Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jeffrey Scott Cross and Version 3 by Rita Xu.
Crude glycerol is the main byproduct of biodiesel manufacturing from oleaginous crops and other biomass-derived oils. Approximately 10% crude glycerol is produced with every batch of biodiesel. Worldwide, there is a glut of glycerol and the price of it has decreased considerably. There are real opportunities for valorizing crude glycerol into higher value-added chemicals which can improve the economic viability of biodiesel production as an alternative fuel. Exploring new potential applications of glycerol in various sectors is needed such as in pharmaceuticals, food and beverages, cosmetics, and as a transportation fuel.
- crude glycerol
- biodiesel
- thermo-, electro-, and biochemical glycerol upgrading
1. Introduction
Renewable energy is a rapidly developing industry in order to reduce the dependence on fossil fuels, oil, and natural gas as main resources for energy generation. In addition to energy savings, the shift to a civilization powered by renewable energy sources including waste, solar, wind, biomass, tidal, wave, and geothermal energy becomes an even more significant option in global use of energy. Based on the International Energy Agency (IEA) [1] report at the COP26 Climate Change Conference, it is anticipated that between 2021 and 2026, there will be a 50% increase in renewable capacity compared to the period between 2015 and 2020 which is due to aggressive support from government policies towards clean energy goals and the replacement of non-renewable fossil fuels. Renewable energy sources are anticipated to increase at the quickest rate of all energy sources according to the World Energy Outlook [2]. Furthermore, this type of energy is also expected to dominate two-thirds of the total global primary energy supply in 2050 based on the REmap Case, held in Abu Dhabi [3]. Municipal solid waste (MSW), including biomass and plastic waste, is the only energy source in this spectrum that is based on carbon. Figure 1 illustrates a breakdown of renewables in the energy sector, where the key role of bioenergy (30%) [3] comprised of biomass industry, biomass buildings, liquid biofuels and biogas as well as biomass power, which is mainly sourced from biomass and waste. Hence, there is much potential for further analysis for green energy development.
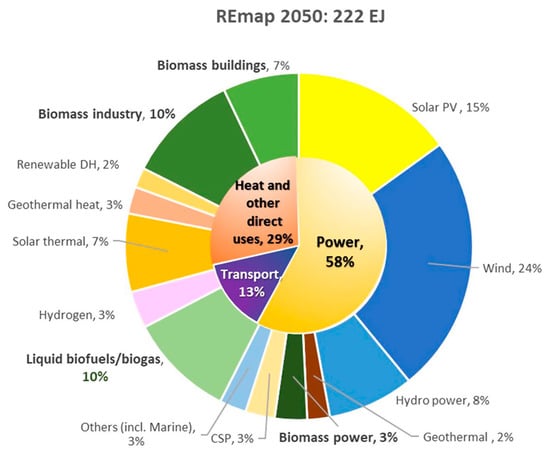
Figure 1. Percentage of total final energy consumption by renewables, according to the IRENA’s Remap CASE 2050 (%). Note: Excludes non-energy use. DH refers to district heat. CSP refers to concentrated solar power. PV refers to photovoltaic.
Crude glycerol, which is widely produced as a byproduct of biodiesel production, has become of particular interest as a potential feedstock to convert into value-added biofuels, e.g., bio ethanol [4][5][4,5], green methanol [6], bio propane [7], and hydrogen [8][9][8,9]. Several publications report that glycerol yield is between 10 and 20% of the overall amount of biodiesel synthesized. In other words, for every 100 kg of biodiesel produced, roughly 10 kg of glycerol is also generated [10]. According to the most recent figures available, European Union (EU) countries recorded annual biodiesel production increased from 3.7 million tons of oil in 2006 to approximately 11 million tons of oil in 2015 and forecasted to increase slightly by 2050 [11]. This level of production will yield more than 1.2 million tons of crude glycerol as the main co-product. Given that crude glycerol exhibits poor characteristics including low heating value (16–22 MJ/kg), high oxygen content (52 wt.%), and poor combustion performance, the expanding biodiesel production in the future will undoubtedly result in an increasing supply and disposal problem. When disposed without proper treatment, there will be social and environmental problems, as along with glycerol, biodiesel washing wastewaters, methanol, and solid byproducts are also generated [12]. According to Nitayavardhana and Khanal [13], large scale biodiesel producers mainly valorized the solid bio-residues as compost or animal food whereas the crude glycerol was refined into pure form and utilized for commercial uses. However, the refinement process of crude glycerol is expensive and economically unviable [13]; as a result, many studies have been reported on crude glycerol upgrading into various valuable byproducts. Thus, researchers and industrialists believe that this approach will improve the economics of biodiesel by reducing its manufacturing costs.
