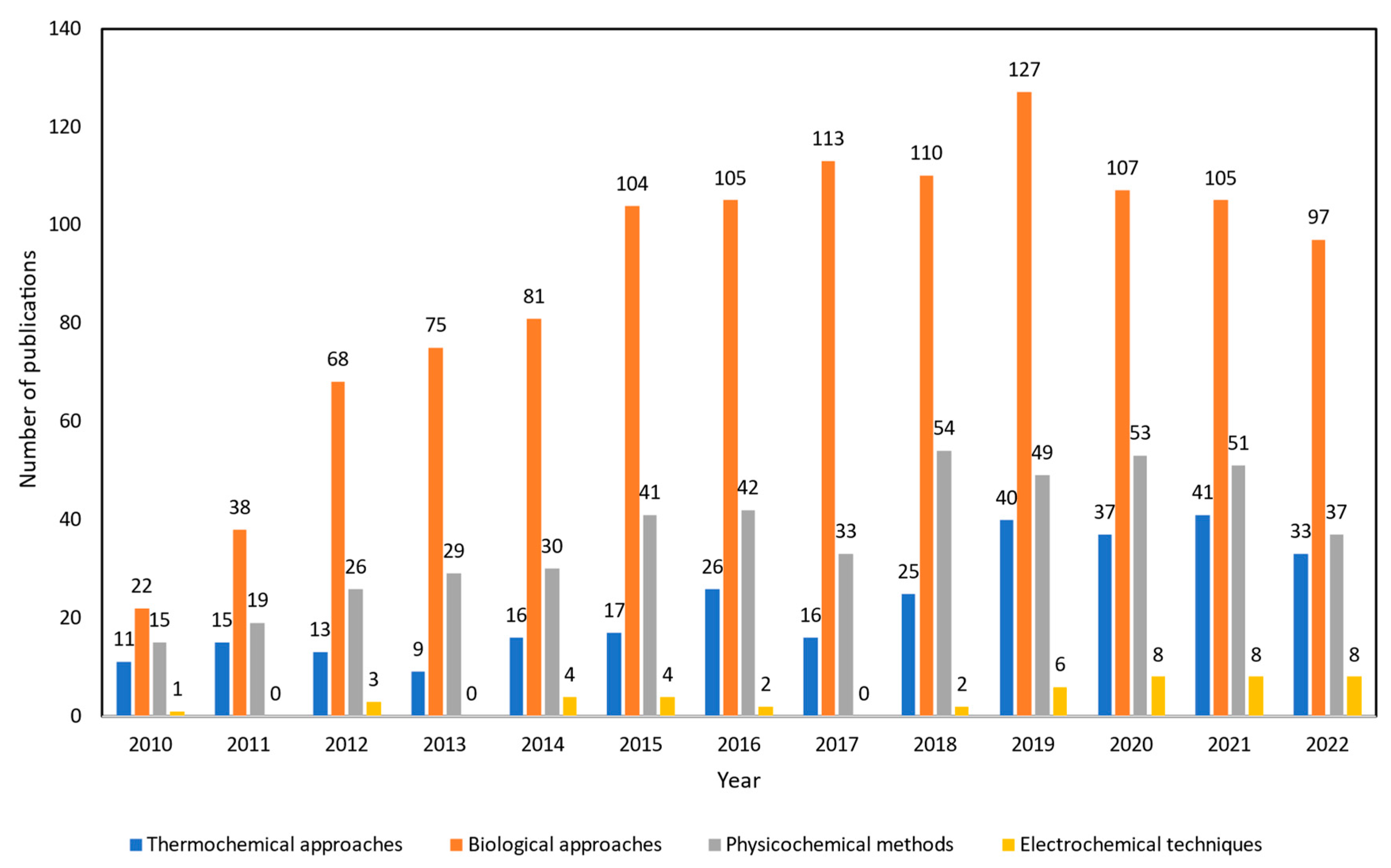
Figure 2. Number of publications about crude glycerol upgrading from 2010 to 2022. The Web of Science (WoS) search engine was used for the data collection by using specific key words. For thermochemical approaches, “(gasification OR pyrolysis OR supercritical water OR catalytic pyrolysis OR microwave assisted pyrolysis OR steam reforming OR aqueous phase reforming) AND crude glycerol” was applied. For biological approaches, “(fermentation OR microbial conversion) AND crude glycerol” was used. For physicochemical methods, “(emulsification OR esterification OR transesterification OR etherification) AND crude glycerol” was applied, and for electrochemical techniques, “(electrochemical OR electrocatalyst OR electrooxidation) AND crude glycerol” was utilized for the search.
Compared to other technologies, thermochemical technologies have been created and used conventionally. Almost 299 published studies have been recorded between 2010 and 2022 that investigate the efficiency of pyrolysis, gasification, supercritical water reforming, and aqueous phase reforming processes in valorizing waste/crude glycerol into various chemicals. Thermochemical approaches have been widely used in upgrading crude glycerol in industrial scale. However, its relatively high process temperature and pressure, increased manufacturing costs, and complex maintenance costs for long-term uses are the current knowledge gaps within this upgrading process. Plus, to some extent, there are needs for additional hydrogen external supply, microwave radiation heater, and use of expensive catalysts depending on intended end-products, which make it less favorable in terms of technoeconomic availability. On the other hand, electrochemical techniques are currently being explored as the number of works are increasing, as observed in Figure 2. There are only around 46 literature studies that have been reported which is due to this electrochemical crude glycerol upgrading technology still being underdeveloped and facing various issues. Thus, there is significant need to further study and compare the applicability of each mechanism to be applied in the industrial scale of crude glycerol upgrading.
2. Glycerol and Its Properties
Referred to both as propane-1, 2, 3-triol and glycerin, glycerol is a basic trihydroxy sugar alcohol, shown in Figure 3, which was identified in 1779 by Swedish scientist Carl Wilhelm Scheele. Three hydrophilic hydroxyl groups bonded to carbon make up polyhydric alcohol glycerol, a feature that makes it stable, with diverse reactions and functions [16]. Glycerol is currently employed in the manufacturing of a variety of food and drink products, as a solvent for food flavors and colors, medical products, personal hygiene products, fuel additives, and anti-freeze chemicals.
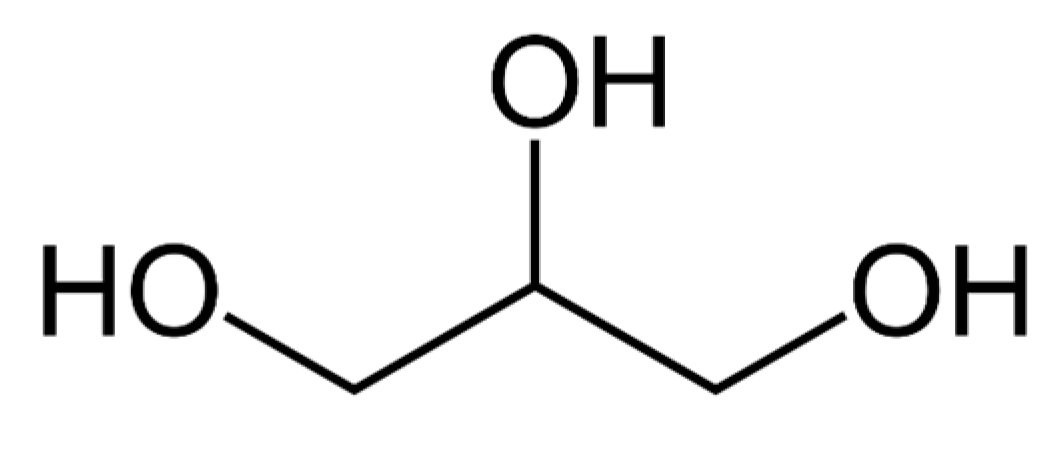
Figure 3. The structure of glycerol.
Glycerol can be found in three different forms: commercially produced glycerol, purified/refined glycerol, and crude/waste glycerol. The purity of refined and synthesized glycerol is higher than that of crude glycerol. While synthetic glycerol is produced in a separate way, often from propene, crude and pure glycerol are byproducts of the manufacturing of biodiesel.
3. Glycerol Upgrading
Since glycerol is available as a comparatively low-cost, high-volume biomass-derived waste, the valorization of crude glycerol which is underutilized is necessary for the economic viability of biodiesel manufacturing and waste fats/oil mass production. Due to its non-toxic, edible, biodegradable, and multifunctional qualities, glycerol is one of the top building block chemicals for organic synthesis from biomass [17]. The highly desirable molecule glycerol is utilized to make a wide range of beneficial chemical intermediates. Traditionally, one of the primary technologies for valorizing crude glycerol was through direct combustion approach. Such practice is inconvenient due to the production of acrolein, a hazardous combustion product of crude glycerol with low boiling point (53 °C) and high autoignition temperature (234 °C), and excess salt content [18]. When compared to other feedstocks, the volatile organic compound (VOC) emissions were notably greater, albeit with swirl refractory burners. Owing to its toxic potential, the acrolein produced throughout this technique has caused a lot of environmental concern [19]. Additionally, this technique of valorization falls short of utilizing full prospects of crude glycerol, which may be exploited through different high-end technologies.
