Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irene Cánovas-Cervera | -- | 3480 | 2023-02-22 10:03:42 | | | |
| 2 | Catherine Yang | + 32 word(s) | 3512 | 2023-02-23 02:07:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cánovas-Cervera, I.; Nacher-Sendra, E.; Osca-Verdegal, R.; Dolz-Andrés, E.; Beltrán-García, J.; Rodríguez-Gimillo, M.; Ferrando-Sánchez, C.; Carbonell, N.; García-Giménez, J.L. The Intricate Role of Non-Coding RNAs in DIC. Encyclopedia. Available online: https://encyclopedia.pub/entry/41522 (accessed on 07 February 2026).
Cánovas-Cervera I, Nacher-Sendra E, Osca-Verdegal R, Dolz-Andrés E, Beltrán-García J, Rodríguez-Gimillo M, et al. The Intricate Role of Non-Coding RNAs in DIC. Encyclopedia. Available at: https://encyclopedia.pub/entry/41522. Accessed February 07, 2026.
Cánovas-Cervera, Irene, Elena Nacher-Sendra, Rebeca Osca-Verdegal, Enric Dolz-Andrés, Jesús Beltrán-García, María Rodríguez-Gimillo, Carolina Ferrando-Sánchez, Nieves Carbonell, José Luis García-Giménez. "The Intricate Role of Non-Coding RNAs in DIC" Encyclopedia, https://encyclopedia.pub/entry/41522 (accessed February 07, 2026).
Cánovas-Cervera, I., Nacher-Sendra, E., Osca-Verdegal, R., Dolz-Andrés, E., Beltrán-García, J., Rodríguez-Gimillo, M., Ferrando-Sánchez, C., Carbonell, N., & García-Giménez, J.L. (2023, February 22). The Intricate Role of Non-Coding RNAs in DIC. In Encyclopedia. https://encyclopedia.pub/entry/41522
Cánovas-Cervera, Irene, et al. "The Intricate Role of Non-Coding RNAs in DIC." Encyclopedia. Web. 22 February, 2023.
Copy Citation
Disseminated Intravascular Coagulation (DIC) is a type of tissue and organ dysregulation in sepsis, due mainly to the effect of the inflammation on the coagulation system. MicroRNAs, long non-coding RNAs, and circular RNAs are studied in relation to DIC. Specifically, the axis between these non-coding RNAs and the corresponding affected pathway has been identified, including inflammation, alteration of the coagulation cascade, and endothelial damage.
non-coding RNAs
miRNAs
lncRNAs
biomarkers
sepsis
DIC
1. The Role of microRNAs in DIC
MiRNAs have been proposed as potential biomarkers for several pathologies since they play a key role in different molecular processes and thus can potentially determine the affected pathways due to diseases [1]. Moreover, miRNAs are stable in the cell and in exosomes, and thus are found in several body fluids which makes them easily accessible for measuring them [2]. Consequently, on-going research has studied the potential of miRNAs to improve both diagnosis and prognosis in several diseases, including trauma-induced coagulopathy and sepsis.
The inflamma-miR miR-155 was found to be upregulated the cardiac tissue of mice treated with lipopolysaccharides (LPS) [3] and in plasma samples from septic patients. These authors showed the potential use of circulating miR-155 to identify septic patients with cardiac injury from septic patients without cardiac injury, obtaining an AUC (area under the curve) of 0.863 in the receiver operating curve (ROC) [3]. Importantly, overexpression of the inflamma-miR miR-155 has proven to induce spontaneous endothelial damage and alteration of the endothelial barrier function, thus leading to vascular leakage in a claudin-1-dependent manner in several models of endotoxemia [4]. In addition, recent research has proposed miR-155 as a master regulator of the coagulation factor XIII in several diseases [5]. As described in the previous section, factor XIII participates in the crosslinking process of fibrin, stabilizing the blood clot. Therefore, these interesting findings provide an intriguing question about the possibility of modulating the expression of miR-155 to control the expression of factor XIII in patients who are suspected to have DIC.
One of the most-studied miRNAs in sepsis is miR-122, identified as a diagnostic and prognostic biomarker in the serum of patients with sepsis [6][7]. Specifically, miR-122-5p has been associated with coagulation abnormalities related to sepsis [8]. Wang et al. described an overexpression of miR-122-5p in abnormal coagulation patients. Additionally, coagulation-related parameters such as aPTT, fibrinogen, and antithrombin III were correlated with the level of miR-122 [8], so it can be stated that miR-122 levels correlate with non-normal and worrisome coagulation during sepsis [8]. Particularly, results suggest that miR-122 may regulate antithrombin III expression [8], thus affecting the anticoagulant and anti-inflammatory properties of antithrombin III. In other studies, miR-122 levels in septic patients were analyzed in both surviving and deceased patients and it was observed that miR-122 was overexpressed in non-surviving septic patients [6]. Wang et al. performed a similar study where they also differentiated between patients with normal and non-normal coagulopathy [8]. It was shown that miR-122 levels were significantly higher in the group of patients with non-normal coagulopathy. Moreover, Wang et al. demonstrated that miR-122 levels in patients with sepsis and non-normal coagulation had elevated miRNAs up to 13 days after admission, so miR-122 could be used as a coagulation index for the detection of coagulation disorders in patients with sepsis [8].
Ma et al. have shown the role of miR-125b in sepsis-induced cardiomyopathy [9] and how its overexpression in mouse hearts improved survival by improving heart function. The proposed mechanism consisted of suppressing ICAM-1 and VCAM-1 and a decrease in inflammatory cytokines [9]. These findings are relevant since it was found that miR-125b is upregulated in sepsis and showed a positive correlation with enhanced disease severity, inflammation, and increased mortality in sepsis patients [10]. The previous results further reinforce the role of miR-125 in controlling key steps in the coagulation cascade, through its role in regulating endothelial adhesion proteins, such as ICAM-1 and VCAM-1, and directly regulating the expression of coagulation factors.
Upstream to factor IX in the intrinsic pathway, factor XI can be regulated by miR-181a-5p. It was shown how miR-181a-5p caused a slight, yet significant, decrease in factor XI mRNA levels, thus reducing the protein levels of factor XI [11]. The assays demonstrated a direct interaction between miR-181a-5p and the 3′-UTR of the messenger for factor XI. Importantly, factor XI mRNA levels were inversely and significantly correlated with miR-181a-5p levels, specifically in healthy livers, demonstrating that miR-181a-5p can directly impact the coagulation pathway [12].
