
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kunwar Pal | -- | 4256 | 2023-02-22 06:43:16 | | | |
| 2 | Kunwar Pal | + 3524 word(s) | 7780 | 2023-02-22 07:16:14 | | | | |
| 3 | Peter Tang | -3012 word(s) | 4768 | 2023-02-22 08:01:02 | | |
Video Upload Options
Plastic waste poses a serious threat to the environment and it has been increasing at an alarming rate. In 2022, global plastic waste generation was reported to be around 380 million tonnes as compared to 353 million tonnes in 2019. Production of liquid fuel from plastic waste is regarded as a viable method for disposing of the plastic and utilizing its energy. A wide range of technologies have been explored for turning plastic waste into fuel, including the conventional pyrolysis, incineration, gasification and advanced oxidation.
1. Introduction
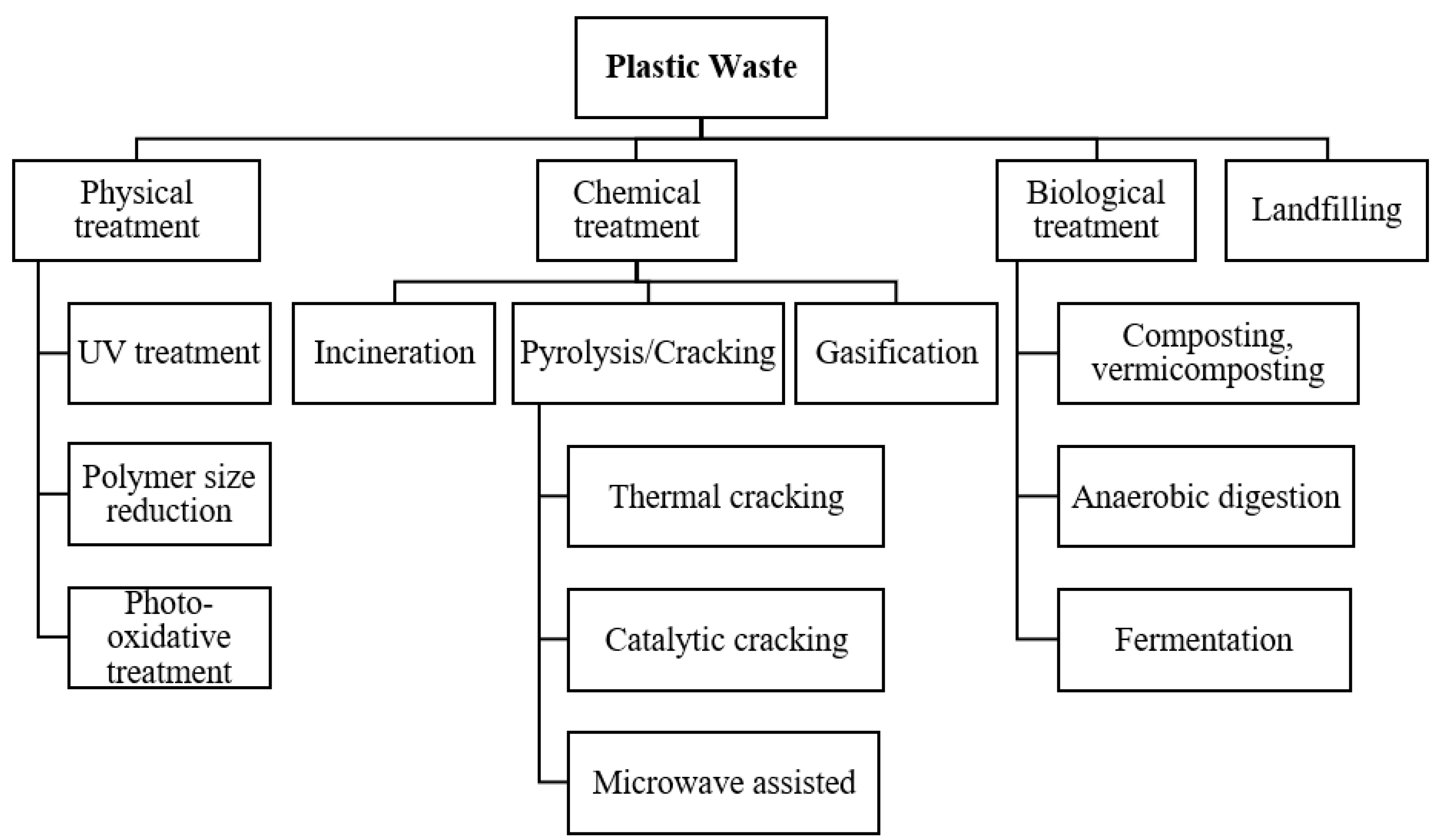
2. Conventional Techniques for Thermal Treatment of Plastic Waste
2.1. Pyrolysis
2.1.1. Thermal Pyrolysis
|
S. No. |
Feed |
Reactor |
Temperature (°C) |
Yield (wt%) |
Reference |
||
|---|---|---|---|---|---|---|---|
|
Liquid |
Gas |
Char |
|||||
|
1. |
HDPE |
Fixed bed |
550 |
70 |
23 |
7 |
[23] |
|
2. |
Mixed |
Semi batch |
500 |
75.8 |
10 |
14.2 |
[24] |
|
3. |
HDPE |
Batch |
440 |
74 |
9 |
17 |
[25] |
|
4. |
PE |
Steel micro |
350 |
80.9 |
17.2 |
1.9 |
[26] |
|
5. |
PP |
Steel micro |
350 |
67.8 |
30 |
1.6 |
[26] |
|
6. |
Mixed |
Semi batch |
500 |
65.2 |
34 |
0.8 |
[27] |
|
7. |
PET |
Fixed bed |
500 |
38.89 |
52.13 |
8.98 |
[28] |
|
8. |
PET |
Parr mini bench top |
500 |
15.0 |
32.0 |
53.0 |
[29] |
|
9. |
HDPE |
Horizontal steel |
350 |
80.88 |
17.24 |
1.88 |
[26] |
|
10. |
HDPE |
Semi batch |
450 |
91.2 |
4.1 |
4.7 |
[30] |
|
11. |
HDPE |
Batch |
550 |
84.70 |
16.30 |
- |
[31] |
|
12. |
HDPE |
Fluidized bed |
650 |
68.50 |
31.50 |
- |
[32] |
|
13. |
HDPE |
Semi-batch |
400 |
82 |
16 |
2 |
[33] |
|
14. |
HDPE |
Fluidized bed |
500 |
85 |
10 |
5 |
[34] |
|
15. |
PS |