In this regard, extensive research has been conducted over the past ten years on the chemical conversion of glycerol into specialized compounds with high added value [20]. Additionally, because glycerol has a high degree of functionalization, it can be used as a precursor for the production of a variety of common chemicals, including syngas, alkenes, alcohols, diols, ethers, acids, esters, acrylates, and even polyglycerols by the use of a variety of processes, involving thermochemical approaches of pyrolysis, biological techniques—fermentation and microbial conversion, physicochemical methods of etherification, trans- and esterification, electro oxidation, hydrogenolysis, dehydration, carboxylation, halogenation, polymerization, and glycerol acetalization [21], as depicted in Figure 4. Thus, in this section, existing glycerol upgrading technologies will be summarized and compared in terms of its state-of-the-art, intended end-products, conversion efficiency, and their process condition.
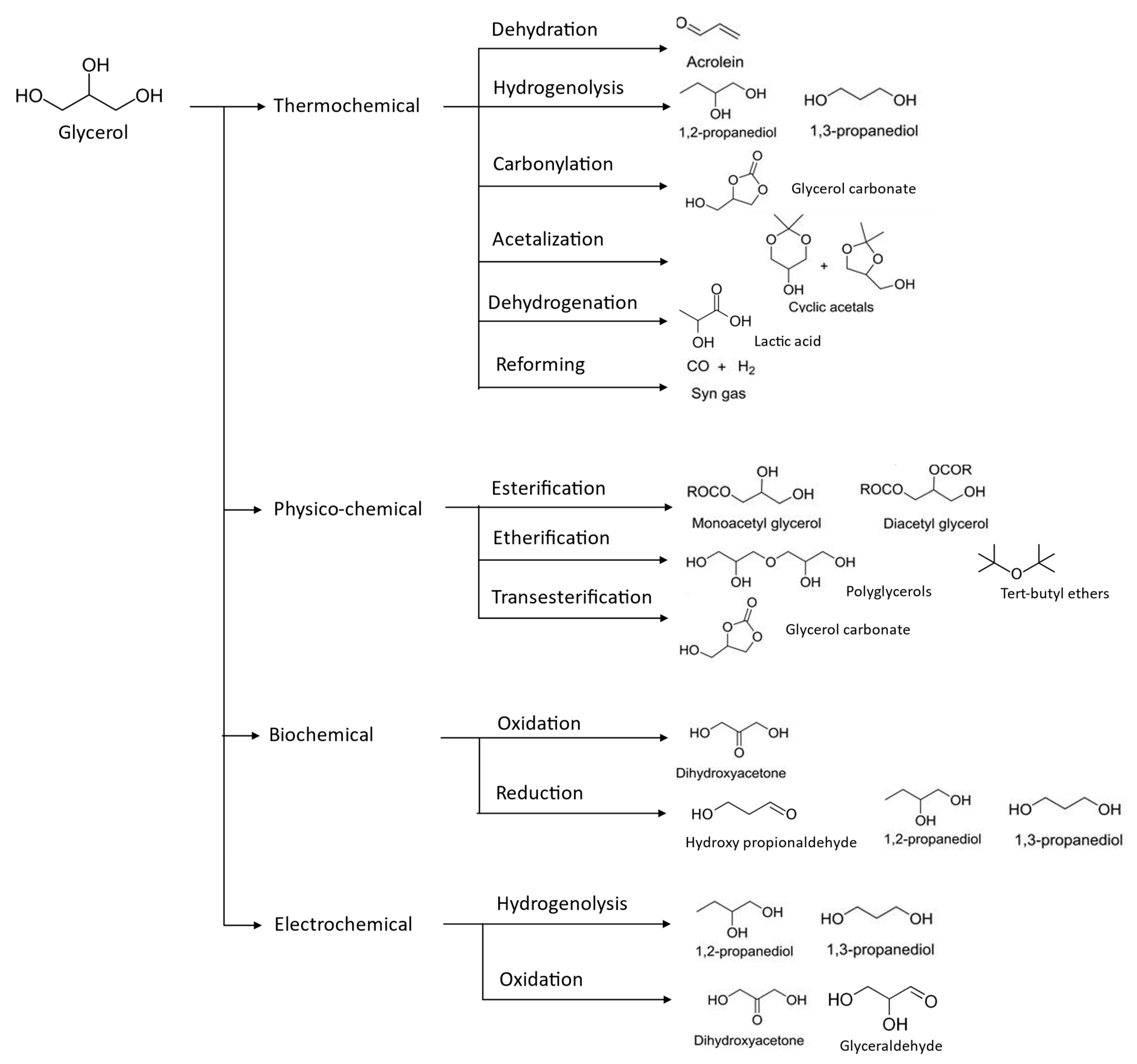
Figure 4. Valorization of glycerol compounds into various intended end-products through different chemical reaction.
3.1. Biochemical Approaches
3.1.1. Microbial Fermentation—Anaerobic and Aerobic Digestion
Crude glycerol can also be converted biologically by fermenting with various microbial biocatalysts including yeast, fungi, bacteria, mixed culture from wastewater, and microalgae under aerobic or anaerobic conditions to produce valuable chemicals. Recent studies have concentrated on using crude glycerol as a carbon source in microbial fermentation to produce green compounds and other platform chemicals [22][23][24]. Thus, such bioconversions via biochemical approaches with the aid of microbes are a promising resource and subsequently enhances the techno economical value of the biodiesel industry. Some advantages of biological conversion via aerobic or anaerobic fermentation are due to their superior yield, selectivity, and product recovery [25]. However, due to factors including pathogenicity, stringent anaerobic conditions, contaminants in the substrate, and lower kinetic reaction which have resulted in longer reaction times may impair their growth and conversion rates. Plus, the capacity for these organisms to be manufactured at an industry scale may also hinder their further application.
- 1.
-
Bacteria
Clostridium, Klebsiella, Komagataella, Lactobacillus, Lipomyces, Escherichia, Candida, and Raoultella are a few often used glycerol-consuming species of bacteria used to manufacture valuable products either in aerobic, anaerobic, or microaerobic condition [25]. The oxidative and reductive metabolic pathways, which also result in the formation of organic acids and alcohols, are used to assimilate glycerol [26]. Clostridium butyricum [26][27][28][29] and pasteurianam [30][31][32], Klebsiella oxytoca [33][34] and pneumonia [35][36][37][38][39], Citrobacter freundii [40][41] and werkmanii [42], Lactobacillus diolivorans [43], Enterobactor sp. [44] as well as engineered Escherichia coli [45][46][47] have been widely utilized in glycerol conversion into 1,2- and 1,3-PDO. From the aforementioned previous studies of various bacteria, it can be summarized that high PDO yields have been recorded, approximately 0.62–1.09 mol/mol glycerol, with inconsistent process productivity of 0.92–10.3 g/L/h [27][28][29][30][48]. Tang et al. [47] have fabricated an engineered E. coli with additional genes for the production of 1,3-PDO, B12-independent glycerol dehydratase (DhaB1), and its activating factor (DhaB2) from C. butyricum. The final PDO yield, productivity, and conversion rate recorded were 1.09 mol/mol, 2.61 g/L/h and 90.2% (g/g), respectively. Johnson and Rehmann [31] investigated the effect of decreasing pH during fermentation of crude glycerol with C. pasteurianum which led to the decreased in cell growth rate, CO2 production, and slower fermentations, thus resulting in higher butanol and PDO yields in a continuous process.
For lactic acid generation, engineered E. coli has been widely used in converting crude glycerol as an alternative to nicotinamide adenine dinucleotide (NAD+) regeneration in the absence of external electron acceptors [49]. The biological route of crude glycerol upgrading to lactic acid assisted by microbes stimulates the development of one specific configuration of either D- or L-, because of the excellent selectivity of lactate dehydrogenase (LDH) [50]. Based on Mazumdar et al. [49][51], E. coli has been engineered to improve its efficiency towards the conversion of glycerol to D- and L-lactic acid under microaerobic and anaerobic conditions. The overall yield of 0.85 and 0.93 mol/mol glycerol has been recorded for D- and L-lactic acid, respectively, with 34 g/L and 50 g/L of each final concentration, whereas Hong et al. [52] utilized E. coli AC-521 to transform glycerol into lactic acid under the aerobic condition at optimized fermentation conditions of 42 °C, pH 6.5, and 0.85 min−1 (KLA). The overall lactic acid concentration and glycerol consumption peaked at 88 h of fermentation, resulting in 86.0 g/L lactic acid yield with 0.97 g/L/h productivity as well as a yield of 0.9 mol/mol glycerol. Other than E. coli, there are also studies relating to the utilization of L. casei NCIM 2125 [53], K. phafii Glpard [54], and yeast S. cerevisiae [55] under fed-batch fermentation for lactic acid production.