In the extrinsic pathway, we can find factor X in the coagulation cascade. It has been found that miR-24 is overexpressed following trauma. However, it negatively correlates with factor X of the coagulation cascade in trauma patients, such that miR-24 is suspected to be able to inhibit factor X synthesis after trauma or trauma-induced coagulopathy [13]. Furthermore, miR-24 can decrease factor X and factor XII mRNA levels through negative regulation of nuclear factor, specifically nuclear factor-4 alpha in liver cells [13]. After performing the corresponding study, the authors concluded that miR-24 overexpression in patients after trauma was involved in the hypocoagulation state by inhibiting factor X and/or factor XII synthesis [13]. Thus, it can be stated that miR-24 plays an anticoagulant role as well as being a suitable biomarker for trauma-induced coagulopathy. MiR-24 could become a new therapeutic treatment for these patients since it has been shown in vitro that pretreatment with miR-24 can significantly suppress factor X levels [13].
Zhang et al. have studied the expression of miR-19a-3p in relation to DIC [14]. Their research revealed the potential of this miRNA as a therapeutic target due to its capability of inhibiting the coagulation cascade by targeting TF. As described above, TF by forming a complex with factor VIIa initiates clotting by activating factor X and factor IX. Both in vitro and in vivo results showed a great improvement in coagulation when miR-19a-3p was administered as a therapy. MiR-19a was able to inhibit the coagulation pathway by directly targeting TF through 3′-UTR binding sites [14]. The PI3K/AKT pathway is directly related to TF expression and coagulation. Factor Xa regulates TF expression in endothelial cells via mitogen-activated protein kinase (MAPK)- and nuclear factor kappa B (NF-κB)-dependent pathways [15][16]. Indirectly, TF regulation by miR-19 was dependent on the NF-κB/IκB pathway and the AKT pathway [14][15]. Therefore, miR-19 seems an interesting axis connecting the coagulation pathway, inflammation pathway, and AKT pathway.
Here it is important to note that recent literature showed how miR-19a was down-regulated while TF was shown to be up-regulated in newborns with sepsis-induced DIC relative to the control group [14].
TF expression can also be modulated by miR-223. In this regard, it was demonstrated that miR-223 is one of the most abundant miRNAs in platelets and platelet-derived extracellular vesicles [17][18]. Therefore, this miRNA could be transferred to platelets from monocytic cells and regulate TF expression as it binds to the 3′-UTR of the TF mRNA transcript and thereby inhibits its expression [19][20].
The before mentioned miRNAs clearly show the impact of miRNA expression in the control of the coagulation process. However, further studies to assess the impact of miRNAs in DIC are needed. In this regard, only an integrative analysis of molecular mechanisms in the context of the control of coagulation factors and endothelial adhesion proteins and mediators that clearly contribute to the coagulation cascade may help to understand the intricate mechanisms underlying sepsis-associated DIC.
2. The Role of Long Non-Coding RNAs in DIC
LncRNAs are defined as long transcripts of RNA of more than 200 nucleotides that are not associated with the expression of proteins [21].
Recent studies have shown that sepsis-related cardiac dysfunction can alter the expression of such ncRNAs [22][23]. Moreover, several lncRNAs have been described in sepsis and cardiovascular diseases [22][23]. As such, it is vital that their role becomes established regarding the dysregulation that takes place during DIC. This will in turn permit the description of lncRNA as biomarkers in diagnosis and prognosis, as well as possible therapeutic targets.
Epigenetic changes due to sepsis-related molecular pathways may also influence ncRNAs. Specifically, lncXR_343955 was found to be differentially methylated and may regulate the expression of different CAMs [24]. LINC00341 has also been associated with VCAM expression, inhibiting it [25]. Since these lncRNAs are linked to the expression of CAMs, they indirectly influence the PI3K/AKT pathway [25]. Thus, elucidating their role in the dysregulation of coagulation in DIC may allow the description of different pathological pathways associated with the disease.
The role of lncRNA cancer susceptibility candidate 2 (lncCASC2) has been studied in sepsis by Wang et al. [26]. Its expression was found to be inversely linked to sepsis severity and mortality, proving its use as a potential biomarker [26]. Interestingly, lncCASC2 was also negatively correlated with inflammatory cytokines. As such, its impact on the coagulation pathway, and specifically on endothelial damage, may be worth exploring. LncCASC2 has also been related to inflammation, sponging the inflamma-miR miR-155 [26]. Additionally, the overexpression of lncCASC2 inhibited the NF-κB activation pathway [26]. It is noteworthy that the TF promoter contains binding sites for NF-κB, which entails that the procoagulant activity of TF endothelial cells and subsequent thrombin generation directly depends on the NF-κB [27].
Similarly, lncRNA growth-arrest-specific transcript 5 (lncGAS5) was also associated with the diagnosis and mortality of sepsis [28]. Interestingly, lncGAS5 has also been linked to the NF-κB pathway in sepsis [29]. Moreover, lncGAS5 was also found to impact the PI3K/AKT pathway by targeting miR-223 and miR-21 [30].
These studies show the potential role of lncCASC2 and lncGAS5 in indicating the state of the NF-κB pathway and, in turn, endothelial damage. Thus, these lncRNAs should be studied further in the context of DIC to establish their potential as regulatory elements in those pathways regulating coagulation.
LncH19 was one of the first lncRNA described and as such is one of the most extensively studied lncRNAs. Although most research published on this lncRNA is related to cancer due to its oncogenic primary description, a recent study has established lncH19 as a potential prognosis biomarker in sepsis [31]. Additionally, lncH19 showed a mild correlation with cytokines IL-6 and TNF-α, as well as coagulation dysfunction.
Recently, miR-125a was found to be part of a regulatory axis with lncRNA Metastasis Associated Lung Adenocarcinoma Transcript 1 (lncMALAT1) [32]. In this study, Liu et al. demonstrated the utility of both lncMALAT1 and miR-125a to diagnose sepsis and predict organ injury, inflammation severity, and mortality. In another study, lncMALAT1 was found to promote the progression of acute lung injury related to sepsis, by sponging other inflamma-miRs such as miR-146 and thus indirectly promoting the NF-κB pathway [33] which downstream may affect the PI3K/AKT axis. Furthermore, lncRNA nuclear paraspeckle assembly transcript 1 (lncNEAT1) was also associated with miR-125a [34]. The overexpression of lncNEAT1 was correlated with a greater severity and a higher probability of mortality [34][35].