Thus, there is a growing number of research papers to examine alternative technologies to improve crude glycerol into higher quality fuels with lower capital costs that are environmentally benign. Interest in this subject has been growing rapidly by the frequency of publications from 2010 to 2022 as shown in Figure 2. The number of publications of upgrading crude glycerol via biological approaches is the highest, more than 1152 studies. From previous studies, it is mentioned that this kind of technology is favored because of cheap start-up and running costs as well as mild operating conditions. However, the longer reaction time, high solvent usage, and complexity in handling microorganisms which act as biocatalysts are the main drawbacks. Moreover, the number of studies using physicochemical methods was reported as more than 479 published articles. Works relating to esterification, transesterification, and etherification of crude glycerol were prominent techniques to emulsify blended fuels from crude glycerol which can be directly used as transportation fuels. However, several hindrances do affect their development including the challenges to scale up the methodologies as batch processes to an industrial scale. Additionally, due to the high oxygen concentration of feedstock and the time-consuming process [14][15][14,15], esterification and transesterification are still insufficient as a single process for upgrading crude glycerol.
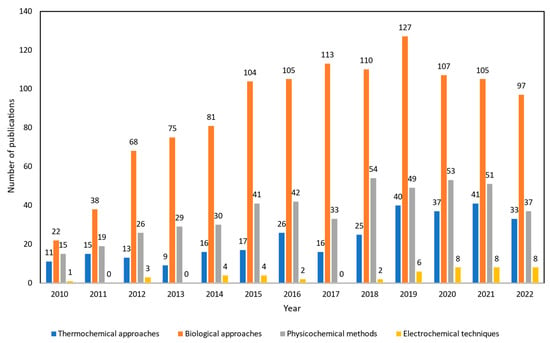
Figure 2. Number of publications about crude glycerol upgrading from 2010 to 2022. The Web of Science (WoS) search engine was used for the data collection by using specific key words. For thermochemical approaches, “(gasification OR pyrolysis OR supercritical water OR catalytic pyrolysis OR microwave assisted pyrolysis OR steam reforming OR aqueous phase reforming) AND crude glycerol” was applied. For biological approaches, “(fermentation OR microbial conversion) AND crude glycerol” was used. For physicochemical methods, “(emulsification OR esterification OR transesterification OR etherification) AND crude glycerol” was applied, and for electrochemical techniques, “(electrochemical OR electrocatalyst OR electrooxidation) AND crude glycerol” was utilized for the search.
Compared to other technologies, thermochemical technologies have been created and used conventionally. Almost 299 published studies have been recorded between 2010 and 2022 that investigate the efficiency of pyrolysis, gasification, supercritical water reforming, and aqueous phase reforming processes in valorizing waste/crude glycerol into various chemicals. Thermochemical approaches have been widely used in upgrading crude glycerol in industrial scale. However, its relatively high process temperature and pressure, increased manufacturing costs, and complex maintenance costs for long-term uses are the current knowledge gaps within this upgrading process. Plus, to some extent, there are needs for additional hydrogen external supply, microwave radiation heater, and use of expensive catalysts depending on intended end-products, which make it less favorable in terms of technoeconomic availability. On the other hand, electrochemical techniques are currently being explored as the number of works are increasing, as observed in Figure 2. There are only around 46 literature studies that have been reported which is due to this electrochemical crude glycerol upgrading technology still being underdeveloped and facing various issues. Thus, there is significant need to further study and compare the applicability of each mechanism to be applied in the industrial scale of crude glycerol upgrading.
2. Glycerol and Its Properties
Referred to both as propane-1, 2, 3-triol and glycerin, glycerol is a basic trihydroxy sugar alcohol, shown in Figure 3, which was identified in 1779 by Swedish scientist Carl Wilhelm Scheele. Three hydrophilic hydroxyl groups bonded to carbon make up polyhydric alcohol glycerol, a feature that makes it stable, with diverse reactions and functions [16]. Glycerol is currently employed in the manufacturing of a variety of food and drink products, as a solvent for food flavors and colors, medical products, personal hygiene products, fuel additives, and anti-freeze chemicals.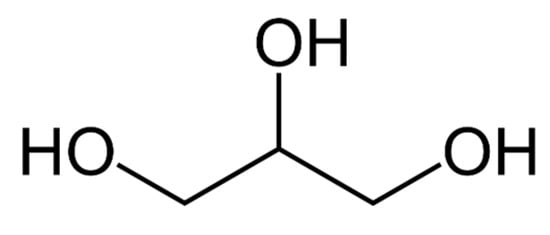
Figure 3. The structure of glycerol.