The potential butanol producer, according to previous studies [30][48], C. pasteurianam under optimal condition can generate 0.43 mol/mol of butanol with 0.074 g/L/h of process productivity in 120 h using crude glycerol as a substrate. In a different study by Saini et al. [56], E. coli was used to assist the fermentation of crude glycerol into butanol with 0.35 mol/mol of butanol yield. Several type of bacteria were also utilized in the production of ethanol via fermentation of crude glycerol which includes E. coli SS1 [57], E. aerogenes TISTR 1468 [5], Pachysolen tannophilus [58], and engineered K. pneumonia [59] where they resulted in 1.00, 0.59, 0.56, and 0.89 mol/mol product yields, respectively. In addition, there is also a study of glycerol upgrading aided by Saccharomyces cerevisiae yeast [60] with an overall yield of 2.4 g/L ethanol recorded which proves the possibility of yeast in ethanol production.
- 2.
-
Microbial mixed cultures and other bacteria
Mixed culture communities have also been studied for their potential to upgrade crude glycerol into other value-added products which include hydrogen gas and PHAs. According to the literature review, the production of H2 was dominantly covered by fermentation of mixed culture extracted from wastewater or resulted from the mixing of rare strain types. Theoretically, crude glycerol fermentation provides a better capacity to generate hydrogen gas at the end of its reaction as compared to glucose fermentation due to it generating more NADH on a 3-carbon basis where one mole of hydrogen gas per mole of excess NADH. According to previous work, Mabutyana and Pott [61] extensively studied the homofermentative H2 production via co-fermentation of glycerol with phenolic compounds assisted by Rhodopseudomonas palustris under anaerobic conditions at temperature of 35 °C equipped with tungsten light bulbs. According to Varrone et al. [62] and Chen et al. [63], the application of enriched activity sludge or microbial mixed culture efficiently enhanced the conversion of crude glycerol into H2 with an approximate yield of 0.52–0.96 mol/mol glycerol, whereas Paenibacillus macerans [64] and Thermoanaerobacterium sp. [8], in another study, were also utilized in the fermentation of crude glycerol to produce H2 which resulted in 0.81 and 0.30 mol/mol of yield. In addition, crude glycerol may be used as a substrate for anaerobic digestion to produce biogas. In the acidogenesis and acetogenesis phases, it is degraded to volatile fatty acids (VFAs), and in the final methanogenesis stage, methane is produced from either acetic acid, CO2, or H2 by the methanogenic community [65]. For further development, anaerobic process optimization to produce hydrogen from crude glycerol is very essential and needs to be explored thoroughly.
- 3.
-
Fungi
Fungi are another promising biocatalyst to enhance the microbial conversion of crude glycerol into other specific valuable products such as single-cell oil (SCO). According to Chatzifragkou et al. [27], throughout the fermentation process, fungi tend to build up lipids inside their mycelia. Andre et al. [24] studied the yield of SCO and oxalic acid from the fermentation of crude glycerol with Lentinula edodes strains and Aspergillus niger strains in carbon-limited and nitrogen-limited conditions, respectively. The maximum yield of lipid of 3.5 g/L and 0.1 g/g biomass was recorded. The SCO product was dominantly composed of oleic acids. Galactomyces geotrichum and ascomycetous fungus were utilized in SCO production of crude glycerol. Overall lipid yield of 0.44 g/g of dry biomass was accumulated, 4 times higher than pure glycerol control, with 38.0% of glycerol conversion throughout the process. A similar trend was also shown by Chatzifragkou et al. [27], who produced an improvement in lipid yield of 11.6 g/L with 70% of fat in biomass via application of eukaryotic microbes, Thamnidium elegans, for SCO production. In this study, acetic acid and mannitol were also generated as their side-products.
- 4.
-
Yeast
Succinic, citric, itaconic, and malic acids may be generated from glycerol utilizing fungus of the genera Rhizopus, Aspergillus, and Ustilago [66][67]. However, recently, yeasts also exhibit the promising potential to catabolize crude glycerol via an aerobic route into citric and succinic acid. Yarrowia lipolytica exhibits the ability in upgrading crude glycerol into citric acid under the aerobic condition with optimal process parameters [68][69][70]. Li et al. [71] investigated the effect of co-fermentation of crude glycerol with various agro-residues into succinic acid under aerobic condition using Y. lipolytica. At the end of the process, 53.6 g/L of succinic acid concentration, process productivity of 1.5 g/L/h, and an overall yield of 0.5 mol/mol were produced.
3.1.2. Bio-Electrochemical Fermentation
Bio-electrochemical fermentation is one of the emerging metabolic pathways which combines both biochemical and electrochemical approaches in upgrading crude glycerol. This technology exploits microbes to catalyze redox reactions in an electrochemical reactor under mild processing conditions [72]. Microbial catalysts on the electrode have been utilized to enhance the electrochemical reaction as well as increase the rate and yields of glycerol conversion. In this approach, the electric current supplied was utilized to permit the fermentation of crude glycerol as the feedstock. By improving cells’ capacity to regenerate NAD+ into NADH, the cathodic current will stimulate microbial reduction processes and thus be able to change fermentation profiles. As mentioned in the previous study [73], a crucial NAD+/NADH ratio greater than 4 has been linked to high PDO productivity and a high specific growth rate of K. pneumoniae. However, there are still a very small number of investigations using glycerol bio-electro fermentations within the cathode [72][73][74][75][76]. Zhou et al. [72] studied the carbon and electron fluxes during the bio-electro fermentation of crude glycerol using batch biocathodes and showed an increase in 1,3-PDO generation, while in the study by Choi et al. [77], C. pasteurianum was used and successfully demonstrated a shift in the microbial metabolism with improved PDO production when an electrical potential is applied. Recently, Xafenias et al. [74] reported a significant increase in PDO synthesis with aid of Clostridiaceae. High PDO concentrations of 42 g/L were recorded which shows the potential of this approach to be further studied.
3.2. Thermochemical Approaches
3.2.1. Gasification Pyrolysis
Pyrolysis is a simple method of upgrading glycerol, yet it is just as significant as other processes. This method involves pyrolyzing feedstocks under specific processing conditions—high temperature (>600 °C), high pressure, in the deoxygenated environment inside a continuous or batch reactor [78][79], resulting from the thermochemical conversion of carbon contained in the feedstock of crude glycerol. The following processes take place to generate gaseous end-products [80]: (1) pyrolysis and devolatilization of feedstocks at relatively low temperature; (2) further degradation of the primary byproducts by continuous heating; and (3) coking gasification which leads to the production of high value-added syngas including carbon monoxide (CO), ethylene, methane, and hydrogen (H2).
Dehydration and hydrogenation reactions dominantly happened throughout this approach. In the previous work of Skoulou and Zabaniotou [81], they used mixed crude glycerol, crude olive oil, and grains in a fixed bed reactor operating at 750–850 °C. Co-gasification attempts were made to increase the amount of hydrogen produced from the gas produced during the gasification process. According to the findings, combining crude glycerol with olive particles at a biomass weight ratio of 49% resulted in gas emissions of between 0.4 and 1.2 Nm3/kg [78][81]. In another work, based on Figure 5, Blass et al. [82] employed gasification pyrolysis of glycerol with H2 in a reactor containing dehydration, hydrogenation and upgrading stages in series with help of HZSM-5 and Pd/α-Al2O3 catalysts at a temperature of 400 °C to produce a mixture of acetaldehyde, acrolein and hydroxypropanone, propanal, and olefins. Propanal condensed over Brønsted acid sites to produce C4–5 olefins, which then underwent high conversion to produce C2–3 olefins, which accounts for this. During this staged process, a C–C bond formed, and negligible carbon was dissipated as CO as a byproduct. Thus, due to environmental concerns of greenhouse gases production, industrial scaleup is limited.