Batch |
581 |
89.5 |
9.9 |
0.6 |
[35] |
|
16. |
PS |
Semi-batch |
400 |
90 |
6 |
4 |
[33] |
|
17. |
PP |
Batch |
380 |
80.1 |
6.6 |
13.3 |
[36] |
|
18. |
PS |
Batch |
500 |
96.73 |
3.27 |
- |
[37] |
|
19. |
LDPE |
Batch |
430 |
75.6 |
8.2 |
7.5 |
[38] |
|
20. |
PP |
Semi-batch |
400 |
85 |
13 |
2 |
[33] |
|
21. |
HDPE |
Semi-batch |
450 |
91.2 |
4.1 |
4.7 |
[30] |
|
22. |
LDPE |
Fluidized bed |
600 |
51.0 |
24.2 |
- |
[39] |
|
23. |
HDPE |
Batch |
450 |
74.5 |
5.8 |
19.7 |
[40] |
|
24. |
PP |
Semi-batch |
450 |
92.3 |
4.1 |
3.6 |
[41] |
|
25. |
HDPE |
CSBR |
650 |
46.0 |
18.0 |
- |
[42] |
|
26. |
Mixed |
CSBR |
450–600 |
- |
- |
100% wax |
[43] |
|
27. |
LDPE and PP |
Fluidized bed |
680 |
56.7 |
42.8 |
0.5 |
[44] |
|
28. |
Mixed |
Fluidized bed |
677 |
57.8 |
35.3 |
6.9 |
[45] |
|
29. |
Mixed |
Fluidized bed |
600 |
49.0 |
43.0 |
8.0 |
[46] |
|
30. |
LDPE |
Fixed bed |
500 |
95.0 |
5.0 |
- |
[47] |
|
31. |
PP |
Batch |
430 |
80.7 |
4.3 |
6.1 |
[48] |
|
32. |
LDPE |
Batch |
550 |
93.1 |
14.6 |
- |
[31] |
|
33. |
HDPE |
Fluidized bed |
650 |
68.5 |
31.5 |
- |
[32] |
2.1.2. Microwave-Assisted Pyrolysis
|
S. No. |
Feed |
Microwave Power Range (kW) |
Yield (wt.%) |
||
|---|---|---|---|---|---|
|
Liquid |
Gas |
Char |
|||
|
1. |
HDPE |
3 |
83.92 |
15.68 |
0.40 |
|
2. |
PP |
3 |
70.82 |
13.29 |
15.89 |
|
3. |
PVC |
3 |
3.44 |
81.87 |
14.69 |
|
4. |
PET |
1.8–3 |
35.32 |
26.48 |
38.20 |
|
5. |
PS |
3–6 |
89.25 |
8.92 |
|
2.1.3. Catalytic Pyrolysis
2.2. Gasification
2.2.1. Gasifying Medium
2.2.2. Classification of Gasifiers
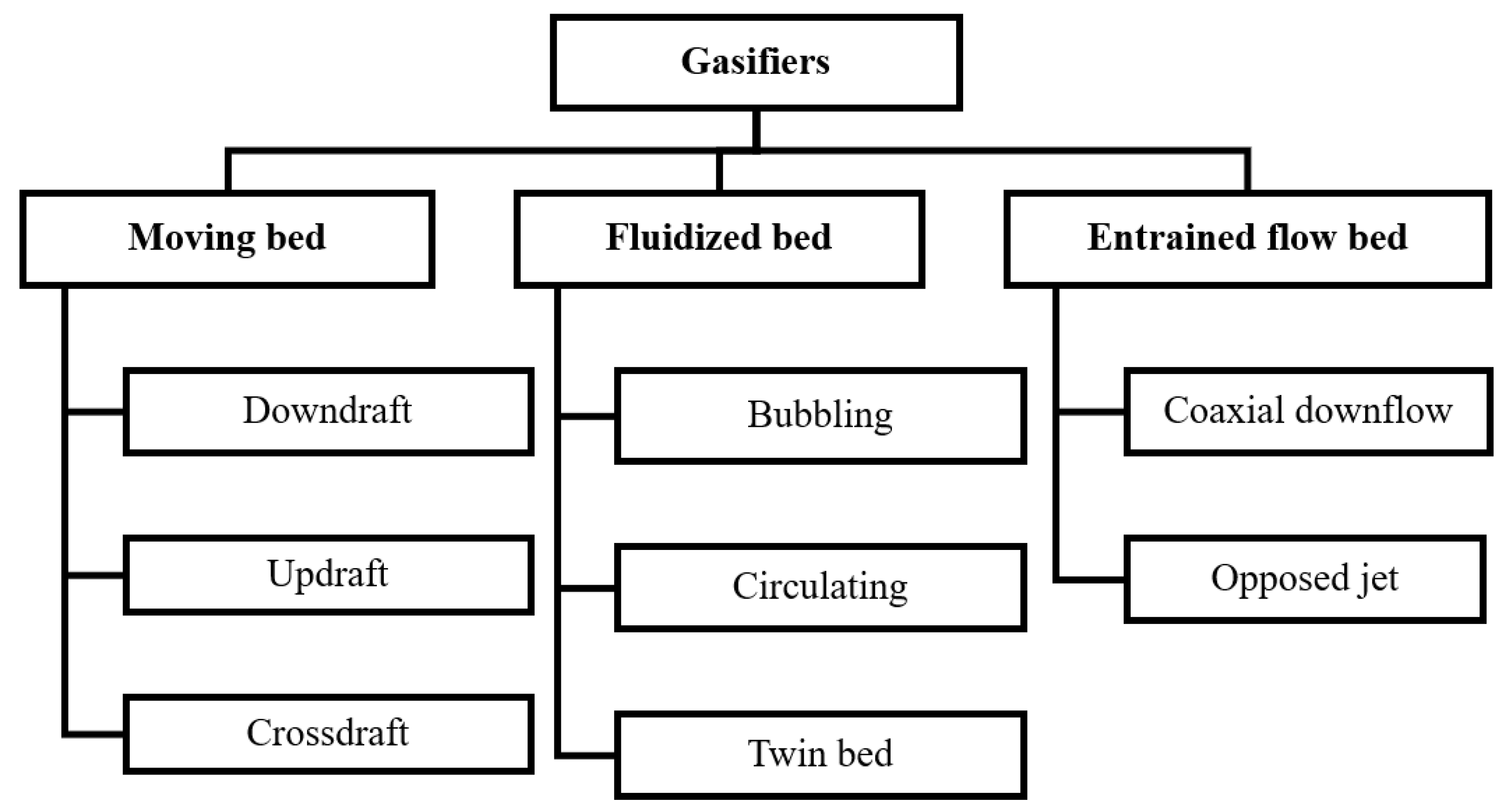
|
S. No. |
Parameter |
Fixed or Moving Bed |
Fluidized Bed |
Entrained Bed |
|---|---|---|---|---|
|
1. |
Feed size |
Less than 51 mm |
Less than 6 mm |
Less than 0.15 mm |
|
2. |
Tolerance for fines |
Limited |
Good |
Excellent |
|
3. |
Tolerance for coarse |
Very good |
Good |
Poor |
|
4. |
Gas exit temperature |
450–650 °C |
800–1000 °C |
Greater than 1200 °C |
|