3. Glycerol Upgrading
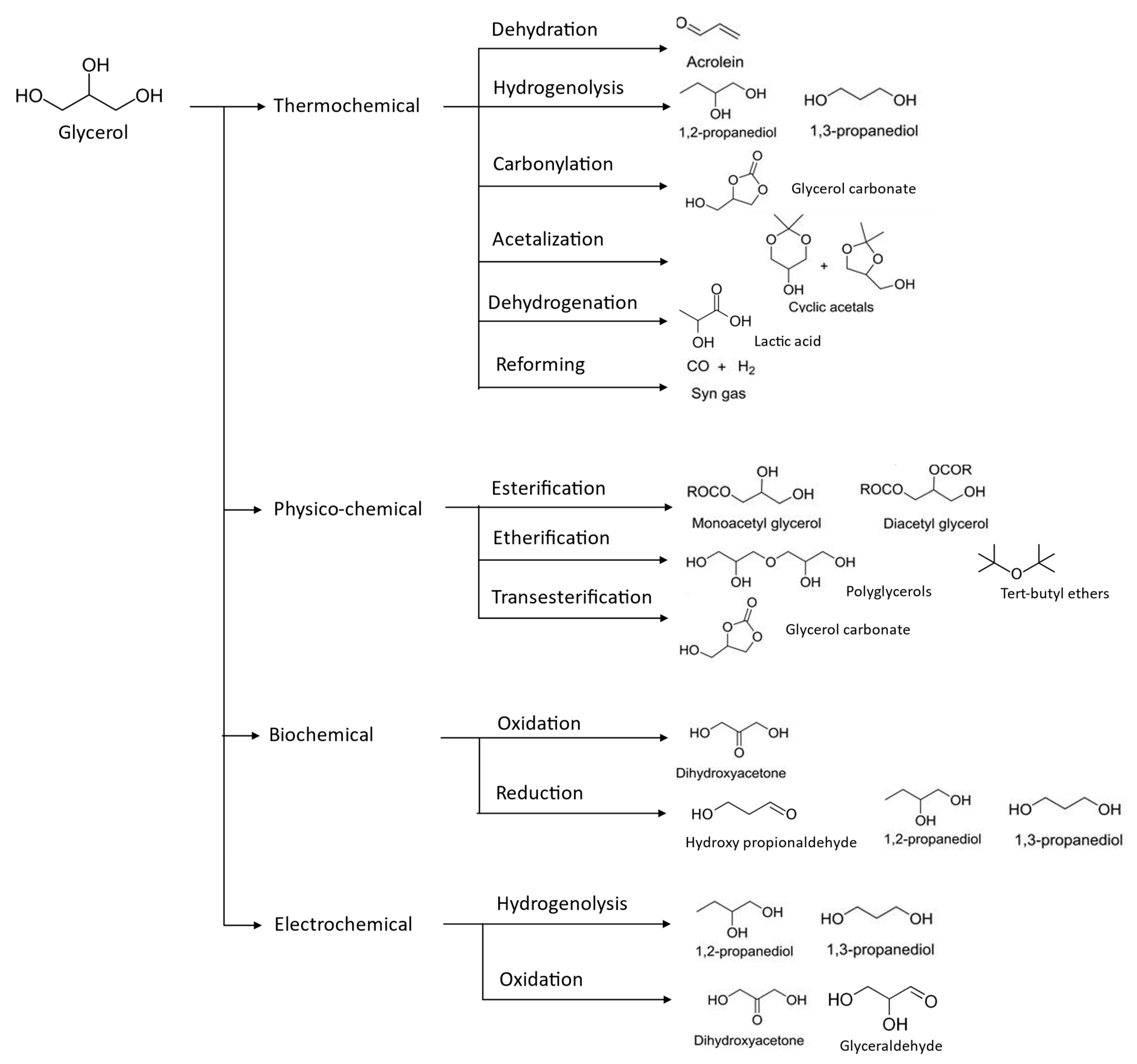
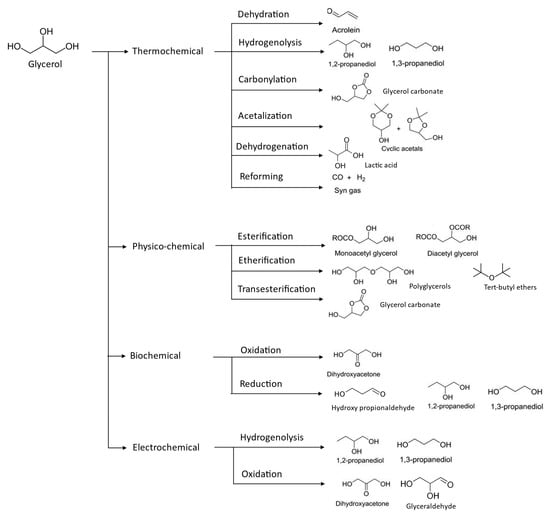
Figure 4. Valorization of glycerol compounds into various intended end-products through different chemical reaction.