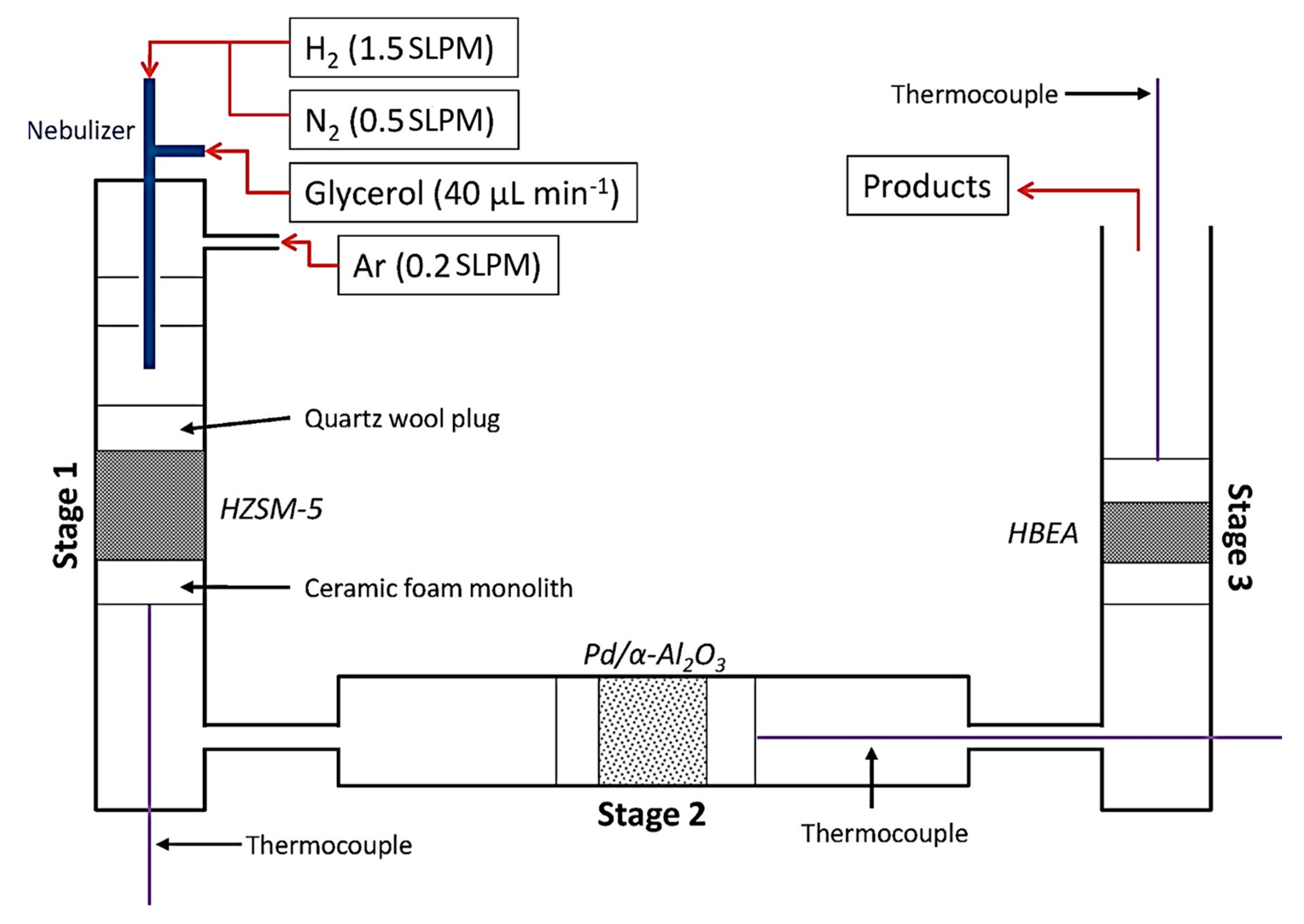
Figure 5. Schematic illustration of three staged reactor used for gasification pyrolysis of crude glycerol feed.
3.2.2. Fast Pyrolysis
Fast pyrolysis is a method that has been adopted to decompose biomass into essential chemical feedstocks and biofuels usually with presence of catalyst. For the manufacture of highly stable liquid fuels, the catalyst-assisted cracking process is executed at temperatures between 400 and 800 °C with a short residence time of between 0.5 and 3 s [79][81][83]. The yield and characteristics of the end-products of the process are also significantly influenced by different types of feedstocks, environment, and particularly catalyst species [84][85]. Hence, optimizing the parameters promotes enhanced byproducts yield such hydrocarbon oils with shorter chain (C1–12), gases, and char [86][87]. Conventionally, hydrotreating of bio-oils and hydrocarbon wastes, derived from engine oil, transmission oil, and hydraulic oil, which fractured into high value-added products via thermal decomposition of pyrolysis and treated with external hydrogen gas has become a key technology in upgrading bio-oil into transportation fuels [88]. However, from the researchers' knowledge, there are no works on hydrotreating of crude glycerol which may be due to its high operating costs, and it is not worth it in waste upcycling, in term of its techno economic view.
There are several previous published works on upgrading crude glycerol via fast pyrolysis assisted with catalysts. He et al. [83] performed catalytic fast pyrolysis of crude glycerol into bio-based BTX in a tandem micro-reactor using ZSM-5/bentonite catalysts which operating at 520–536 °C. The study resulted in only 8.1 wt.% of bio-BTX yield (15% carbon yield) based on crude glycerol feed with fresh catalyst. In their work, the reduced end-product yields are mainly due to coking issues. The majority of the coke (10.5 wt.%) deposit was on the ZSM-5 planes, which not only reduced the number of Lewis and Brønsted acid sites but also clogged the pores, deactivating the catalyst. From previous published works, relatively poor BTX yields (2 wt.%) were also recorded, with different catalysts—Al2O3 [89] and Pd-Ru catalysts [90]. However, in other works [91][92], zeolites favored the production of acrolein (86%) from catalytic conversion of glycerol with higher glycerol conversion.
3.2.3. Supercritical Fluids
One of the alternative thermochemical conversion techniques for crude glycerol utilizes supercritical fluids, including water, ethanol, methanol, and CO2, as the processing medium. This method has drawn a lot of interest recently and was demonstrated to be an appropriate reaction medium for biomass reforming. Either with or without the assistance of a catalyst, supercritical fluid reforming of crude glycerol and model compounds was already explored [9][93][94][95][96][97]. As an additional point, crude glycerol incorporates 6.5% of water content; thus, the prior drying step can be neglected via this hydrothermal approach which reduced its processing cost.
Lighter weight liquid constituents and permanent gases, mixture of H2, CO, CO2, methane, and higher hydrocarbons will be produced during this glycerol upgrading process, with or without a catalyst. In addition, the rate of conversion, reaction selectivity, and the nature of the gas obtained are acknowledged to be impacted by the inclusion of a catalyst–homogenous catalysts (metal salt and acid catalysts lead to enhanced glycerol conversion and acrolein production). The utilization of various catalysts within this approach has been discussed in previous literature works by Markocic et al. [98] and Pavlovic et al. [99]. With the utilization of catalysts, the energy required for the procedure is reduced and it proceeds at lower operating temperatures and minimizes capital expenditures.
According to previous studies [94][95][98][100][101], it has been observed that the main solvent for crude glycerol upgrading via supercritical fluid reforming is water, while there are not many works utilizing CO2 [93], and methanol [102]. According to Markocic et al. [98], utilization of water is due to the unique properties of supercritical water itself, when temperature and pressure of the normal water both reached its critical point (Tc = 373 °C, Pc = 22.1 MPa). The solubility of additional molecules of glycerol in water is thus improved by the decreasing dielectric constant, and supercritical water takes on the characteristics of a nonpolar solvent [103]. Other than that, reduced dynamic viscosity of supercritical water also leads to improved diffusion coefficients of dissolved substances. Therefore, polar, and ionic reactions as well as free radical reactions may occur at this state. In sub- and supercritical water, crude glycerol conversion proceeds quickly, and the high solubility of the intermediates in supercritical water prevents the development of tar and coke, and high product yields are produced at comparatively low temperatures [100][101].
3.2.4. Steam Reforming
Crude glycerol can be upgraded into H2 using the steam reforming method. In steam reforming of crude glycerol, there are four main steps—feedstocks refinement, reforming, water-gas-shift (WGS) reaction, and end-product refining [104]. In the first step of raw material purification, sulfur and chloride impurities are removed. Then, in a fixed-bed reactor, the reforming process is commonly conducted. Thus, the raw material is brought into contact with steam while being catalyzed by a heterogeneous catalyst, resulting in the production of syngas and other gaseous byproducts. The catalytic WGS reactor collects this gas mixture, where the CO combines with the steam to produce more H2. Finally, high purity H2 is produced by purifying the resulting gas stream using various techniques, including pressure swing adsorption (PSA) and/or membrane systems. This strategy is suitable to prepare chemicals made of oxygenated hydrocarbons, including waste/bio-glycerol. These processes may be complemented by undesirable ones, which including methanation, dehydration, carbon precursor reactions, and dehydrogenation, depending on the operational circumstances.
Since scaling up the steam reforming strategy to an industrial level would not necessitate substantial changes to the present infrastructures used for natural gas reforming, this method can be regarded as one of the most promising glycerol upgrading technologies [105]. Industrial steam reforming exploits γ-Al2O3 supported Ni catalysts as its catalyst option due to their increased availability and lower cost compared to precious metal-based catalysts [106][107][108][109][110]. Furthermore, Ni-based catalytic systems are capable of cleaving C–C, O–H, and C–H bonds as well as the WGS reaction, with typical NiO contents of 10 to 25 wt.% [111]. WGS can eliminate the adsorbed CO from the surface of the catalyst by converting it into CO2, the first three bonds must be broken in the reforming phase [106][109]. Due to its improved mechanical and chemical resistance, large surface area (SBET), and favorable metal dispersion, the γ-Al2O3 support is also commonly employed. However, coke deposition and particle sintering cause some deactivation in these systems. The first occurrence is often attributed to the γ-Al2O3 acidic characteristic, whereas sintering is attributed, among other aspects, to metal phases and γ-Al2O3 hydrothermal disturbances [106][112]. Within steam reforming operation parameters, these changes are often connected to the transition of γ-Al2O3 into other stable phases.
3.2.5. Aqueous Phase Reforming
With a similar objective as steam reforming technology, the catalytic method of aqueous phase reforming was developed to convert oxygenated hydrocarbons generated from biomass into H2-rich gas. However, when compared to other catalytic thermochemical approaches such as pyrolysis, gasification, or steam reforming, aqueous phase reforming exhibited various advantages [113][114]. Aqueous phase reforming chemically converts the liquid phase-feedstocks at ambient processing conditions, low temperature, around 150 to 300 °C, due to the partial evaporation of the water. Operating pressure, approximately 1.5–7 MPa, is used during the reformation of crude glycerol, such that ample pressure was exerted to allow the H2-rich effluent to be in situ filtered via swing adsorption approach or, in some cases, using membrane technology which addresses its storage issue and efficiently isolated CO2 from the main products. With extremely low CO concentrations (100–1000 ppm), it is possible to generate H2 gas in a batch reactor assisted with metal catalysts, produced higher amount of H2 as compared to the existing steam reforming technique. In conclusion, this approach functions at minimal pressure, at a low temperature, and with less costly technology [104]. Additionally, they are simple to integrate into safe, environmentally friendly energy production systems.