5. |
Feedstock tolerance |
Low rank coal |
Low rank coal and excellent for biomass |
Any coal including caking but unsuitable for biomass |
|
6. |
Oxidant requirements |
Low |
Moderate |
High |
|
7. |
Reaction zone temperature |
1090 °C |
800–1000 °C |
1990 °C |
|
8. |
Steam requirement |
High |
Moderate |
Low |
|
9. |
Nature of ash produced |
Dry |
Dry |
Slagging |
|
10. |
Cold-gas efficiency |
80% |
89% |
80% |
|
11. |
Application |
Small capacities |
Medium size units |
Large capacities |
|
12. |
Problem areas |
Tar production and utilization of fines |
Carbon conversion |
Raw-gas cooling |
2.2.3. Other Gasifier Types
Spouted Bed
Plasma Gasifier
Pyrolysis-Reforming Process
2.2.4. Gasification Reactions
|
Reaction Type |
Reaction |
Heat of Reaction (kJ/mol) |
|---|---|---|
|
R1: Combustion reaction |
C + ½ O2 |
−122 |
|
R2: Combustion reaction |
CO + ½ O2 |
−283 |
|
R3: Combustion reaction |
H2 + ½ O2 |
−248 |
|
R4: Water gas reaction |
C + H2O |
+136 |
|
R5: Water gas shift reaction |
CO + H2O |
−35 |
|
R6: Steam reforming of methane |
CH4 + H2O |
+206 |
|
R7: Boudouard reaction |
C + CO2 |
+171 |
|
R8: Hydrogasification |
C + 2H2 |
−74.8 |
|
S. No. |
Feed |
Reactor Type |
Conditions [Equivalence Ratio (ER), Temperature (T), Steam to Plastic (S/P)] |
Gasifying Medium |
Gas Yield (m3kg−1) |
Gas Composition (% vol) |
Reference |
|---|---|---|---|---|---|---|---|
|
1. |
PE |
Bubbling fluidized bed |
ER: 0.2–0.31, T: 845–897 °C |
Air |
3–4.3 |
H2: 9.1–9.5, CO: 2.2–2.8, CO2: 9.1–10.4, CH4: 7.1–10.4 |
[65] |
|
2. |
PE |
Bubbling fluidized bed |
ER: 0.3, T: 750 °C |
Air |
3.6 |
H2: 2.7, CO: 6.1, CO2: 8.8, CH4: 7.0 |
[66] |
|
3. |
PP |
Fluidized bed |
ER: 0.32–0.36 T: 850 °C |
Air |
4.5 |
H2: 5, CO: 5, CO2: 12, CH4: 3 |
[67] |
|
4. |
PP |
Fluidized bed |
ER: 0.2–0.45 T: 690–950 °C |
Air |
2.0–3.8 |
H2: 4–5, CO: 15–20, CO2: 9–15, CH4: 4–6 |
[58] |
|
5. |
Waste PE |
Bubbling fluidized bed |
ER: 0.3, T: 750 °C |
Air |
3.7 |