LncRNAs related to protease-activated receptor 1 (PAR-1) should also be studied due to their effects on the coagulation cascade after secretion from the endothelial cell. One such lncRNA is a non-protein-coding RNA upstream of F2R/PAR1 (ncRuPAR) that has been found to target PAR-1 and reduce indirectly the PI3K/AKT pathway [36]. This lncRNA has also been associated with the expression of VEGF, inhibiting it [37].
Another interesting lncRNA is potassium voltage-gated channel subfamily Q member 1 (KCNQ1) opposite strand/antisense transcript 1 (lncKCNQ1OT1), which has been found to sponge miR-24-3p [38]. This miRNA, as discussed in the previous section, has been studied extensively in both sepsis and DIC. LncKCNQ1OT1 was found to be under-expressed in sepsis patients, while also having a negative correlation to clinical scores related to coagulation [38] Additionally, Luo et al. first determined through in silico analysis, and later proved using experimental procedures, the relationship between lncKCNQ1OT1, miR-24, and vWF [39]. Such an axis should be studied further in the context of DIC since vWF is also a key contributor to the coagulation pathway.
Lastly, some lncRNAs have specifically been associated with factors in the coagulation cascade. Coagulation factor XI antisense RNA 1 (F11-AS1) targets miR-3146 and promotes the expression of phosphatase and tensin homolog (PTEN) [40], which participates in the PI3K/AKT pathway.
3. The Role of Circular RNAs in DIC
One of the most important characteristics of circRNAs is that these circular nucleic acids are present in most mammalian tissues and are highly expressed in specific tissues, being also present in plasma [41][42]. Moreover, circRNAs have shown differential expression levels at distinct development stages [43][44]. Due to their potential role in regulating transcriptional programs, circRNAs have been proposed as potential biomarkers for several diseases [45], including sepsis, as recently reviewed [46]. Interestingly, several circRNAs have been described to function as miRNA “sponges”, reducing the availability of such miRNAs. This in turn causes modulation of gene expression, transcription, and protein translation [47], so circRNAs are feasible key modulatory factors and mediators participating in mechanisms underlying pathological processes such as DIC.
Despite the advances in this field, it is noteworthy that further biological contextualization of these circRNAs by exploring gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways may contribute to demonstrating that these circRNAs are key mediators in inflammatory response (e.g., cytosine receptor interaction, TNF-α signaling pathway, NF-κB signaling pathway, and chemokine signaling pathway), leukocyte rolling and adhesion on endothelial cells, platelet function (activation, aggregation, and adhesion), and DIC. Moreover, even though the potential of circRNA biomarkers has been established [48], they are mainly studied and evaluated in the context of cancer, with little research focusing on the role of circRNA in sepsis and DIC. Though the researchers will focus on established relationships directly with sepsis, some connections provided by cancer studies may be useful to find relations between described circRNAs and their potential regulation of miRNAs and molecular pathways related to sepsis and DIC.
In sepsis, some circRNAs have been postulated as promising biomarkers. This is the case of circRNA guanine nucleotide exchange factor for Ras-like small GTPases (RasGEF) domain family member 1B (circRasGEF1B), an LPS-inducible circRNA in animal models of sepsis, which modulates the stability of ICAM-1 mRNA [49]. It was observed by Nie et al. that LPS stimulation leads to the activation of endothelial cells, promoting the release of inflammatory mediators (e.g., IL-1β, IL-18, TNF-α, chemokines, monocyte chemoattractant protein-1 (MCP-1), etc.), which further promote vascular endothelial damage [50]. The study of Nie et al. proposes that during sepsis, circRNAs can modulate vascular function by influencing the increment of nitric oxide levels and by down-regulating the antioxidant defense system [50]. Besides the role of circRasGEF1B in regulating ICAM-1 levels, both circ_0007456 and circRasGEF1B have been directly linked to the expression of ICAM-1 [49][51]. Closely related to the expression of ICAM-1 is the VCAM-1, both being downstream proteins of the PI3K/AKT pathway. Several studies have indicated that circRNA nephrocystin 4 (circNPHP4) expression has a significant impact on the expression of both molecules, specifically by sponging miR-1231 [52]. In addition, VCAM-1 also interacts with E-selectin and Lai et al. have found a relation between the expression of circ_0079662, miR-324, and HOXA9 [53].
CircRNA fatty acid desaturase 2 (circFADS2) is an important circRNA that has been found in low levels in septic patients [54]. CircFADS2 counteracts the function of miR-133a and miR-122a. Surprisingly, although circFADS2 blocks the function of miR-133a, this miRNA is usually increased in severe septic patients with unfavorable prognoses [55]. Furthermore, circFADS2 can suppress cell apoptosis by down-regulating miR-122a through the interaction with the miR-498/mammalian target of rapamycin (mTOR) axis to protect chondrocytes from apoptosis induced by LPS and inflammation [55]. This is quite exciting because it was described how mTOR is required for Bcl-3 activation, which in turn participates in the condensation of fibrin by activated platelets [56].
The role of AKT/mTOR axis in platelet activity and thrombosis is being uncovered [57]. Particularly, abnormal autophagic death of endothelial cells has been shown to affect plaque and promote thrombosis, which is related to this axis; thus it is feasible that circ_0030042 could play an essential role in vascular function [58].
Also related to the AKT/mTOR axis, Zeng et al. demonstrated that the overexpression of circRNA angiomotin like 1 (circAmotl1) protects against cardiomyopathy by binding to 3-phosphoinositide-dependent kinase 1 (PDK1) and AKT1 in vivo [59].
The AKT/mTOR axis is also directly involved in controlling the expression of TF to initiate thrombosis [60]. Interestingly, as described above, miR-19a-3p targets TF so it has been directly related to DIC. Thus, elucidating the possible circRNAs that may regulate this miRNA can be useful in the design of therapeutic approaches. CircRNA hippocampus abundant transcript 1 (circHIAT1) or circ_0000096 has been proven to modulate the expression of miR-19a-3p in cervical cancer [61]. The decrease in circHIAT1 increases miR-19a-3p expression which in turn produces the increase in AKT and mTOR. Therefore, the ability of circHIAT1 to sponge miR-19a-3p may directly affect the pathway. Another study carried out by Lu et al. related, in type 2 diabetes, the increase in miR-19a-3p to a decrease in circ_0063425 and the PI3K/AKT pathway [62].