3.2.6. Microwave-Assisted Pyrolysis
The microwave absorbent must be capable of absorbing microwave energy and heat to the requisite temperature in order to pyrolyze waste [115]. Based on a study by Leong et al. [116], using a carbonaceous catalyst and temperatures between 300–800 °C, a microwave heating technique utilized to convert crude glycerol collected from biodiesel plants into biofuels. The product yields in each phase of such a process is determined by the processing conditions including residence time, process temperature, and type of catalysts employed during the reaction, all of which affect the reaction system and relative activation energy. From previous studies [115][116][117][118], it is observed that there was a decreased total mass of gaseous products due to the catalyst’s inclination toward hydrogen gas selectivity. Other than that, temperature reduction and longer residence times increased the overall energy production. The findings demonstrated that crude glycerol has the potential to produce syngas and bio-oil for use in bioenergy [118].
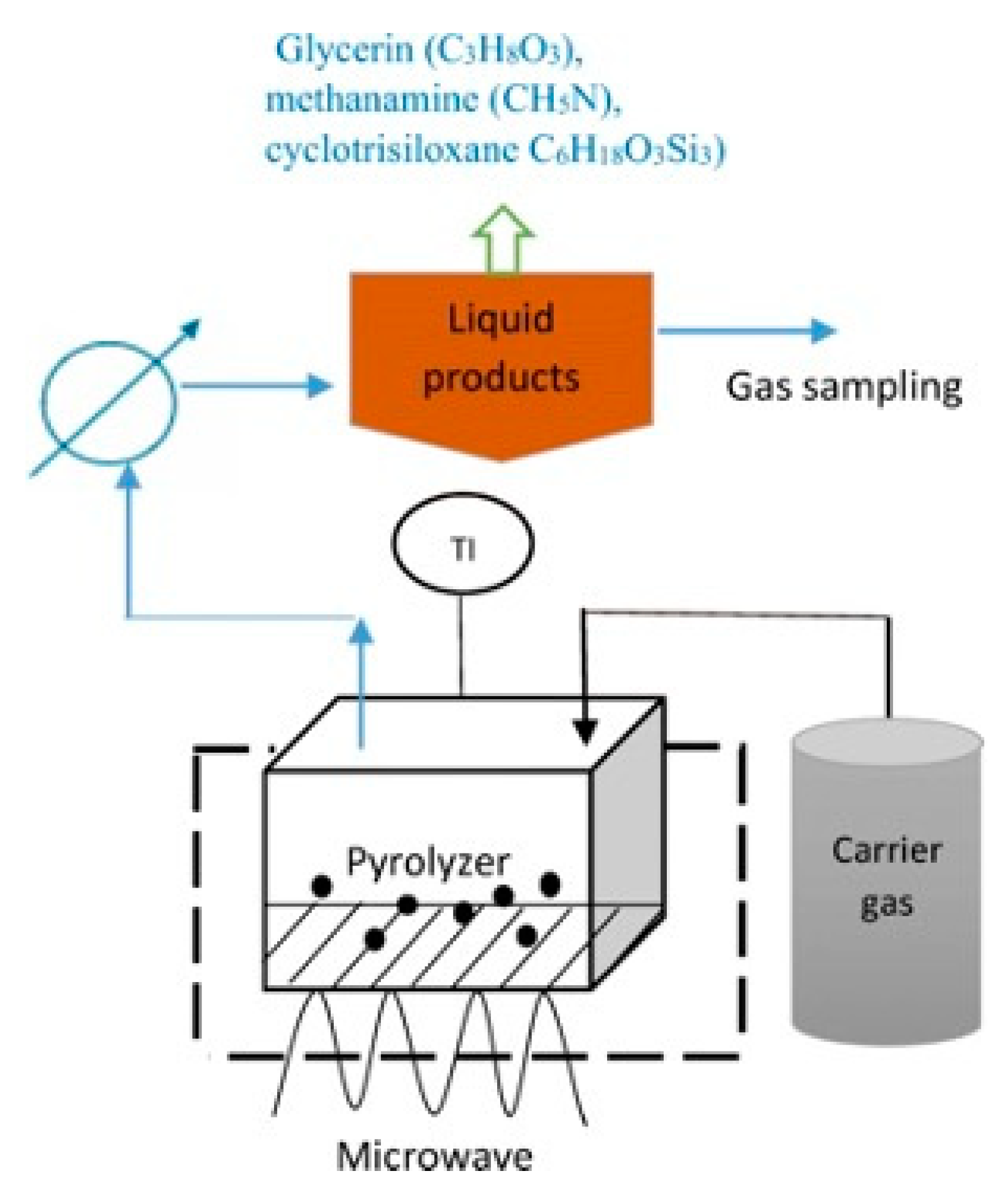
Figure 6. Microwave-assisted pyrolysis of crude glycerol by magnetron microwave in a quartz reactor with the presence of activated carbon as catalyst.
References
- IEA Renewable Electricity Growth Is Accelerating Faster than Ever Worldwide, Supporting the Emergence of the New Global Energy Economy. Available online: https://www.iea.org/news/renewable-electricity-growth-is-accelerating-faster-than-ever-worldwide-supporting-the-emergence-of-the-new-global-energy-economy (accessed on 25 July 2022).
- International Energy Agency. World Energy Outlook; OECD/IEA: Paris, France, 2009; ISBN 926428205X.
- Gielen, D.; Gorini, R.; Wagner, N.; Leme, R.; Gutierrez, L.; Prakash, G.; Asmelash, E.; Janeiro, L.; Gallina, G.; Vale, G.; et al. Global Energy Transformation: A Roadmap to 2050; IRENA: Abu Dhabi, United Arab Emirates, 2019.
- Loaces, I.; Rodríguez, C.; Amarelle, V.; Fabiano, E.; Noya, F. Improved Glycerol to Ethanol Conversion by E. Coli Using a Metagenomic Fragment Isolated from an Anaerobic Reactor. J. Ind. Microbiol. Biotechnol. 2016, 43, 1405–1416.
- Boonyawanich, S.; Haosagul, S.; Pisutpaisal, N. Ethanol Production from Crude glycerol Using Glucose as Co-Carbon Source. Biomass Convers. Biorefinery 2021, 2021, 1–10.
- Haider, M.H.; Dummer, N.F.; Knight, D.W.; Jenkins, R.L.; Howard, M.; Moulijn, J.; Taylor, S.H.; Hutchings, G.J. Efficient Green Methanol Synthesis from Glycerol. Nat. Chem. 2015, 7, 1028–1032.
- Hulteberg, C.; Nörregård, Ö.; Brandin, J.; Leveau, A. Bio Propane: Tailoring WO3/ZrO2 Catalyst for the Dehydration of Glycerol to Acrolein. In Proceedings of the 17th Nordic Symposium on Catalysis, Lund, Sweden, 14–16 June 2016; Lund University Publications: Lund, Sweden, 2016.
- Sittijunda, S.; Reungsang, A. Media Optimization for Biohydrogen Production from Crude glycerol by Anaerobic Thermophilic Mixed Cultures. Int. J. Hydrogen Energy 2012, 37, 15473–15482.
- Yu-Wu, Q.M.; Weiss-Hortala, E.; Barna, R.; Boucard, H.; Bulza, S. Glycerol and Bioglycerol Conversion in Supercritical Water for Hydrogen Production. Environ. Technol. 2012, 33, 2245–2255.
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, Consumption, Prices, Characterization and New Trends in Combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493.
- Ennetta, R.; Soyhan, H.S.; Koyunoğlu, C.; Demir, V.G. Current Technologies and Future Trends for Biodiesel Production: A Review. Arab. J. Sci. Eng. 2022, 47, 15133–15151.
- Varanda, M.G.; Pinto, G.; Martins, F. Life Cycle Analysis of Biodiesel Production. Fuel Process. Technol. 2011, 92, 1087–1094.
- Nitayavardhana, S.; Khanal, S.K. Biodiesel-Derived Crude Glycerol Bioconversion to Animal Feed: A Sustainable Option for a Biodiesel Refinery. Bioresour. Technol. 2011, 102, 5808–5814.
- Zheng, L.; Xia, S.; Hou, Z.; Zhang, M.; Hou, Z. Transesterification of Glycerol with Dimethyl Carbonate over Mg-Al Hydrotalcites. Chin. J. Catal. 2014, 35, 310–318.
- Helwani, Z.; Othman, M.; Aziz, N.; Kim, J.; Fernando, W. Solid Heterogeneous Catalysts for Transesterification of Triglycerides with Methanol: A Review. Appl. Catal. Gen. 2009, 363, 1–10.
- Ayoub, M.; Abdullah, A.Z. Critical Review on the Current Scenario and Significance of Crude Glycerol Resulting from Biodiesel Industry towards More Sustainable Renewable Energy Industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686.