H2: 3, CO: 8.7, CO2: 7.4, CH4: 8.7 |
[66] |
|
6. |
Mixed plastic wastes |
Bubbling fluidized bed |
ER: 0.25, T: 887 °C |
Air |
3.3 |
H2: 5.9, CO: 4.5, CO2: 10.3, CH4: 6.6 |
[68] |
|
7. |
PE |
Bubbling fluidized bed |
ER: 0.2–0.29 T: 807–850 °C |
Air |
4.2–6.2 |
H2: 30, CO: 18.4–20.9, CO2: 1.6–2.2, CH4: 3.4–1.5 |
[65] |
|
8. |
Waste plastic mixture |
Fixed bed |
ER: 0.15–0.6 T: 700–900 °C |
Air |
1.2–1.5 |
H2: 29–41, CO: 22–33, CO2: 8.2–22, CH4: 4.3–10 |
[69] |
|
9. |
Waste polyolefins |
Bubbling fluidized bed |
ER: 0.25–0.35, T: 750 °C |
Air |
3.2–4.4 |
H2: 3, CO: 8.5–10, CO2: 6.5–7.8, CH4: 8.5–10 |
[66] |
|
10. |
Waste plastic mixture |
Bubbling fluidized bed |
ER: 0.22–0.31 T: 869–914 °C |
Air |
2.5–3.2 |
H2: 6.6–6.8, CO: 3.7–4.8, CO2: 11–11.6, CH4: 6.3–7.3 |
[65] |
|
11. |
PP |
Fluidized bed |
ER: 0.32–0.36 T: 850 °C |
Air |
5.3 |
H2: 6, CO: 7, CO2: 16, CH4: 8 |
[67] |
|
12. |
Mixed plastic and cellulosic material |
Bubbling fluidized bed |
ER: 0.24, T: 869 °C |
Air |
2.73 |
H2: 6, CO: 6.6, CO2: 12.7, CH4: 6.5 |
[68] |
|
13. |
Recycled plastic |
Bubbling fluidized bed |
ER: 0.25, T: 877 °C |
Air |
3.5 |
H2: 6, CO: 6.6, CO2: 12.7, CH4: 6.5 |
[70] |
|
14. |
PE |
Fluidized bed |
S/P: 2, T: 850 °C |
Steam |
1.2 |
H2: 38, CO: 7, CO2: 8, CH4: 30 |
[71] |
|
15. |
PP |
Fluidized bed |
S/P: 2, T: 850 °C |
Steam |
1 |
H2: 34, CO: 4, CO2: 8, CH4: 40 |
[71] |
|
16. |
PP + PE |
Fluidized bed |
S/P: 2, T: 835 °C |
Steam |
2.1 |
H2: 46, CO: 22, CO2: 5, CH4: 16 |
[71] |
|
17. |
PE + PET |
Fluidized bed |
S/P: 1.2, T: 850 °C |
Steam |
1 |
H2: 27, CO: 20, CO2: 29, CH4: 15 |
[71] |
|
18. |
PE + PS |
Fluidized bed |
S/P: 1.8, T: 850 °C |
Steam |
1.4 |
H2: 52, CO: 24, CO2: 7, CH4: 12 |
[71] |
|
19. |
PE |
Spouted bed |
S/P: 1, T: 800–900 °C |
Steam |
2.5-3.4 |
H2: 57–60, CO: 24–28, CO2: 1–3, CH4: 6–7 |
[57] |
|
20. |
PE |
Spouted bed |
S/P: 1, T: 900 °C |
Steam |
3.2 |
H2: 58, CO: 27, CO2: 3, CH4: 7 |
[57] |
|
21. |
PE |
Spouted bed |