These studies show that circ_0000096 and circ_0063425 regulate the expression of miR-19a-3p resulting in a modulation of the PI3K/AKT/mTOR pathway. These signaling molecules have been shown to regulate the expression of TF [63]. Thus, the aforementioned circRNAs should be studied further in the context of the coagulation cascade to determine their possible involvement in DIC-related dysfunction. This idea of the important role of circRNAs in DIC acquires relevance because there is evidence of the important role of the PI3K/AKT pathway in the suppression of LPS-induced inflammation and coagulation in the endotoxemic mice model [16]. Downstream to the PI3K/AKT/mTOR pathway, AKT targets Forkhead box O (FOXO) transcription factors [64]. In this pathway, Yuan et al. predicted through in silico analysis that hsa_circ_0039466 sponges miR-96 in hepatocellular carcinoma which impacts FOXO1 expression [65]; thus hsa_circ_0039466 may also indirectly regulate TF, as the researchers have previously described for hsa_circ_0000096 and hsa_circ_0063425. Additionally, circRNA PTEN (circPTEN) or circ_0094342 modulates the inflamma-miR miR-155 and miR-330, regulating the expression of PTEN/PI3K/AKT [66].
As described above, miR-122-5p has been related to coagulation malfunctioning in sepsis, and altered levels of this miRNA have been found in patients with altered coagulation parameters (i.e., aPTT, fibrinogen, and antithrombin) [8]. Therefore, it is necessary to explore the possible expression dysfunction of the circRNA sponges.
Hsa_circ_0005963 or ciRS-122 sponges miR-122, decreasing its expression and increasing expression of PKM2 [67], like hsa_circ_000826 which sponges miR-330 [68]. CircPTN has also been found to sponge miR-122 and, in turn, modulate the expression of SOX6 [69].
Notably, some studies have focused on the relation between miR-122 and the cytoplasmic polyadenylation element-binding protein 1 (CPEB1)/PTEN/AMPK/mTOR axis, which the researchers have shown is a relevant metabolic axis related to coagulation. Specifically, circRNA_002581 has been found to directly influence the expression of CPEB1 [70][71] which, besides controlling this axis, has been found to regulate the pathological expression of VEGF [72]. Another relevant circRNA related to VEGF is circRNA fibronectin type III domain containing 3B (circFndc3b) [73]. CircFndc3b has been found in cardiac endothelial cells to be contributing to the overexpression of VEGF which in turn increases its angiogenic activity in endothelial cells [73]. As is widely known, VEGF is involved in the hemostasis of endothelial cells by participating in several processes such as vascular permeability, VEGF stimulates endothelial cell proliferation, migration, and tube formation [74]. Recent studies suggest that VEGF is involved in the thrombotic process by stimulating the expression of TF in vascular endothelial cells [75]. Interestingly, TF can also stimulate the transcription of the gene encoding VEGF through a positive feedback process [76]. Importantly, VEGF, via the formation of the TF–factor VIIa complex, can trigger the extrinsic coagulation cascade [77][78][79]. In addition, VEGF can also increase the expression of thrombomodulin (TM) in endothelial cells. When complexed with thrombin in the membrane of endothelial cells this increases levels of APC, which is an inhibitor of the coagulation cascade, through the neutralization of activated factor V and activated factor VIII. Moreover, VEGF also increases the activation of thrombin-activatable fibrinolysis inhibitor (TAFI) by >1000-fold, which is involved in the inhibition of clot lysis [80].
Therefore, the increase in knowledge of the control of VEGF by circFndc3b may result in further interest in the comprehension of the epigenetic regulation of the coagulation process, mainly in those syndromes such as DIC.
Lastly, several circRNA have been linked to vWF, such as hsa_circ_0025119 [81], hsa_circ_0000698, hsa_circ_0002775, hsa_circ_0005585, and hsa_circ_0043837 [82]. These last circRNAs have also been found to sponge miR-16, which directly impacts vWF expression.
After reviewing the recent advances, the researchers can identify common pathways that are likely altered and regulated by specific ncRNAs. Most notably, the PI3K/AKT pathway and associated proteins and pathways are altered after NF-κB activation and by the effect of TF. Several circRNAs have been associated with the dysregulation of these pathways, such as circHIAT1, circFADS2, circ_0000096, and circ_0063425. Moreover, the expression of VEGF is altered and is likely modulated by diverse circRNAs including circ_002581 and circFndc3b (Figure 1).
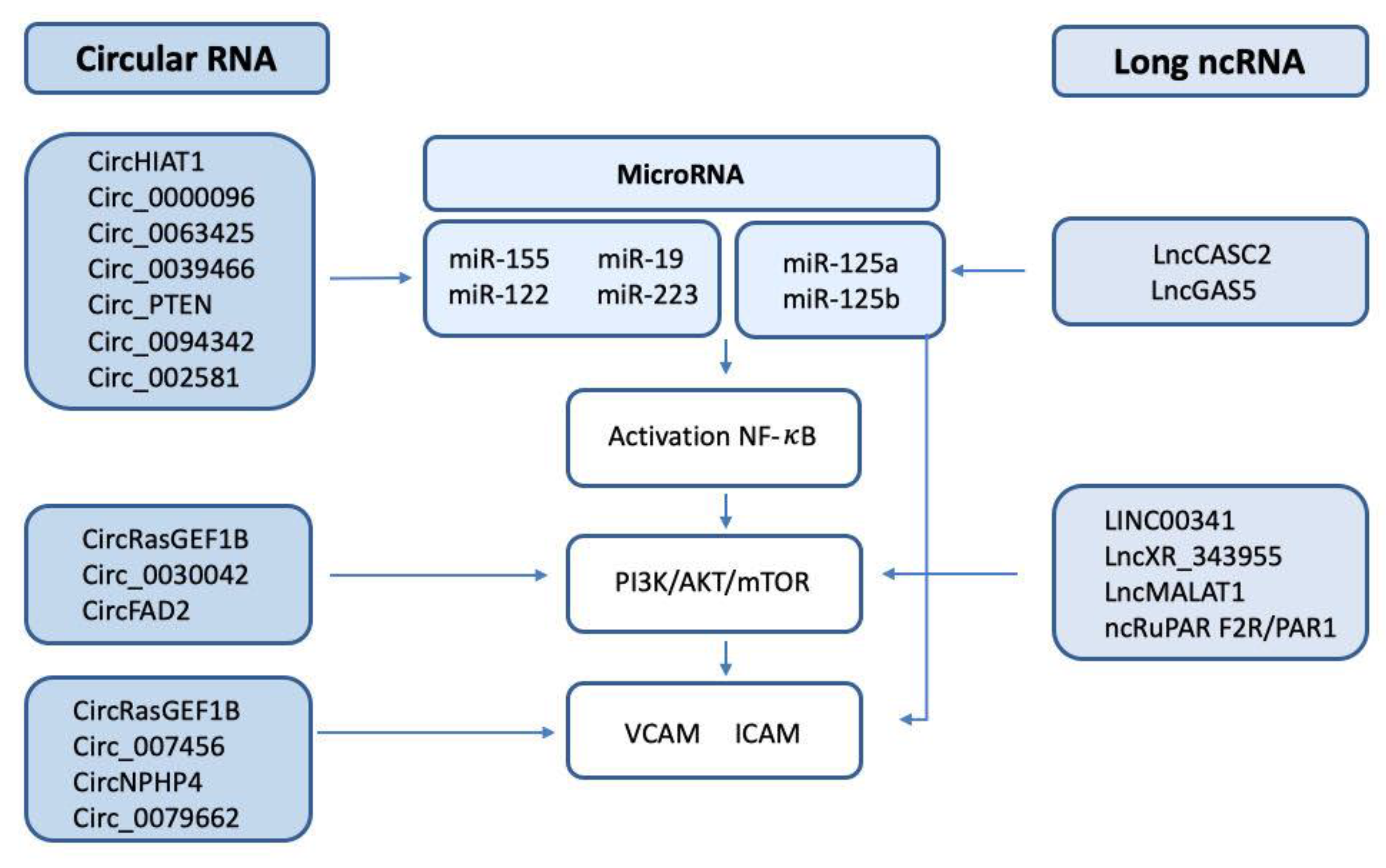
Figure 1. Scheme of the circRNA/lncRNA/miRNA involved in the regulation of NF-κB, the PI3K/AKT/mTOR pathway, and the endothelial adhesion factors VCAM and ICAM.