- Samudrala, S.P. Glycerol Transformation to Value-Added 1,3-Propanediol Production: A Paradigm for a Sustainable Biorefinery Process. In Glycerine Production and Transformation—An Innovative Platform for Sustainable Biorefinery and Energy; BoD—Books on Demand: Norderstedt, Germany, 2019.
- Gupta, M.; Kumar, N. Scope and Opportunities of Using Glycerol as an Energy Source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556.
- Hejna, A.; Kosmela, P.; Formela, K.; Piszczyk, Ł.; Haponiuk, J.T. Potential Applications of Crude Glycerol in Polymer Technology–Current State and Perspectives. Renew. Sustain. Energy Rev. 2016, 66, 449–475.
- Kenar, J.A. Glycerol as a Platform Chemical: Sweet Opportunities on the Horizon? Lipid Technol. 2007, 19, 249–253.
- Zheng, Y.; Chen, X.; Shen, Y. Commodity Chemicals Derived from Glycerol, an Important Biorefinery Feedstock. Chem. Rev. 2008, 110, 1807.
- Rodrigues, C.V.; Santana, K.O.; Nespeca, M.G.; Rodrigues, A.V.; Pires, L.O.; Maintinguer, S.I. Energy Valorization of Crude Glycerol and Sanitary Sewage in Hydrogen Generation by Biological Processes. Int. J. Hydrogen Energy 2020, 45, 11943–11953.
- Sedghi, R.; Shahbeik, H.; Rastegari, H.; Rafiee, S.; Peng, W.; Nizami, A.-S.; Gupta, V.K.; Chen, W.-H.; Lam, S.S.; Pan, J. Turning Biodiesel Glycerol into Oxygenated Fuel Additives and Their Effects on the Behavior of Internal Combustion Engines: A Comprehensive Systematic Review. Renew. Sustain. Energy Rev. 2022, 167, 112805.
- André, A.; Diamantopoulou, P.; Philippoussis, A.; Sarris, D.; Komaitis, M.; Papanikolaou, S. Biotechnological Conversions of Bio-Diesel Derived Crude glycerol into Added-Value Compounds by Higher Fungi: Production of Biomass, Single Cell Oil and Oxalic Acid. Ind. Crops Prod. 2010, 31, 407–416.
- Pradima, J.; Kulkarni, M.R. Review on Enzymatic Synthesis of Value Added Products of Glycerol, a by-Product Derived from Biodiesel Production. Resour.-Effic. Technol. 2017, 3, 394–405.
- Loureiro-Pinto, M.; González-Benito, G.; Coca, M.; Lucas, S.; García-Cubero, M.T. Valorization of Crude Glycerol from the Biodiesel Industry to 1,3-propanediol by Clostridium Butyricum DSM 10702: Influence of Pretreatment with Ion Exchange Resins. Can. J. Chem. Eng. 2016, 94, 1242–1248.
- Chatzifragkou, A.; Papanikolaou, S.; Dietz, D.; Doulgeraki, A.I.; Nychas, G.-J.E.; Zeng, A.-P. Production of 1,3-Propanediol by Clostridium Butyricum Growing on Biodiesel-Derived Crude Glycerol through a Non-Sterilized Fermentation Process. Appl. Microbiol. Biotechnol. 2011, 91, 101–112.
- González-Pajuelo, M.; Andrade, J.; Vasconcelos, I. Production of 1,3-Propanediol by Clostridium Butyricum VPI 3266 in Continuous Cultures with High Yield and Productivity. J. Ind. Microbiol. Biotechnol. 2005, 32, 391–396.
- Wilkens, E.; Ringel, A.K.; Hortig, D.; Willke, T.; Vorlop, K.-D. High-Level Production of 1,3-Propanediol from Crude Glycerol by Clostridium Butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063.
- Jensen, T.Ø.; Kvist, T.; Mikkelsen, M.J.; Christensen, P.V.; Westermann, P. Fermentation of Crude Glycerol from Biodiesel Production by Clostridium Pasteurianum. J. Ind. Microbiol. Biotechnol. 2012, 39, 709–717.
- Johnson, E.E.; Rehmann, L. The Role of 1,3-Propanediol Production in Fermentation of Glycerol by Clostridium Pasteurianum. Bioresour. Technol. 2016, 209, 1–7.
- Pyne, M.E.; Sokolenko, S.; Liu, X.; Srirangan, K.; Bruder, M.R.; Aucoin, M.G.; Moo-Young, M.; Chung, D.A.; Chou, C.P. Disruption of the Reductive 1,3-Propanediol Pathway Triggers Production of 1, 2-Propanediol for Sustained Glycerol Fermentation by Clostridium Pasteurianum. Appl. Environ. Microbiol. 2016, 82, 5375–5388.
- Metsoviti, M.; Paraskevaidi, K.; Koutinas, A.; Zeng, A.-P.; Papanikolaou, S. Production of 1,3-Propanediol, 2,3-Butanediol and Ethanol by a Newly Isolated Klebsiella Oxytoca Strain Growing on Biodiesel-Derived Glycerol Based Media. Process Biochem. 2012, 47, 1872–1882.
- Yang, G.; Tian, J.; Li, J. Fermentation of 1,3-Propanediol by a Lactate Deficient Mutant of Klebsiella Oxytoca under Microaerobic Conditions. Appl. Microbiol. Biotechnol. 2007, 73, 1017–1024.
- Laura, M.; Monica, T.; Dan-Cristian, V. The Effect of Crude Glycerol Impurities on 1,3-Propanediol Biosynthesis by Klebsiella Pneumoniae DSMZ 2026. Renew. Energy 2020, 153, 1418–1427.
- Cheng, K.-K.; Zhang, J.-A.; Liu, D.-H.; Sun, Y.; Liu, H.-J.; Yang, M.-D.; Xu, J.-M. Pilot-Scale Production of 1,3-Propanediol Using Klebsiella Pneumoniae. Process Biochem. 2007, 42, 740–744.
- Oh, B.-R.; Hong, W.-K.; Heo, S.-Y.; Luo, L.H.; Kondo, A.; Seo, J.-W.; Kim, C.H. The Production of 1,3-Propanediol from Mixtures of Glycerol and Glucose by a Klebsiella Pneumoniae Mutant Deficient in Carbon Catabolite Repression. Bioresour. Technol. 2013, 130, 719–724.
- Yang, X.; Kim, D.S.; Choi, H.S.; Kim, C.K.; Thapa, L.P.; Park, C.; Kim, S.W. Repeated Batch Production of 1,3-Propanediol from Biodiesel Derived Crude glycerol by Klebsiella Pneumoniae. Chem. Eng. J. 2017, 314, 660–669.
- Yang, X.; Choi, H.S.; Lee, J.H.; Lee, S.K.; Han, S.O.; Park, C.; Kim, S.W. Improved Production of 1,3-Propanediol from Biodiesel-Derived Crude Glycerol by Klebsiella Pneumoniae in Fed-Batch Fermentation. Chem. Eng. J. 2018, 349, 25–36.
- Metsoviti, M.; Zeng, A.-P.; Koutinas, A.A.; Papanikolaou, S. Enhanced 1,3-Propanediol Production by a Newly Isolated Citrobacter Freundii Strain Cultivated on Biodiesel-Derived Crude glycerol through Sterile and Non-Sterile Bioprocesses. J. Biotechnol. 2013, 163, 408–418.
- Celińska, E.; Drożdżyńska, A.; Jankowska, M.; Białas, W.; Czaczyk, K.; Grajek, W. Genetic Engineering to Improve 1,3-Propanediol Production in an Isolated Citrobacter Freundii Strain. Process Biochem. 2015, 50, 48–60.
- Maervoet, V.E.; Beauprez, J.; De Maeseneire, S.L.; Soetaert, W.K.; De Mey, M. Citrobacter Werkmanii, a New Candidate for the Production of 1,3-Propanediol: Strain Selection and Carbon Source Optimization. Green Chem. 2012, 14, 2168–2178.
- Vivek, N.; Pandey, A.; Binod, P. Biological Valorization of Pure and Crude Glycerol into 1,3-Propanediol Using a Novel Isolate Lactobacillus Brevis N1E9. 3.3. Bioresour. Technol. 2016, 213, 222–230.
- Kongjan, P.; Jariyaboon, R.; Reungsang, A.; Sittijunda, S. Co-Fermentation of 1,3-Propanediol and 2,3-Butanediol from Crude Glycerol Derived from the Biodiesel Production Process by Newly Isolated Enterobacter sp.: Optimization Factors Affecting. Bioresour. Technol. Rep. 2021, 13, 100616.
- Clomburg, J.M.; Gonzalez, R. Metabolic Engineering of Escherichia coli for the Production of 1, 2-propanediol from Glycerol. Biotechnol. Bioeng. 2011, 108, 867–879.
- Przystałowska, H.; Lipiński, D.; Słomski, R. Biotechnological Conversion of Glycerol from Biofuels to 1,3-Propanediol Using Escherichia coli. Acta Biochim. Pol. 2015, 62, 23–34.