3.1. Biochemical Approaches
3.1.1. Microbial Fermentation—Anaerobic and Aerobic Digestion
- 1.
-
BacteriaClostridium, Klebsiella, Komagataella, Lactobacillus, Lipomyces, Escherichia, Candida, and Raoultella are a few often used glycerol-consuming species of bacteria used to manufacture valuable products either in aerobic, anaerobic, or microaerobic condition [25][85]. The oxidative and reductive metabolic pathways, which also result in the formation of organic acids and alcohols, are used to assimilate glycerol [26][86]. Clostridium butyricum [26][27][28][29][86,87,88,89] and pasteurianam [30][31][32][90,91,92], Klebsiella oxytoca [33][34][93,94] and pneumonia [35][36][37][38][39][38,95,96,97,98], Citrobacter freundii [40][41][99,100] and werkmanii [42][101], Lactobacillus diolivorans [43][102], Enterobactor sp. [44][39] as well as engineered Escherichia coli [45][46][47][103,104,105] have been widely utilized in glycerol conversion into 1,2- and 1,3-PDO. From the aforementioned previous studies of various bacteria, it can be summarized that high PDO yields have been recorded, approximately 0.62–1.09 mol/mol glycerol, with inconsistent process productivity of 0.92–10.3 g/L/h [27][28][29][30][48][87,88,89,90,106]. Tang et al. [47][105] have fabricated an engineered E. coli with additional genes for the production of 1,3-PDO, B12-independent glycerol dehydratase (DhaB1), and its activating factor (DhaB2) from C. butyricum. The final PDO yield, productivity, and conversion rate recorded were 1.09 mol/mol, 2.61 g/L/h and 90.2% (g/g), respectively. Johnson and Rehmann [31][91] investigated the effect of decreasing pH during fermentation of crude glycerol with C. pasteurianum which led to the decreased in cell growth rate, CO2 production, and slower fermentations, thus resulting in higher butanol and PDO yields in a continuous process.For lactic acid generation, engineered E. coli has been widely used in converting crude glycerol as an alternative to nicotinamide adenine dinucleotide (NAD+) regeneration in the absence of external electron acceptors [49][112]. The biological route of crude glycerol upgrading to lactic acid assisted by microbes stimulates the development of one specific configuration of either D- or L-, because of the excellent selectivity of lactate dehydrogenase (LDH) [50][33]. Based on Mazumdar et al. [49][51][111,112], E. coli has been engineered to improve its efficiency towards the conversion of glycerol to D- and L-lactic acid under microaerobic and anaerobic conditions. The overall yield of 0.85 and 0.93 mol/mol glycerol has been recorded for D- and L-lactic acid, respectively, with 34 g/L and 50 g/L of each final concentration, whereas Hong et al. [52][126] utilized E. coli AC-521 to transform glycerol into lactic acid under the aerobic condition at optimized fermentation conditions of 42 °C, pH 6.5, and 0.85 min−1 (KLA). The overall lactic acid concentration and glycerol consumption peaked at 88 h of fermentation, resulting in 86.0 g/L lactic acid yield with 0.97 g/L/h productivity as well as a yield of 0.9 mol/mol glycerol. Other than E. coli, there are also studies relating to the utilization of L. casei NCIM 2125 [53][110], K. phafii Glpard [54][115], and yeast S. cerevisiae [55][34] under fed-batch fermentation for lactic acid production.The potential butanol producer, according to previous studies [30][48][90,106], C. pasteurianam under optimal condition can generate 0.43 mol/mol of butanol with 0.074 g/L/h of process productivity in 120 h using crude glycerol as a substrate. In a different study by Saini et al. [56][42], E. coli was used to assist the fermentation of crude glycerol into butanol with 0.35 mol/mol of butanol yield. Several type of bacteria were also utilized in the production of ethanol via fermentation of crude glycerol which includes E. coli SS1 [57][114], E. aerogenes TISTR 1468 [5], Pachysolen tannophilus [58][36], and engineered K. pneumonia [59][108] where they resulted in 1.00, 0.59, 0.56, and 0.89 mol/mol product yields, respectively. In addition, there is also a study of glycerol upgrading aided by Saccharomyces cerevisiae yeast [60][120] with an overall yield of 2.4 g/L ethanol recorded which proves the possibility of yeast in ethanol production.