S/P: 1, T: 900 °C |
Steam |
3.3 |
H2: 59, CO: 26, CO2: 2, CH4: 8 |
[57] |
|
22. |
PP |
Fixed bed |
T: 850 °C |
Steam |
1.9 |
H2: 38, CO: 45, CO2: 8, CH4: 9 |
[72] |
|
23. |
HDPE |
Fixed bed |
T: 850 °C |
Steam |
2.4 |
H2: 35, CO: 43, CO2: 10, CH4: 11 |
[72] |
|
24. |
PS |
Fixed bed |
T: 850 °C |
Steam |
1.3 |
H2: 29, CO: 43, CO2: 26, CH4: 1.7 |
[72] |
|
25. |
Waste plastic |
Plasma |
T: 1200 °C |
Steam |
3.5 |
H2: 62, CO: 34 |
[73] |
|
26. |
PP |
Fixed bed/fixed bed |
T: 400/580–680 °C |
Pyrolysis and steam reforming |
5.4-8.8 |
H2: 70, CO: 9–11, CO2: 16–19, CH4: 1.4–1.5 |
[74] |
|
27. |
PP |
Fixed bed/fixed bed |
T: 400–600/630 °C |
Pyrolysis and steam reforming |
5.4-5.6 |
H2: 71–72, CO: 8–9, CO2: 19, CH4: 0.9–1.5 |
[74] |
|
28. |
PS |
Spouted bed/fluidized bed |
T: 500/700 °C |
Pyrolysis and steam reforming |
5 |
H2: 65, CO: 14, CO2: 21, CH4: <0.1 |
[75] |
|
29. |
PE |
Fixed bed/fixed bed |
T: 500/800 °C |
Pyrolysis and steam reforming |
4.35 |
H2: 67, CO: 24, CO2: 9, CH4: 1 |
[76] |
|
30. |
PP |
Fluidized bed/fluidized bed |
T: 650/850 °C |
Pyrolysis and steam reforming |
4.1 |
H2: 65, CO: 12, CO2: 21, CH4: 1.6 |
[62] |
3. Advanced Oxidation Techniques for Treatment of Plastic Waste
3.1 Photocatalytic Oxidation
3.2. Electrocatalytic Oxidation
Conversion of plastics through electrocatalytic oxidation can be brought about using two different methods: direct oxidation and indirect oxidation. Direct oxidation refers to the electrophilic attack on a polymer by ·OH produced by water discharge on the anode surface. When strong oxidising intermediates dominate in the plastic conversion process, it is referred to as indirect oxidation. With an external voltage (0.55 V) applied in H3PO4 solution at 200 °C, polyvinyl alcohol (PVA) was successfully converted to H2 (9.5 mol/min) [77]. Also, for the production of carboxylic acid (75%), electrocatalytic degradation of PVC was carried out on TiO2/C cathode (−0.7 V) at 100 °C [78].