- Tang, X.; Tan, Y.; Zhu, H.; Zhao, K.; Shen, W. Microbial Conversion of Glycerol to 1,3-Propanediol by an Engineered Strain of Escherichia coli. Appl. Environ. Microbiol. 2009, 75, 1628–1634.
- Khanna, S.; Goyal, A.; Moholkar, V.S. Production of N-Butanol from Biodiesel Derived Crude Glycerol Using Clostridium Pasteurianum Immobilized on Amberlite. Fuel 2013, 112, 557–561.
- Mazumdar, S.; Blankschien, M.D.; Clomburg, J.M.; Gonzalez, R. Efficient Synthesis of L-Lactic Acid from Glycerol by Metabolically Engineered Escherichia coli. Microb. Cell Factories 2013, 12, 7.
- Sharninghausen, L.S.; Campos, J.; Manas, M.G.; Crabtree, R.H. Efficient Selective and Atom Economic Catalytic Conversion of Glycerol to Lactic Acid. Nat. Commun. 2014, 5, 5084.
- Mazumdar, S.; Clomburg, J.M.; Gonzalez, R. Escherichia coli Strains Engineered for Homofermentative Production of D-Lactic Acid from Glycerol. Appl. Environ. Microbiol. 2010, 76, 4327–4336.
- Hong, A.; Cheng, K.; Peng, F.; Zhou, S.; Sun, Y.; Liu, C.; Liu, D. Strain Isolation and Optimization of Process Parameters for Bioconversion of Glycerol to Lactic Acid. J. Chem. Technol. Biotechnol. 2009, 84, 1576–1581.
- Sumitha, V.; Christy Mathelin, R.; Sivanandham, M. Effect of Major and Minor Nutrients on Lactic Acid Production Using Biodiesel Waste-Derived Crude Glycerol as a Carbon Source by Lactobacillus Casei NCIM 2125. Energy Sources Part Recovery Util. Environ. Eff. 2018, 40, 1322–1331.
- Tamires Moreira Melo, N.; Pontes, G.C.; Procópio, D.P.; de Gois e Cunha, G.C.; Eliodório, K.P.; Costa Paes, H.; Basso, T.O.; Parachin, N.S. Evaluation of Product Distribution in Chemostat and Batch Fermentation in Lactic Acid-Producing Komagataella Phaffii Strains Utilizing Glycerol as Substrate. Microorganisms 2020, 8, 781.
- Baek, S.-H.; Kwon, E.Y.; Kim, Y.H.; Hahn, J.-S. Metabolic Engineering and Adaptive Evolution for Efficient Production of D-Lactic Acid in Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2737–2748.
- Saini, M.; Wang, Z.W.; Chiang, C.-J.; Chao, Y.-P. Metabolic Engineering of Escherichia coli for Production of N-Butanol from Crude Glycerol. Biotechnol. Biofuels 2017, 10, 173.
- Soo, C.-S.; Yap, W.-S.; Hon, W.-M.; Ramli, N.; Shah, U.K.M.; Phang, L.-Y. Co-Production of Hydrogen and Ethanol by Escherichia coli SS1 and Its Recombinant. Electron. J. Biotechnol. 2017, 30, 64–70.
- Liu, X.; Jensen, P.R.; Workman, M. Bioconversion of Crude Glycerol Feedstocks into Ethanol by Pachysolen Tannophilus. Bioresour. Technol. 2012, 104, 579–586.
- Oh, B.-R.; Heo, S.-Y.; Lee, S.-M.; Hong, W.-K.; Park, J.M.; Jung, Y.R.; Kim, D.-H.; Sohn, J.-H.; Seo, J.-W.; Kim, C.H. Production of 2-Butanol from Crude Glycerol by a Genetically-Engineered Klebsiella Pneumoniae Strain. Biotechnol. Lett. 2014, 36, 57–62.
- Yu, K.O.; Kim, S.W.; Han, S.O. Engineering of Glycerol Utilization Pathway for Ethanol Production by Saccharomyces Cerevisiae. Bioresour. Technol. 2010, 101, 4157–4161.
- Mabutyana, L.; Pott, R.W. Photo-Fermentative Hydrogen Production by Rhodopseudomonas Palustris CGA009 in the Presence of Inhibitory Compounds. Int. J. Hydrogen Energy 2021, 46, 29088–29099.
- Varrone, C.; Giussani, B.; Izzo, G.; Massini, G.; Marone, A.; Signorini, A.; Wang, A. Statistical Optimization of Biohydrogen and Ethanol Production from Crude Glycerol by Microbial Mixed Culture. Int. J. Hydrogen Energy 2012, 37, 16479–16488.
- Chen, Y.; Yin, Y.; Wang, J. Comparison of Fermentative Hydrogen Production from Glycerol Using Immobilized and Suspended Mixed Cultures. Int. J. Hydrogen Energy 2021, 46, 8986–8994.
- Gupta, A.; Murarka, A.; Campbell, P.; Gonzalez, R. Anaerobic Fermentation of Glycerol in Paenibacillus Macerans: Metabolic Pathways and Environmental Determinants. Appl. Environ. Microbiol. 2009, 75, 5871–5883.
- Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.-L.; Lam, M.K.; Chen, W.-H. Organic Carbonate Production Utilizing Crude Glycerol Derived as By-Product of Biodiesel Production: A Review. Energies 2020, 13, 1483.
- Iyyappan, J.; Bharathiraja, B.; Baskar, G.; Kamalanaban, E. Process Optimization and Kinetic Analysis of Malic Acid Production from Crude Glycerol Using Aspergillus Niger. Bioresour. Technol. 2019, 281, 18–25.
- Kuenz, A.; Hoffmann, L.; Goy, K.; Bromann, S.; Prüße, U. High-Level Production of Succinic Acid from Crude Glycerol by a Wild Type Organism. Catalysts 2020, 10, 470.
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The Citric Acid Production from Raw Glycerol by Yarrowia Lipolytica Yeast and Its Regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397.
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia Lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36.
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric Acid Production from Glycerol-Containing Waste of Biodiesel Industry by Yarrowia Lipolytica in Batch, Repeated Batch, and Cell Recycle Regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979.
- Li, C.; Gao, S.; Yang, X.; Lin, C.S.K. Green and Sustainable Succinic Acid Production from Crude Glycerol by Engineered Yarrowia Lipolytica via Agricultural Residue Based in Situ Fibrous Bed Bioreactor. Bioresour. Technol. 2018, 249, 612–619.
- Zhou, M.; Chen, J.; Freguia, S.; Rabaey, K.; Keller, J. Carbon and Electron Fluxes during the Electricity Driven 1,3-Propanediol Biosynthesis from Glycerol. Environ. Sci. Technol. 2013, 47, 11199–11205.
- Dennis, P.G.; Harnisch, F.; Yeoh, Y.K.; Tyson, G.W.; Rabaey, K. Dynamics of Cathode-Associated Microbial Communities and Metabolite Profiles in a Glycerol-Fed Bioelectrochemical System. Appl. Environ. Microbiol. 2013, 79, 4008–4014.
- Xafenias, N.; Anunobi, M.O.; Mapelli, V. Electrochemical Startup Increases 1,3-Propanediol Titers in Mixed-Culture Glycerol Fermentations. Process Biochem. 2015, 50, 1499–1508.
- Roume, H.; Arends, J.B.; Ameril, C.P.; Patil, S.A.; Rabaey, K. Enhanced Product Recovery from Glycerol Fermentation into 3-Carbon Compounds in a Bioelectrochemical System Combined with in Situ Extraction. Front. Bioeng. Biotechnol. 2016, 4, 73.
- Selembo, P.A.; Perez, J.M.; Lloyd, W.A.; Logan, B.E. High Hydrogen Production from Glycerol or Glucose by Electrohydrogenesis Using Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2009, 34, 5373–5381.
- Choi, O.; Kim, T.; Woo, H.M.; Um, Y. Electricity-Driven Metabolic Shift through Direct Electron Uptake by Electroactive Heterotroph Clostridiumpasteurianum. Sci. Rep. 2014, 4, 6961.
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C.C. Catalytic Conversion of Glycerol for Sustainable Production of Solketal as a Fuel Additive: A Review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031.
- Harussani, M.; Sapuan, S.; Rashid, U.; Khalina, A.; Ilyas, R. Pyrolysis of Polypropylene Plastic Waste into Carbonaceous Char: Priority of Plastic Waste Management amidst COVID-19 Pandemic. Sci. Total Environ. 2022, 803, 149911.
- Li, X.-L.; Zhou, Q.; Pan, S.-X.; He, Y.; Chang, F. A Review of Catalytic Upgrading of Biodiesel Crude glycerol to Valuable Products. Curr. Green Chem. 2020, 7, 259–266.
- Skoulou, V.K.; Zabaniotou, A.A. Co-Gasification of Crude Glycerol with Lignocellulosic Biomass for Enhanced Syngas Production. J. Anal. Appl. Pyrolysis 2013, 99, 110–116.
- Blass, S.D.; Hermann, R.J.; Persson, N.E.; Bhan, A.; Schmidt, L.D. Conversion of Glycerol to Light Olefins and Gasoline Precursors. Appl. Catal. Gen. 2014, 475, 10–15.