- 2.
-
Microbial mixed cultures and other bacteriaMixed culture communities have also been studied for their potential to upgrade crude glycerol into other value-added products which include hydrogen gas and PHAs. According to the literature review, the production of H2 was dominantly covered by fermentation of mixed culture extracted from wastewater or resulted from the mixing of rare strain types. Theoretically, crude glycerol fermentation provides a better capacity to generate hydrogen gas at the end of its reaction as compared to glucose fermentation due to it generating more NADH on a 3-carbon basis where one mole of hydrogen gas per mole of excess NADH. According to previous work, Mabutyana and Pott [61][116] extensively studied the homofermentative H2 production via co-fermentation of glycerol with phenolic compounds assisted by Rhodopseudomonas palustris under anaerobic conditions at temperature of 35 °C equipped with tungsten light bulbs. According to Varrone et al. [62][118] and Chen et al. [63][41], the application of enriched activity sludge or microbial mixed culture efficiently enhanced the conversion of crude glycerol into H2 with an approximate yield of 0.52–0.96 mol/mol glycerol, whereas Paenibacillus macerans [64][117] and Thermoanaerobacterium sp. [8], in another study, were also utilized in the fermentation of crude glycerol to produce H2 which resulted in 0.81 and 0.30 mol/mol of yield. In addition, crude glycerol may be used as a substrate for anaerobic digestion to produce biogas. In the acidogenesis and acetogenesis phases, it is degraded to volatile fatty acids (VFAs), and in the final methanogenesis stage, methane is produced from either acetic acid, CO2, or H2 by the methanogenic community [65][127]. For further development, anaerobic process optimization to produce hydrogen from crude glycerol is very essential and needs to be explored thoroughly.
- 3.
-
FungiFungi are another promising biocatalyst to enhance the microbial conversion of crude glycerol into other specific valuable products such as single-cell oil (SCO). According to Chatzifragkou et al. [27][87], throughout the fermentation process, fungi tend to build up lipids inside their mycelia. Andre et al. [24][84] studied the yield of SCO and oxalic acid from the fermentation of crude glycerol with Lentinula edodes strains and Aspergillus niger strains in carbon-limited and nitrogen-limited conditions, respectively. The maximum yield of lipid of 3.5 g/L and 0.1 g/g biomass was recorded. The SCO product was dominantly composed of oleic acids. Galactomyces geotrichum and ascomycetous fungus were utilized in SCO production of crude glycerol. Overall lipid yield of 0.44 g/g of dry biomass was accumulated, 4 times higher than pure glycerol control, with 38.0% of glycerol conversion throughout the process. A similar trend was also shown by Chatzifragkou et al. [27][87], who produced an improvement in lipid yield of 11.6 g/L with 70% of fat in biomass via application of eukaryotic microbes, Thamnidium elegans, for SCO production. In this study, acetic acid and mannitol were also generated as their side-products.
- 4.
-
YeastSuccinic, citric, itaconic, and malic acids may be generated from glycerol utilizing fungus of the genera Rhizopus, Aspergillus, and Ustilago [66][67][128,129]. However, recently, yeasts also exhibit the promising potential to catabolize crude glycerol via an aerobic route into citric and succinic acid. Yarrowia lipolytica exhibits the ability in upgrading crude glycerol into citric acid under the aerobic condition with optimal process parameters [68][69][70][121,122,123]. Li et al. [71][124] investigated the effect of co-fermentation of crude glycerol with various agro-residues into succinic acid under aerobic condition using Y. lipolytica. At the end of the process, 53.6 g/L of succinic acid concentration, process productivity of 1.5 g/L/h, and an overall yield of 0.5 mol/mol were produced.