A plastic polymer is reduced when it receives electrons from the cathode (TiO2/C), which undergoes dechlorination at a high temperature. Additionally, the polymer is oxidised with ·OH to produce carbonyl and hydroxyl groups, which subsequently breaks down into tiny molecules (e.g., alcohols, carboxylic acids and esters). Finally, these chemicals partially mineralize to CO2 and H2O. Electrocatalytic breakdown of plastic wastes may result in a single product that could be turned directly into fuels. Electrolysis alone cannot yield as many fuel components as pyrolysis and electrolysis combined.
3.3. Fenton Oxidation
References
- Weiland, F.; Lundin, L.; Celebi, M.; van der Vlist, K.; Moradian, F. Aspects of chemical recycling of complex plastic waste via the gasification route. Waste Manag. 2021, 126, 65–77.
- Bai, B.; Jin, H.; Fan, C.; Cao, C.; Wei, W.; Cao, W. Experimental investigation on liquefaction of plastic waste to oil in supercritical water. Waste Manag. 2019, 89, 247–253.
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018.
- Brems, A.; Dewil, R.; Baeyens, J.; Zhang, R. Gasification of Plastic Waste as Waste-to-Energy or Waste-to-Syngas Recovery Route. In Solid Waste as a Renewable Resource; Apple Academic Press: Palm Bay, FL, USA, 2015; pp. 241–263. ISBN 9780429154485.
- Arena, U.; Di Gregorio, F.; Amorese, C.; Mastellone, M.L. A techno-economic comparison of fluidized bed gasification of two mixed plastic wastes. Waste Manag. 2011, 31, 1494–1504.
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent advances in the gasification of waste plastics. A critical overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596.
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492.
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Current state and future prospects of plastic waste as source of fuel: A review. Renew. Sustain. Energy Rev. 2015, 50, 1167–1180.
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manage. 2017, 197, 177–198.
- Mohanraj, C.; Senthilkumar, T.; Chandrasekar, M. A review on conversion techniques of liquid fuel from waste plastic materials. Int. J. Energy Res. 2017, 41, 1534–1552.
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326.
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428.
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546.
- Pichler, C.M.; Bhattacharjee, S.; Rahaman, M.; Uekert, T.; Reisner, E. Conversion of Polyethylene Waste into Gaseous Hydrocarbons via Integrated Tandem Chemical-Photo/Electrocatalytic Processes. ACS Catal. 2021, 11, 9159–9167.
- Weber, R.S.; Ramasamy, K.K. Electrochemical oxidation of lignin and waste plastic. ACS Omega 2020, 5, 27735–27740.
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828.
- Miandad, R.; Barakat, M.A.; Aburiazaiza, A.S.; Rehan, M.; Nizami, A.S. Catalytic pyrolysis of plastic waste: A review. Process Saf. Environ. Prot. 2016, 102, 822–838.
- Eze, W.U.; Madufor, I.C.; Onyeagoro, G.N.; Obasi, H.C. The effect of Kankara zeolite-Y-based catalyst on some physical properties of liquid fuel from mixed waste plastics (MWPs) pyrolysis. Polym. Bull. 2020, 77, 1399–1415.
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of plastic waste: Opportunities and challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804.
- Kaminsky, W.; Kim, J.S. Pyrolysis of mixed plastics into aromatics. J. Anal. Appl. Pyrolysis 1999, 51, 127–134.
- Olazar, M.; Lopez, G.; Amutio, M.; Elordi, G.; Aguado, R.; Bilbao, J. Influence of FCC catalyst steaming on HDPE pyrolysis product distribution. J. Anal. Appl. Pyrolysis 2009, 85, 359–365.
- Eze, W.U.; Umunakwe, R.; Obasi, H.C.; Ugbaja, M.I.; Uche, C.C.; Madufor, I.C. Plastics waste management: A review of pyrolysis technology. Clean Technol. Recycl. 2021, 1, 50–69.
- Al-Salem, S.M. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC). Process Saf. Environ. Prot. 2019, 127, 171–179.
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: Product yield analysis and their characterization. J. Energy Inst. 2019, 92, 1647–1657.
- Sharma, B.K.; Moser, B.R.; Vermillion, K.E.; Doll, K.M.; Rajagopalan, N. Production, characterization and fuel properties of alternative diesel fuel from pyrolysis of waste plastic grocery bags. Fuel Process. Technol. 2014, 122, 79–90.
- Ahmad, I.; Ismail Khan, M.; Khan, H.; Ishaq, M.; Tariq, R.; Gul, K.; Ahmad, W. Pyrolysis Study of Polypropylene and Polyethylene Into Premium Oil Products. Int. J. Green Energy 2015, 12, 663–671.