- He, S.; Muizebelt, I.; Heeres, A.; Schenk, N.; Blees, R.; Heeres, H. Catalytic Pyrolysis of Crude Glycerol over Shaped ZSM-5/Bentonite Catalysts for Bio-BTX Synthesis. Appl. Catal. B Environ. 2018, 235, 45–55.
- Isahak, W.N.R.W.; Hisham, M.W.; Yarmo, M.A.; Hin, T.Y. A Review on Bio-Oil Production from Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923.
- Khosravanipour Mostafazadeh, A.; Solomatnikova, O.; Drogui, P.; Tyagi, R.D. A Review of Recent Research and Developments in Fast Pyrolysis and Bio-Oil Upgrading. Biomass Convers. Biorefin. 2018, 8, 739–773.
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in Waste Oil to Sustainable Energy, with Emphasis on Pyrolysis Techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753.
- Harussani, M.M.; Rashid, U.; Sapuan, S.M.; Abdan, K. Low-Temperature Thermal Degradation of Disinfected COVID-19 Non-Woven Polypropylene—Based Isolation Gown Wastes into Carbonaceous Char. Polymers 2021, 13, 3980.
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-Oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720.
- Shahnazari, A. Catalytic Co-Conversion of Glycerol and Proton-Donor Species to Gasoline-Range Aromatics over Alumina. Ph.D. Thesis, University of New Brunswick, Fredericton, NB, Canada, 2016; p. 113. Available online: https://unbscholar.lib.unb.ca/islandora/object/unbscholar%3A8006/datastream/PDF/download/citation.pdf (accessed on 1 February 2023).
- Suib, S.L. New and Future Developments in Catalysis: Catalytic Biomass Conversion; Newnes: Oxford, UK, 2013; ISBN 0-444-53879-8.
- Hoang, T.Q.; Zhu, X.; Danuthai, T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Conversion of Glycerol to Alkyl-Aromatics over Zeolites. Energy Fuels 2010, 24, 3804–3809.
- Suh, Y.-W.; Jang, H.-S.; Bae, K.-B. Method for Producing Bio-Aromatics from Glycerol. U.S. Patent 9,834,489, 5 December 2017.
- Cui, M.; Mi, M.; Zhang, Y.; Xu, W.; Wang, M.; Shao, R.; Ding, J. Dehydration of Glycerol over H6P2W18O62/γ-Al2O3 Prepared by Supercritical Method. New J. Chem. 2022, 47, 1342–1348.
- Ott, L.; Bicker, M.; Vogel, H. Catalytic Dehydration of Glycerol in Sub-and Supercritical Water: A New Chemical Process for Acrolein Production. Green Chem. 2006, 8, 214–220.
- Müller, J.B.; Vogel, F. Tar and Coke Formation during Hydrothermal Processing of Glycerol and Glucose. Influence of Temperature, Residence Time and Feed Concentration. J. Supercrit. Fluids 2012, 70, 126–136.
- Onwudili, J.A.; Williams, P.T. Hydrothermal Reforming of Bio-Diesel Plant Waste: Products Distribution and Characterization. Fuel 2010, 89, 501–509.
- Van Bennekom, J.; Venderbosch, R.; Assink, D.; Heeres, H. Reforming of Methanol and Glycerol in Supercritical Water. J. Supercrit. Fluids 2011, 58, 99–113.
- Markočič, E.; Kramberger, B.; van Bennekom, J.G.; Heeres, H.J.; Vos, J.; Knez, Ž. Glycerol Reforming in Supercritical Water; a Short Review. Renew. Sustain. Energy Rev. 2013, 23, 40–48.
- Pavlovic, I.; Knez, Z.; Skerget, M. Hydrothermal Reactions of Agricultural and Food Processing Wastes in Sub-and Supercritical Water: A Review of Fundamentals, Mechanisms, and State of Research. J. Agric. Food Chem. 2013, 61, 8003–8025.
- Remón, J.; Zhu, G.; Budarin, V.L.; Clark, J.H. Analysis and Optimisation of a Microwave-Assisted Hydrothermal Process for the Production of Value-Added Chemicals from Glycerol. Green Chem. 2018, 20, 2624–2636.
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Jena, U.; Brewer, C.E. Integrated Extraction and Catalytic Upgrading of Biocrude Oil from Co-Hydrothermal Liquefaction of Crude Glycerol and Algae. Energy Fuels 2021, 35, 12165–12174.
- Ezhova, N.; Korosteleva, I.; Kolesnichenko, N.; Kuz’min, A.; Khadzhiev, S.; Vasil’eva, M.; Voronina, Z. Glycerol Carboxylation to Glycerol Carbonate in the Presence of Rhodium Complexes with Phosphine Ligands. Pet. Chem. 2012, 52, 91–96.
- Krammer, P.; Mittelstädt, S.; Vogel, H. Investigating the Synthesis Potential in Supercritical Water. Chem. Eng. Technol. Ind. Chem. Equip.-Process Eng. 1999, 22, 126–130.
- El Doukkali, M.; Iriondo, A.; Gandarias, I. Enhanced Catalytic Upgrading of Glycerol into High Value-Added H2 and Propanediols: Recent Developments and Future Perspectives. Mol. Catal. 2020, 490, 110928.
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722.
- Charisiou, N.; Papageridis, K.; Tzounis, L.; Sebastian, V.; Hinder, S.; Baker, M.; AlKetbi, M.; Polychronopoulou, K.; Goula, M. Ni Supported on CaO-MgO-Al2O3 as a Highly Selective and Stable Catalyst for H2 Production via the Glycerol Steam Reforming Reaction. Int. J. Hydrogen Energy 2019, 44, 256–273.
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Dou, B.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. Ni/Y2O3–ZrO2 Catalyst for Hydrogen Production through the Glycerol Steam Reforming Reaction. Int. J. Hydrogen Energy 2020, 45, 10442–10460.
- Dahdah, E.; Estephane, J.; Gennequin, C.; Aboukais, A.; Abi-Aad, E.; Aouad, S. Zirconia Supported Nickel Catalysts for Glycerol Steam Reforming: Effect of Zirconia Structure on the Catalytic Performance. Int. J. Hydrogen Energy 2020, 45, 4457–4467.
- Iriondo, A.; Barrio, V.; Cambra, J.; Arias, P.; Guemez, M.; Sanchez-Sanchez, M.; Navarro, R.; Fierro, J. Glycerol Steam Reforming over Ni Catalysts Supported on Ceria and Ceria-Promoted Alumina. Int. J. Hydrogen Energy 2010, 35, 11622–11633.
- Zamzuri, N.H.; Mat, R.; Amin, N.A.S.; Talebian-Kiakalaieh, A. Hydrogen Production from Catalytic Steam Reforming of Glycerol over Various Supported Nickel Catalysts. Int. J. Hydrogen Energy 2017, 42, 9087–9098.
- El-Bousiffi, M.; Gunn, D. A Dynamic Study of Steam-Methane Reforming. Int. J. Heat Mass Transf. 2007, 50, 723–733.
- Yan, Z.; Liu, S.; Zhang, Y.; Wang, T.; Luo, S.; Chu, W.; Jing, F. The Role of Zr in NiZrAl Oxides Catalyst and the Evaluation on Steam Reforming of Glycerol for Hydrogen Product. Catal. Today 2019, 319, 229–238.
- Davda, R.; Shabaker, J.; Huber, G.; Cortright, R.; Dumesic, J.A. A Review of Catalytic Issues and Process Conditions for Renewable Hydrogen and Alkanes by Aqueous-Phase Reforming of Oxygenated Hydrocarbons over Supported Metal Catalysts. Appl. Catal. B Environ. 2005, 56, 171–186.
- El Doukkali, M.; Iriondo, A.; Arias, P.; Requies, J.; Gandarías, I.; Jalowiecki-Duhamel, L.; Dumeignil, F. A Comparison of Sol–Gel and Impregnated Pt or/and Ni Based γ-Alumina Catalysts for Bioglycerol Aqueous Phase Reforming. Appl. Catal. B Environ. 2012, 125, 516–529.
- Lam, S.S.; Mahari, W.A.W.; Jusoh, A.; Chong, C.T.; Lee, C.L.; Chase, H.A. Pyrolysis Using Microwave Absorbents as Reaction Bed: An Improved Approach to Transform Used Frying Oil into Biofuel Product with Desirable Properties. J. Clean. Prod. 2017, 147, 263–272.
- Leong, S.K.; Lam, S.S.; Ani, F.N.; Ng, J.-H.; Chong, C.T. Production of Pyrolyzed Oil from Crude Glycerol Using a Microwave Heating Technique. Int. J. Technol. 2016, 7, 323–331.
- Ganesapillai, M.; Manara, P.; Zabaniotou, A. Effect of Microwave Pretreatment on Pyrolysis of Crude Glycerol–Olive Kernel Alternative Fuels. Energy Convers. Manag. 2016, 110, 287–295.
- Ng, J.-H.; Leong, S.K.; Lam, S.S.; Ani, F.N.; Chong, C.T. Microwave-Assisted and Carbonaceous Catalytic Pyrolysis of Crude Glycerol from Biodiesel Waste for Energy Production. Energy Convers. Manag. 2017, 143, 399–409.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
3 times
(View History)
Update Date:
24 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