3.1.2. Bio-Electrochemical Fermentation
Bio-electrochemical fermentation is one of the emerging metabolic pathways which combines both biochemical and electrochemical approaches in upgrading crude glycerol. This technology exploits microbes to catalyze redox reactions in an electrochemical reactor under mild processing conditions [72][130]. Microbial catalysts on the electrode have been utilized to enhance the electrochemical reaction as well as increase the rate and yields of glycerol conversion. In this approach, the electric current supplied was utilized to permit the fermentation of crude glycerol as the feedstock. By improving cells’ capacity to regenerate NAD+ into NADH, the cathodic current will stimulate microbial reduction processes and thus be able to change fermentation profiles. As mentioned in the previous study [73][44], a crucial NAD+/NADH ratio greater than 4 has been linked to high PDO productivity and a high specific growth rate of K. pneumoniae. However, there are still a very small number of investigations using glycerol bio-electro fermentations within the cathode [72][73][74][75][76][43,44,45,130,131]. Zhou et al. [72][130] studied the carbon and electron fluxes during the bio-electro fermentation of crude glycerol using batch biocathodes and showed an increase in 1,3-PDO generation, while in the study by Choi et al. [77][132], C. pasteurianum was used and successfully demonstrated a shift in the microbial metabolism with improved PDO production when an electrical potential is applied. Recently, Xafenias et al. [74][43] reported a significant increase in PDO synthesis with aid of Clostridiaceae. High PDO concentrations of 42 g/L were recorded which shows the potential of this approach to be further studied.3.2. Thermochemical Approaches
3.2.1. Gasification Pyrolysis
Pyrolysis is a simple method of upgrading glycerol, yet it is just as significant as other processes. This method involves pyrolyzing feedstocks under specific processing conditions—high temperature (>600 °C), high pressure, in the deoxygenated environment inside a continuous or batch reactor [78][79][47,133], resulting from the thermochemical conversion of carbon contained in the feedstock of crude glycerol. The following processes take place to generate gaseous end-products [80][134]: (1) pyrolysis and devolatilization of feedstocks at relatively low temperature; (2) further degradation of the primary byproducts by continuous heating; and (3) coking gasification which leads to the production of high value-added syngas including carbon monoxide (CO), ethylene, methane, and hydrogen (H2).Dehydration and hydrogenation reactions dominantly happened throughout this approach. In the previous work of Skoulou and Zabaniotou [81][48], they used mixed crude glycerol, crude olive oil, and grains in a fixed bed reactor operating at 750–850 °C. Co-gasification attempts were made to increase the amount of hydrogen produced from the gas produced during the gasification process. According to the findings, combining crude glycerol with olive particles at a biomass weight ratio of 49% resulted in gas emissions of between 0.4 and 1.2 Nm3/kg [78][81][47,48]. In another work, based on Figure 5, Blass et al. [82][46] employed gasification pyrolysis of glycerol with H2 in a reactor containing dehydration, hydrogenation and upgrading stages in series with help of HZSM-5 and Pd/α-Al2O3 catalysts at a temperature of 400 °C to produce a mixture of acetaldehyde, acrolein and hydroxypropanone, propanal, and olefins. Propanal condensed over Brønsted acid sites to produce C4–5 olefins, which then underwent high conversion to produce C2–3 olefins, which accounts for this. During this staged process, a C–C bond formed, and negligible carbon was dissipated as CO as a byproduct. Thus, due to environmental concerns of greenhouse gases production, industrial scaleup is limited.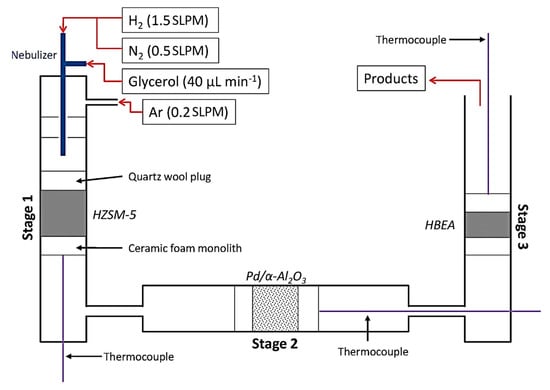 Figure 5. Schematic illustration of three staged reactor used for gasification pyrolysis of crude glycerol feed.