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Influence of time and temperature on pyrolysis of plastic wastes in a semi-batch reactor. Chem. Eng. J. 2011, 173, 62–71.
- Fakhrhoseini, S.M.; Dastanian, M. Predicting pyrolysis products of PE, PP, and PET using NRTL activity coefficient model. J. Chem. 2013, 2013, 7–9.
- Williams, P.T.; Slaney, E. Analysis of products from the pyrolysis and liquefaction of single plastics and waste plastic mixtures. Resour. Conserv. Recycl. 2007, 51, 754–769.
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H. Evaluation of pyrolysis product of virgin high density polyethylene degradation using different process parameters in a stirred reactor. Fuel Process. Technol. 2013, 109, 90–95.
- Marcilla, A.; Beltrán, M.I.; Navarro, R. Thermal and catalytic pyrolysis of polyethylene over HZSM5 and HUSY zeolites in a batch reactor under dynamic conditions. Appl. Catal. B Environ. 2009, 86, 78–86.
- Mastral, F.J.; Esperanza, E.; Garciía, P.; Juste, M. Pyrolysis of high-density polyethylene in a fluidised bed reactor. Influence of the temperature and residence time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15.
- Lee, K.H.; Noh, N.S.; Shin, D.H.; Seo, Y. Comparison of plastic types for catalytic degradation of waste plastics into liquid product with spent FCC catalyst. Polym. Degrad. Stab. 2002, 78, 539–544.
- Luo, G.; Suto, T.; Yasu, S.; Kato, K. Catalytic degradation of high density polyethylene and polypropylene into liquid fuel in a powder-particle fluidized bed. Polym. Degrad. Stab. 2000, 70, 97–102.
- Demirbas, A. Pyrolysis of municipal plastic wastes for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102.
- Sakata, Y.; Uddin, M.A.; Muto, A. Degradation of polyethylene and polypropylene into fuel oil by using solid acid and non-acid catalysts. J. Anal. Appl. Pyrolysis 1999, 51, 135–155.
- Adnan; Shah, J.; Jan, M.R. Thermo-catalytic pyrolysis of polystyrene in the presence of zinc bulk catalysts. J. Taiwan Inst. Chem. Eng. 2014, 45, 2494–2500.
- Uddin, M.A.; Koizumi, K.; Murata, K.; Sakata, Y. Thermal and catalytic degradation of structurally different types of polyethylene into fuel oil. Polym. Degrad. Stab. 1997, 56, 37–44.
- Williams, P.T.; Williams, E.A. Fluidised bed pyrolysis of low density polyethylene to produce petrochemical feedstock. J. Anal. Appl. Pyrolysis 1999, 51, 107–126.
- Miskolczi, N.; Bartha, L.; Deák, G.; Jóver, B.; Kalló, D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis 2004, 72, 235–242.
- Abbas-Abadi, M.S.; Haghighi, M.N.; Yeganeh, H.; McDonald, A.G. Evaluation of pyrolysis process parameters on polypropylene degradation products. J. Anal. Appl. Pyrolysis 2014, 109, 272–277.
- Elordi, G.; Olazar, M.; Lopez, G.; Artetxe, M.; Bilbao, J. Product yields and compositions in the continuous pyrolysis of high-density polyethylene in a conical spouted bed reactor. Ind. Eng. Chem. Res. 2011, 50, 6650–6659.
- Arabiourrutia, M.; Elordi, G.; Lopez, G.; Borsella, E.; Bilbao, J.; Olazar, M. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J. Anal. Appl. Pyrolysis 2012, 94, 230–237.
- Jung, S.H.; Cho, M.H.; Kang, B.S.; Kim, J.S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process. Technol. 2010, 91, 277–284.
- Cho, M.H.; Jung, S.H.; Kim, J.S. Pyrolysis of mixed plastic wastes for the recovery of benzene, toluene, and xylene (BTX) aromatics in a fluidized bed and chlorine removal by applying various additives. Energy Fuels 2010, 24, 1389–1395.
- Grause, G.; Matsumoto, S.; Kameda, T.; Yoshioka, T. Pyrolysis of mixed plastics in a fluidized bed of hard burnt lime. Ind. Eng. Chem. Res. 2011, 50, 5459–5466.
- Bagri, R.; Williams, P.T. Catalytic pyrolysis of polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41.
- Ali, M.F.; Ahmed, S.; Qureshi, M.S. Catalytic coprocessing of coal and petroleum residues with waste plastics to produce transportation fuels. Fuel Process. Technol. 2011, 92, 1109–1120.
- Kaminsky, W. Plastics recycling. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 1992; ISBN 3-52730-385-5.