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Shah, M.Y.; Calin, G.A. The Mix of Two Worlds: Non-Coding RNAs and Hormones. Nucleic Acid Ther. 2013, 23, 2–8.
- Wang, H.; Bei, Y.; Huang, P.; Zhou, Q.; Shi, J.; Sun, Q.; Zhong, J.; Li, X.; Kong, X.; Xiao, J. Inhibition of MiR-155 Protects Against LPS-Induced Cardiac Dysfunction and Apoptosis in Mice. Mol. Ther. Nucleic Acids 2016, 5, e374.
- Etzrodt, V.; Idowu, T.O.; Schenk, H.; Seeliger, B.; Prasse, A.; Thamm, K.; Pape, T.; Müller-Deile, J.; van Meurs, M.; Thum, T.; et al. Role of Endothelial MicroRNA 155 on Capillary Leakage in Systemic Inflammation. Crit. Care 2021, 25, 76.
- Ntanyane Phasha, M.-A.; Soma, P.; Van Rooy, M.; Phulukdaree, A. MicroRNA 155, Factor XIII and Type 2 Diabetes Mellitus and Coronary Heart Disease. Curr. Diabetes Rev. 2023, 19, e190822207740.
- Wang, H.; Zhang, P.; Chen, W.; Feng, D.; Jia, Y.; Xie, L. Serum MicroRNA Signatures Identified by Solexa Sequencing Predict Sepsis Patients’ Mortality: A Prospective Observational Study. PLoS ONE 2012, 7, e38885.
- Wang, H.; Zhang, P.; Chen, W.; Feng, D.; Jia, Y.; Xie, L. Four Serum MicroRNAs Identified as Diagnostic Biomarkers of Sepsis. J. Trauma Acute Care Surg. 2012, 73, 850.
- Wang, H.-J.; Deng, J.; Wang, J.-Y.; Zhang, P.-J.; Xin, Z.; Xiao, K.; Feng, D.; Jia, Y.-H.; Liu, Y.-N.; Xie, L.-X. Serum MiR-122 Levels Are Related to Coagulation Disorders in Sepsis Patients. Clin. Chem. Lab. Med. CCLM 2014, 52, 927–933.
- Ma, H.; Wang, X.; Ha, T.; Gao, M.; Liu, L.; Wang, R.; Yu, K.; Kalbfleisch, J.H.; Kao, R.L.; Williams, D.L.; et al. MicroRNA-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor ΚB Activation and P53-Mediated Apoptotic Signaling. J. Infect. Dis. 2016, 214, 1773–1783.
- Zhu, X. MiR-125b but Not MiR-125a Is Upregulated and Exhibits a Trend to Correlate with Enhanced Disease Severity, Inflammation, and Increased Mortality in Sepsis Patients. J. Clin. Lab. Anal. 2019, 34, e23094.
- Salloum-Asfar, S.; Teruel-Montoya, R.; Arroyo, A.B.; García-Barberá, N.; Chaudhry, A.; Schuetz, E.; Luengo-Gil, G.; Vicente, V.; González-Conejero, R.; Martínez, C. Regulation of Coagulation Factor XI Expression by MicroRNAs in the Human Liver. PLoS ONE 2014, 9, e111713.
- Nourse, J.; Danckwardt, S. A Novel Rationale for Targeting FXI: Insights from the Hemostatic MicroRNA Targetome for Emerging Anticoagulant Strategies. Pharmacol. Ther. 2021, 218, 107676.
- Chen, L.-J.; Yang, L.; Cheng, X.; Xue, Y.-K.; Chen, L.-B. Overexpression of MiR-24 Is Involved in the Formation of Hypocoagulation State after Severe Trauma by Inhibiting the Synthesis of Coagulation Factor X. Dis. Markers 2017, 2017, 3649693.
- Zhang, R.; Lu, S.; Yang, X.; Li, M.; Jia, H.; Liao, J.; Jing, Q.; Wu, Y.; Wang, H.; Xiao, F.; et al. MiR-19a-3p Downregulates Tissue Factor and Functions as a Potential Therapeutic Target for Sepsis-Induced Disseminated Intravascular Coagulation. Biochem. Pharmacol. 2021, 192, 114671.
- Jiang, R.; Wang, N.-P.; Tanaka, K.A.; Levy, J.H.; Guyton, R.A.; Zhao, Z.-Q.; Vinten-Johansen, J. Factor Xa Induces Tissue Factor Expression in Endothelial Cells by P44/42 MAPK and NF-ΚB-Dependent Pathways. J. Surg. Res. 2011, 169, 319–327.
- Schabbauer, G.; Tencati, M.; Pedersen, B.; Pawlinski, R.; Mackman, N. PI3K-Akt Pathway Suppresses Coagulation and Inflammation in Endotoxemic Mice. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1963–1969.