Figure 5. Schematic illustration of three staged reactor used for gasification pyrolysis of crude glycerol feed.3.2.2. Fast Pyrolysis
Fast pyrolysis is a method that has been adopted to decompose biomass into essential chemical feedstocks and biofuels usually with presence of catalyst. For the manufacture of highly stable liquid fuels, the catalyst-assisted cracking process is executed at temperatures between 400 and 800 °C with a short residence time of between 0.5 and 3 s [79][81][83][48,49,133]. The yield and characteristics of the end-products of the process are also significantly influenced by different types of feedstocks, environment, and particularly catalyst species [84][85][135,136]. Hence, optimizing the parameters promotes enhanced byproducts yield such hydrocarbon oils with shorter chain (C1–12), gases, and char [86][87][137,138]. Conventionally, hydrotreating of bio-oils and hydrocarbon wastes, derived from engine oil, transmission oil, and hydraulic oil, which fractured into high value-added products via thermal decomposition of pyrolysis and treated with external hydrogen gas has become a key technology in upgrading bio-oil into transportation fuels [88][139]. However, from the researchers'uthors’ knowledge, there are no works on hydrotreating of crude glycerol which may be due to its high operating costs, and it is not worth it in waste upcycling, in term of its techno economic view.There are several previous published works on upgrading crude glycerol via fast pyrolysis assisted with catalysts. He et al. [83][49] performed catalytic fast pyrolysis of crude glycerol into bio-based BTX in a tandem micro-reactor using ZSM-5/bentonite catalysts which operating at 520–536 °C. The study resulted in only 8.1 wt.% of bio-BTX yield (15% carbon yield) based on crude glycerol feed with fresh catalyst. In their work, the reduced end-product yields are mainly due to coking issues. The majority of the coke (10.5 wt.%) deposit was on the ZSM-5 planes, which not only reduced the number of Lewis and Brønsted acid sites but also clogged the pores, deactivating the catalyst. From previous published works, relatively poor BTX yields (2 wt.%) were also recorded, with different catalysts—Al2O3 [89][140] and Pd-Ru catalysts [90][141]. However, in other works [91][92][142,143], zeolites favored the production of acrolein (86%) from catalytic conversion of glycerol with higher glycerol conversion.3.2.3. Supercritical Fluids
One of the alternative thermochemical conversion techniques for crude glycerol utilizes supercritical fluids, including water, ethanol, methanol, and CO2, as the processing medium. This method has drawn a lot of interest recently and was demonstrated to be an appropriate reaction medium for biomass reforming. Either with or without the assistance of a catalyst, supercritical fluid reforming of crude glycerol and model compounds was already explored [9][93][94][95][96][97]. As an additional point[9,51,52,144, crude glycerol incorporates 6.5% of water content; thus145, the prior drying step can be neglected via this hydrothermal approach which reduced its processing cost146].Lighter weight liquid constituents and permanent gases, mixture of H2, CO, CO2, methane, and higher hydrocarbons will be produced during this glycerol upgrading process, with or without a catalyst. In addition, the rate of conversion, reaction selectivity, and the nature of the gas obtained are acknowledged to be impacted by the inclusion of a catalyst–homogenous catalysts (metal salt and acid catalysts lead to enhanced glycerol conversion and acrolein production). The utilization of various catalysts within this approach has been discussed in previous literature works by Markocic et al. [98][147] and Pavlovic et al. [99][148]. With the utilization of catalysts, the energy required for the procedure is reduced and it proceeds at lower operating temperatures and minimizes capital expenditures.According to previous studies [94][95][98][100][101][52,144,147,149,150], it has been observed that the main solvent for crude glycerol upgrading via supercritical fluid reforming is water, while there are not many works utilizing CO2 [93][51], and methanol [102][53]. According to Markocic et al. [98][147], utilization of water is due to the unique properties of supercritical water itself, when temperature and pressure of the normal water both reached its critical point (Tc = 373 °C, Pc = 22.1 MPa). The solubility of additional molecules of glycerol in water is thus improved by the decreasing dielectric constant, and supercritical water takes on the characteristics of a nonpolar solvent [103][151]. Other than that, reduced dynamic viscosity of supercritical water also leads to improved diffusion coefficients of dissolved substances. Therefore, polar, and ionic reactions as well as free radical reactions may occur at this state. In sub- and supercritical water, crude glycerol conversion proceeds quickly, and the high solubility of the intermediates in supercritical water prevents the development of tar and coke, and high product yields are produced at comparatively low temperatures [100][101][149,150].3.2.4. Steam Reforming
Crude glycerol can be upgraded into H2 using the steam reforming method. In steam reforming of crude glycerol, there are four main steps—feedstocks refinement, reforming, water-gas-shift (WGS) reaction, and end-product refining [104][152]. In the first step of raw material purification, sulfur and chloride impurities are removed. Then, in a fixed-bed reactor, the reforming process is commonly conducted. Thus, the raw material is brought into contact with steam while being catalyzed by a heterogeneous catalyst, resulting in the production of syngas and other gaseous byproducts. The catalytic WGS reactor collects this gas mixture, where the CO combines with the steam to produce more H2. Finally, high purity H2 is produced by purifying the resulting gas stream using various techniques, including pressure swing adsorption (PSA) and/or membrane systems. This strategy is suitable to prepare chemicals made of oxygenated hydrocarbons, including waste/bio-glycerol. These processes may be complemented by undesirable ones, which including methanation, dehydration, carbon precursor reactions, and dehydrogenation, depending on the operational circumstances.Since scaling up the steam reforming strategy to an industrial level would not necessitate substantial changes to the present infrastructures used for natural gas reforming, this method can be regarded as one of the most promising glycerol upgrading technologies [105][153]. Industrial steam reforming exploits γ-Al2O3 supported Ni catalysts as its catalyst option due to their increased availability and lower cost compared to precious metal-based catalysts [106][107][108][109][110][58,154,155,156,157]. Furthermore, Ni-based catalytic systems are capable of cleaving C–C, O–H, and C–H bonds as well as the WGS reaction, with typical NiO contents of 10 to 25 wt.% [111][158]. WGS can eliminate the adsorbed CO from the surface of the catalyst by converting it into CO2, the first three bonds must be broken in the reforming phase [106][109][58,156]. Due to its improved mechanical and chemical resistance, large surface area (SBET), and favorable metal dispersion, the γ-Al2O3 support is also commonly employed. However, coke deposition and particle sintering cause some deactivation in these systems. The first occurrence is often attributed to the γ-Al2O3 acidic characteristic, whereas sintering is attributed, among other aspects, to metal phases and γ-Al2O3 hydrothermal disturbances [106][112][58,159]. Within steam reforming operation parameters, these changes are often connected to the transition of γ-Al2O3 into other stable phases.3.2.5. Aqueous Phase Reforming
With a similar objective as steam reforming technology, the catalytic method of aqueous phase reforming was developed to convert oxygenated hydrocarbons generated from biomass into H2-rich gas. However, when compared to other catalytic thermochemical approaches such as pyrolysis, gasification, or steam reforming, aqueous phase reforming exhibited various advantages [113][114][160,161]. Aqueous phase reforming chemically converts the liquid phase-feedstocks at ambient processing conditions, low temperature, around 150 to 300 °C, due to the partial evaporation of the water. Operating pressure, approximately 1.5–7 MPa, is used during the reformation of crude glycerol, such that ample pressure was exerted to allow the H2-rich effluent to be in situ filtered via swing adsorption approach or, in some cases, using membrane technology which addresses its storage issue and efficiently isolated CO2 from the main products. With extremely low CO concentrations (100–1000 ppm), it is possible to generate H2 gas in a batch reactor assisted with metal catalysts, produced higher amount of H2 as compared to the existing steam reforming technique. In conclusion, this approach functions at minimal pressure, at a low temperature, and with less costly technology [104][152]. Additionally, they are simple to integrate into safe, environmentally friendly energy production systems.3.2.6. Microwave-Assisted Pyrolysis
The microwave absorbent must be capable of absorbing microwave energy and heat to the requisite temperature in order to pyrolyze waste [115][162]. Based on a study by Leong et al. [116][66], using a carbonaceous catalyst and temperatures between 300–800 °C, a microwave heating technique utilized to convert crude glycerol collected from biodiesel plants into biofuels. The product yields in each phase of such a process is determined by the processing conditions including residence time, process temperature, and type of catalysts employed during the reaction, all of which affect the reaction system and relative activation energy. From previous studies [115][116][117][118][65,66,67,162], it is observed that there was a decreased total mass of gaseous products due to the catalyst’s inclination toward hydrogen gas selectivity. Other than that, temperature reduction and longer residence times increased the overall energy production. The findings demonstrated that crude glycerol has the potential to produce syngas and bio-oil for use in bioenergy [118][67].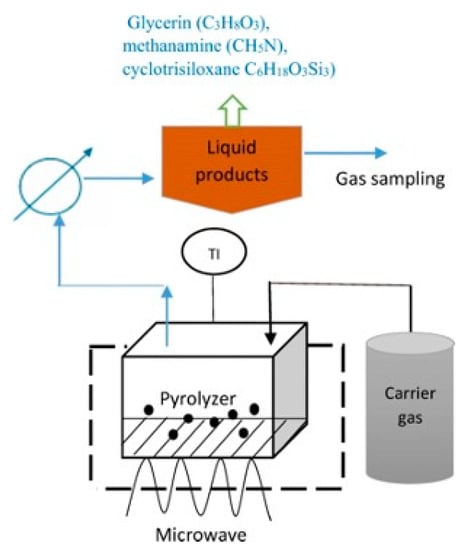 Figure 6.Microwave-assisted pyrolysis of crude glycerol by magnetron microwave in a quartz reactor with the presence of activated carbon as catalyst.
Figure 6.Microwave-assisted pyrolysis of crude glycerol by magnetron microwave in a quartz reactor with the presence of activated carbon as catalyst.