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating MicroRNAs as Novel Biomarkers for Platelet Activation. Circ. Res. 2013, 112, 595–600.
- Laffont, B.; Corduan, A.; Plé, H.; Duchez, A.-C.; Cloutier, N.; Boilard, E.; Provost, P. Activated Platelets Can Deliver MRNA Regulatory Ago2•microRNA Complexes to Endothelial Cells via Microparticles. Blood 2013, 122, 253–261.
- Li, S.; Chen, H.; Ren, J.; Geng, Q.; Song, J.; Lee, C.; Cao, C.; Zhang, J.; Xu, N. MicroRNA-223 Inhibits Tissue Factor Expression in Vascular Endothelial Cells. Atherosclerosis 2014, 237, 514–520.
- Zhang, X.; Yu, H.; Lou, J.R.; Zheng, J.; Zhu, H.; Popescu, N.-I.; Lupu, F.; Lind, S.E.; Ding, W.-Q. MicroRNA-19 (MiR-19) Regulates Tissue Factor Expression in Breast Cancer Cells. J. Biol. Chem. 2011, 286, 1429–1435.
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: LncRNA Localization and Function. J. Cell Biol. 2021, 220, e202009045.
- Wang, C.; Liang, G.; Shen, J.; Kong, H.; Wu, D.; Huang, J.; Li, X. Long Non-Coding RNAs as Biomarkers and Therapeutic Targets in Sepsis. Front. Immunol. 2021, 12, 722004.
- Li, J.; Zhang, Y.; Zhang, D.; Li, Y. The Role of Long Non-Coding RNAs in Sepsis-Induced Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 684348.
- Shen, Z.-J.; Han, Y.-C.; Nie, M.-W.; Wang, Y.-N.; Xiang, R.-L.; Xie, H.-Z. Genome-Wide Identification of Altered RNA M6A Profiles in Vascular Tissue of Septic Rats. Aging 2021, 13, 21610–21627.
- Huang, T.-S.; Wang, K.-C.; Quon, S.; Nguyen, P.; Chang, T.-Y.; Chen, Z.; Li, Y.-S.; Subramaniam, S.; Shyy, J.; Chien, S. LINC00341 Exerts an Anti-Inflammatory Effect on Endothelial Cells by Repressing VCAM1. Physiol. Genom. 2017, 49, 339–345.
- Wang, R.; Zhao, J.; Wei, Q.; Wang, H.; Zhao, C.; Hu, C.; Han, Y.; Hui, Z.; Yang, L.; Dai, Q.; et al. Potential of Circulating LncRNA CASC2 as a Biomarker in Reflecting the Inflammatory Cytokines, Multi-organ Dysfunction, Disease Severity, and Mortality in Sepsis Patients. J. Clin. Lab. Anal. 2022, 36, e24569.
- Yang, X.; Li, L.; Liu, J.; Lv, B.; Chen, F. Extracellular Histones Induce Tissue Factor Expression in Vascular Endothelial Cells via TLR and Activation of NF-ΚB and AP-1. Thromb. Res. 2016, 137, 211–218.
- Zhang, W.; Chen, B.; Chen, W. LncRNA GAS5 Relates to Th17 Cells and Serves as a Potential Biomarker for Sepsis Inflammation, Organ Dysfunctions and Mortality Risk. J. Clin. Lab. Anal. 2022, 36, e24309.
- Gao, H.; Ma, H.; Gao, M.; Chen, A.; Zha, S.; Yan, J. Long Non-Coding RNA GAS5 Aggravates Myocardial Depression in Mice with Sepsis via the MicroRNA-449b/HMGB1 Axis and the NF-ΚB Signaling Pathway. Biosci. Rep. 2021, 41, BSR20201738.
- Zheng, D.; Hou, Y.; Li, Y.; Bian, Y.; Khan, M.; Li, F.; Huang, L.; Qiao, C. Long Non-Coding RNA Gas5 Is Associated With Preeclampsia and Regulates Biological Behaviors of Trophoblast via MicroRNA-21. Front. Genet. 2020, 11, 188.
- Yu, B.; Cui, R.; Lan, Y.; Zhang, J.; Liu, B. Long Non-Coding RNA H19 as a Diagnostic Marker in Peripheral Blood of Patients with Sepsis. Am. J. Transl. Res. 2021, 13, 2923–2930.
- Liu, W.; Geng, F.; Yu, L. Long Non-coding RNA MALAT1/MicroRNA 125a Axis Presents Excellent Value in Discriminating Sepsis Patients and Exhibits Positive Association with General Disease Severity, Organ Injury, Inflammation Level, and Mortality in Sepsis Patients. J. Clin. Lab. Anal. 2020, 34, e23222.
- Liang, W.-J.; Zeng, X.-Y.; Jiang, S.-L.; Tan, H.-Y.; Yan, M.-Y.; Yang, H.-Z. Long Non-Coding RNA MALAT1 Sponges MiR-149 to Promote Inflammatory Responses of LPS-Induced Acute Lung Injury by Targeting MyD88. Cell Biol. Int. 2020, 44, 317–326.
- Yang, Y.; Yang, L.; Liu, Z.; Wang, Y.; Yang, J. Long Noncoding RNA NEAT 1 and Its Target MicroRNA-125a in Sepsis: Correlation with Acute Respiratory Distress Syndrome Risk, Biochemical Indexes, Disease Severity, and 28-day Mortality. J. Clin. Lab. Anal. 2020, 34, e23509.
- Huang, Q.; Huang, C.; Luo, Y.; He, F.; Zhang, R. Circulating LncRNA NEAT1 Correlates with Increased Risk, Elevated Severity and Unfavorable Prognosis in Sepsis Patients. Am. J. Emerg. Med. 2018, 36, 1659–1663.
- Xu, R.; Yu, J.; Song, S.; Sun, D.; Xiu, L.; Xu, J.; Zhao, J.; Liu, X.; Ji, Q.; Yue, X. Long Non-Coding RNA NcRuPAR Regulates Gastric Cancer Cell Proliferation and Apoptosis via Phosphoinositide 3-Kinase/Protein Kinase B Signaling. Int. J. Med. Sci. 2022, 19, 1835–1846.
- Liu, L.; Yan, B.; Yang, Z.; Zhang, X.; Gu, Q.; Yue, X. NcRuPAR Inhibits Gastric Cancer Progression by Down-Regulating Protease-Activated Receptor-1. Tumor Biol. 2014, 35, 7821–7829.
- Jiao, W.; Zhou, X.; Wu, J.; Zhang, X.; Ding, J. Potential of Long Non-coding RNA KCNQ1OT1 as a Biomarker Reflecting Systemic Inflammation, Multiple Organ Dysfunction, and Mortality Risk in Sepsis Patients. J. Clin. Lab. Anal. 2021, 35, e24047.
- Luo, Y.; Fu, Y.; Tan, T.; Hu, J.; Li, F.; Liao, Z.; Peng, J. Screening of LncRNA-MiRNA-MRNA Coexpression Regulatory Networks Involved in Acute Traumatic Coagulation Dysfunction Based on CTD, GeneCards, and PharmGKB Databases. Oxid. Med. Cell. Longev. 2022, 2022, 7280312.
- Du, J.; Chen, M.; Liu, J.; Hu, P.; Guan, H.; Jiao, X. LncRNA F11-AS1 Suppresses Liver Hepatocellular Carcinoma Progression by Competitively Binding with MiR-3146 to Regulate PTEN Expression. J. Cell. Biochem. 2019, 120, 18457–18464.
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885.
- Zhang, Z.; Yang, T.; Xiao, J. Circular RNAs: Promising Biomarkers for Human Diseases. EBioMedicine 2018, 34, 267–274.
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 2013, 495, 333–338.
- Salzman, J. Circular RNA Expression: Its Potential Regulation and Function. Trends Genet. TIG 2016, 32, 309–316.
- Beltrán-García, J.; Osca-Verdegal, R.; Nacher-Sendra, E.; Pallardó, F.V.; García-Giménez, J.L. Circular RNAs in Sepsis: Biogenesis, Function, and Clinical Significance. Cells 2020, 9, 1544.
- Beltrán-García, J.; Osca-Verdegal, R.; Nácher-Sendra, E.; Cardona-Monzonís, A.; Sanchis-Gomar, F.; Carbonell, N.; Pallardó, F.V.; Lavie, C.J.; García-Giménez, J.L. Role of Non-Coding RNAs as Biomarkers of Deleterious Cardiovascular Effects in Sepsis. Prog. Cardiovasc. Dis. 2021, 68, 70–77.
- Huang, A.; Zheng, H.; Wu, Z.; Chen, M.; Huang, Y. Circular RNA-Protein Interactions: Functions, Mechanisms, and Identification. Theranostics 2020, 10, 3503–3517.
- Han, B.; Chao, J.; Yao, H. Circular RNA and Its Mechanisms in Disease: From the Bench to the Clinic. Pharmacol. Ther. 2018, 187, 31–44.
- Ng, W.L.; Marinov, G.K.; Liau, E.S.; Lam, Y.L.; Lim, Y.-Y.; Ea, C.-K. Inducible RasGEF1B Circular RNA Is a Positive Regulator of ICAM-1 in the TLR4/LPS Pathway. RNA Biol. 2016, 13, 861–871.
- Nie, M.-W.; Han, Y.-C.; Shen, Z.-J.; Xie, H.-Z. Identification of CircRNA and MRNA Expression Profiles and Functional Networks of Vascular Tissue in Lipopolysaccharide-Induced Sepsis. J. Cell. Mol. Med. 2020, 24, 7915–7927.
- Shi, M.; Li, Z.-Y.; Zhang, L.-M.; Wu, X.-Y.; Xiang, S.-H.; Wang, Y.-G.; Zhang, Y.-Q. Hsa_circ_0007456 Regulates the Natural Killer Cell-Mediated Cytotoxicity toward Hepatocellular Carcinoma via the MiR-6852-3p/ICAM-1 Axis. Cell Death Dis. 2021, 12, 94.
- Xiong, F.; Mao, R.; Zhang, L.; Zhao, R.; Tan, K.; Liu, C.; Xu, J.; Du, G.; Zhang, T. CircNPHP4 in Monocyte-Derived Small Extracellular Vesicles Controls Heterogeneous Adhesion in Coronary Heart Atherosclerotic Disease. Cell Death Dis. 2021, 12, 948.
- Liu, S.; Gao, J.; Wang, S. HOXA9 Inhibitors Promote Microcirculation of Coronary Arteries in Rats via Downregulating E-Selectin/VCAM-1. Exp. Ther. Med. 2021, 22, 871.
- Niu, F.; Liang, X.; Ni, J.; Xia, Z.; Jiang, L.; Wang, H.; Liu, H.; Shen, G.; Li, X. CircRNA CircFADS2 Is Under-Expressed in Sepsis and Protects Lung Cells from LPS-Induced Apoptosis by Downregulating MiR-133a. J. Inflamm. 2022, 19, 4.
- Tacke, F.; Roderburg, C.; Benz, F.; Cardenas, D.V.; Luedde, M.; Hippe, H.-J.; Frey, N.; Vucur, M.; Gautheron, J.; Koch, A.; et al. Levels of Circulating MiR-133a Are Elevated in Sepsis and Predict Mortality in Critically Ill Patients. Crit. Care Med. 2014, 42, 1096.
- Weyrich, A.S.; Denis, M.M.; Schwertz, H.; Tolley, N.D.; Foulks, J.; Spencer, E.; Kraiss, L.W.; Albertine, K.H.; McIntyre, T.M.; Zimmerman, G.A. MTOR-Dependent Synthesis of Bcl-3 Controls the Retraction of Fibrin Clots by Activated Human Platelets. Blood 2007, 109, 1975–1983.
- Woulfe, D.S. Akt Signaling in Platelets and Thrombosis. Expert Rev. Hematol. 2010, 3, 81–91.
- Yu, F.; Zhang, Y.; Wang, Z.; Gong, W.; Zhang, C. Hsa_circ_0030042 Regulates Abnormal Autophagy and Protects Atherosclerotic Plaque Stability by Targeting EIF4A3. Theranostics 2021, 11, 5404–5417.
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A Circular RNA Binds To and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics 2017, 7, 3842–3855.
- Cong, Y.; Li, Q.; Zhang, X.; Chen, Y.; Yu, K. MTOR Promotes Tissue Factor Expression and Activity in EGFR-Mutant Cancer. Front. Oncol. 2020, 10, 1615.
- Hu, J.; Wang, R.; Liu, Y.; Zhou, J.; Shen, K.; Dai, Y. Baicalein Represses Cervical Cancer Cell Growth, Cell Cycle Progression and Promotes Apoptosis via Blocking AKT/MTOR Pathway by the Regulation of CircHIAT1/MiR-19a-3p Axis. OncoTargets Ther. 2021, 14, 905–916.
- Lu, Y.-K.; Chu, X.; Wang, S.; Sun, Y.; Zhang, J.; Dong, J.; Yan, Y.-X. Identification of Circulating Hsa_circ_0063425 and Hsa_circ_0056891 as Novel Biomarkers for Detection of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e2688–e2699.
- Hu, C.; Huang, L.; Gest, C.; Xi, X.; Janin, A.; Soria, C.; Li, H.; Lu, H. Opposite Regulation by PI3K/Akt and MAPK/ERK Pathways of Tissue Factor Expression, Cell-Associated Procoagulant Activity and Invasiveness in MDA-MB-231 Cells. J. Hematol. Oncol. 2012, 5, 16.
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and Regulation of Apoptosis. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2011, 1813, 1978–1986.
- Yuan, F.; Tang, Y.; Cao, M.; Ren, Y.; Li, Y.; Yang, G.; Ou, Q.; Tustumi, F.; Levi Sandri, G.B.; Raissi, D.; et al. Identification of the Hsa_circ_0039466/MiR-96-5p/FOXO1 Regulatory Network in Hepatocellular Carcinoma by Whole-Transcriptome Analysis. Ann. Transl. Med. 2022, 10, 769.
- Wang, Y.; Wang, Z.; Lu, J.; Zhang, H. Circular RNA Circ-PTEN Elevates PTEN Inhibiting the Proliferation of Non-Small Cell Lung Cancer Cells. Hum. Cell 2021, 34, 1174–1184.
- Wang, X.; Zhang, H.; Yang, H.; Bai, M.; Ning, T.; Deng, T.; Liu, R.; Fan, Q.; Zhu, K.; Li, J.; et al. Exosome-delivered CircRNA Promotes Glycolysis to Induce Chemoresistance through the MiR-122-PKM2 Axis in Colorectal Cancer. Mol. Oncol. 2020, 14, 539–555.
- Zhao, X.; Tian, Z.; Liu, L. CircATP2B1 Promotes Aerobic Glycolysis in Gastric Cancer Cells Through Regulation of the MiR-326 Gene Cluster. Front. Oncol. 2021, 11, 628624.
- Chen, C.; Deng, L.; Nie, D.-K.; Jia, F.; Fu, L.-S.; Wan, Z.-Q.; Lan, Q. Circular RNA Pleiotrophin Promotes Carcinogenesis in Glioma via Regulation of MicroRNA-122/SRY-Box Transcription Factor 6 Axis. Eur. J. Cancer Prev. 2020, 29, 165.
- Jin, X.; Gao, J.; Zheng, R.; Yu, M.; Ren, Y.; Yan, T.; Huang, Y.; Li, Y. Antagonizing CircRNA_002581–MiR-122–CPEB1 Axis Alleviates NASH through Restoring PTEN–AMPK–MTOR Pathway Regulated Autophagy. Cell Death Dis. 2020, 11, 123.
- Jin, X.; Feng, C.; Xiang, Z.; Chen, Y.; Li, Y. CircRNA Expression Pattern and CircRNA-MiRNA-MRNA Network in the Pathogenesis of Nonalcoholic Steatohepatitis. Oncotarget 2016, 7, 66455–66467.
- Calderone, V.; Gallego, J.; Fernandez-Miranda, G.; Garcia-Pras, E.; Maillo, C.; Berzigotti, A.; Mejias, M.; Bava, F.-A.; Angulo-Urarte, A.; Graupera, M.; et al. Sequential Functions of CPEB1 and CPEB4 Regulate Pathologic Expression of Vascular Endothelial Growth Factor and Angiogenesis in Chronic Liver Disease. Gastroenterology 2016, 150, 982–997.
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b Modulates Cardiac Repair after Myocardial Infarction via FUS/VEGF-A Axis. Nat. Commun. 2019, 10, 4317.
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular Permeability Factor/Vascular Endothelial Growth Factor, Microvascular Hyperpermeability, and Angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039.
- Nakasaki, T.; Wada, H.; Shigemori, C.; Miki, C.; Gabazza, E.C.; Nobori, T.; Nakamura, S.; Shiku, H. Expression of Tissue Factor and Vascular Endothelial Growth Factor Is Associated with Angiogenesis in Colorectal Cancer. Am. J. Hematol. 2002, 69, 247–254.
- Gadomska, G.; Ziołkowska, K.; Boinska, J.; Filipiak, J.; Rość, D. Activation of TF-Dependent Blood Coagulation Pathway and VEGF-A in Patients with Essential Thrombocythemia. Medicina 2019, 55, 54.
- Clauss, M.; Gerlach, M.; Gerlach, H.; Brett, J.; Wang, F.; Familletti, P.C.; Pan, Y.C.; Olander, J.V.; Connolly, D.T.; Stern, D. Vascular Permeability Factor: A Tumor-Derived Polypeptide That Induces Endothelial Cell and Monocyte Procoagulant Activity, and Promotes Monocyte Migration. J. Exp. Med. 1990, 172, 1535–1545.
- Mechtcheriakova, D.; Wlachos, A.; Holzmüller, H.; Binder, B.R.; Hofer, E. Vascular Endothelial Cell Growth Factor–Induced Tissue Factor Expression in Endothelial Cells Is Mediated by EGR-1. Blood 1999, 93, 3811–3823.
- Camera, M.; Giesen, P.L.A.; Fallon, J.; Aufiero, B.M.; Taubman, M.; Tremoli, E.; Nemerson, Y. Cooperation Between VEGF and TNF-α Is Necessary for Exposure of Active Tissue Factor on the Surface of Human Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 531–537.
- Bajzar, L.; Nesheim, M.; Morser, J.; Tracy, P.B. Both Cellular and Soluble Forms of Thrombomodulin Inhibit Fibrinolysis by Potentiating the Activation of Thrombin-Activable Fibrinolysis Inhibitor. J. Biol. Chem. 1998, 273, 2792–2798.
- Wu, W.; Zou, J. Studies on the Role of CircRNAs in Osteoarthritis. BioMed Res. Int. 2021, 2021, 8231414.
- Xiao, Q.; Yin, R.; Wang, Y.; Yang, S.; Ma, A.; Pan, X.; Zhu, X. Comprehensive Analysis of Peripheral Exosomal CircRNAs in Large Artery Atherosclerotic Stroke. Front. Cell Dev. Biol. 2021, 9, 685741.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
651
Entry Collection:
Hypertension and Cardiovascular Diseases
Revisions:
2 times
(View History)
Update Date:
23 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