- Xue, Y.; Johnston, P.; Bai, X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energy Convers. Manag. 2017, 142, 441–451.
- Wan, S.; Wang, Y. A review on ex situ catalytic fast pyrolysis of biomass. Front. Chem. Sci. Eng. 2014, 8, 280–294.
- Luo, G.; Resende, F.L.P. In-situ and ex-situ upgrading of pyrolysis vapors from beetle-killed trees. Fuel 2016, 166, 367–375.
- Hu, M.; Guo, D.; Ma, C.; Luo, S.; Chen, X.; Cheng, Q.; Laghari, M.; Xiao, B. A novel pilot-scale production of fuel gas by allothermal biomass gasification using biomass micron fuel (BMF) as external heat source. Clean Technol. Environ. Policy 2016, 18, 743–751.
- Nyakuma, B.B.; Ivase, T.J.P. Emerging trends in sustainable treatment and valorisation technologies for plastic wastes in Nigeria: A concise review. Environ. Prog. Sustain. Energy 2021, 40, e13660.
- Salaudeen, S.A.; Arku, P.; Dutta, A. Gasification of Plastic Solid Waste and Competitive Technologies; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128131404.
- Gil, J.; Corella, J.; Aznar, M.P.; Caballero, M.A. Biomass gasification in atmospheric and bubbling fluidized bed: Effect of the type of gasifying agent on the product distribution. Biomass Bioenergy 1999, 17, 389–403.
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from steam gasification of polyethylene in a conical spouted bed reactor. Fuel 2013, 109, 461–469.
- Xiao, R.; Jin, B.; Zhou, H.; Zhong, Z.; Zhang, M. Air gasification of polypropylene plastic waste in fluidized bed gasifier. Energy Convers. Manag. 2007, 48, 778–786.
- Degnan, T.F. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356.
- Artetxe, M.; Lopez, G.; Amutio, M.; Elordi, G.; Bilbao, J.; Olazar, M. Cracking of high density polyethylene pyrolysis waxes on HZSM-5 catalysts of different acidity. Ind. Eng. Chem. Res. 2013, 52, 10637–10645.
- Garforth, A.A.; Lin, Y.H.; Sharratt, P.N.; Dwyer, J. Production of hydrocarbons by catalytic degradation of high density polyethylene in a laboratory fluidised-bed reactor. Appl. Catal. A Gen. 1998, 169, 331–342.
- Czernik, S.; French, R.J. Production of hydrogen from plastics by pyrolysis and catalytic steam reform. Energy Fuels 2006, 20, 754–758.
- Olazar, M.; Santamaria, L.; Lopez, G.; Fernandez, E.; Cortazar, M.; Arregi, A.; Bilbao, J. Progress on catalyst development for the steam reforming of biomass and waste plastics pyrolysis volatiles: A review. Energy Fuels 2021, 35, 17051–17084.
- Kannan, P.; Al Shoaibi, A.; Srinivasakannan, C. Energy recovery from co-gasification of waste polyethylene and polyethylene terephthalate blends. Comput. Fluids 2013, 88, 38–42.
- Arena, U.; Zaccariello, L.; Mastellone, M.L. Fluidized bed gasification of waste-derived fuels. Waste Manag. 2010, 30, 1212–1219.
- Martínez-Lera, S.; Torrico, J.; Pallarés, J.; Gil, A. Design and first experimental results of a bubbling fluidized bed for air gasification of plastic waste. J. Mater. Cycles Waste Manag. 2013, 15, 370–380.
- Sancho, J.A.; Aznar, M.P.; Toledo, J.M. Catalytic Air Gasification of Plastic Waste (Polypropylene) in Fluidized Bed. Part I: Use of in-Gasifier Bed Additives. Ind. Eng. Chem. Res. 2008, 47, 1005–1010.
- Arena, U.; Di Gregorio, F. Energy generation by air gasification of two industrial plastic wastes in a pilot scale fluidized bed reactor. Energy 2014, 68, 735–743.
- Lee, J.W.; Yu, T.U.; Lee, J.W.; Moon, J.H.; Jeong, H.J.; Park, S.S.; Yang, W.; Lee, U. Do Gasification of mixed plastic wastes in a moving-grate gasifier and application of the producer gas to a power generation engine. Energy Fuels 2013, 27, 2092–2098.
- Zaccariello, L.; Mastellone, M.L. Fluidized-bed gasification of plastic waste, wood, and their blends with coal. Energies 2015, 8, 8052–8068.
- Wilk, V.; Hofbauer, H. Conversion of mixed plastic wastes in a dual fluidized bed steam gasifier. Fuel 2013, 107, 787–799.
- Friengfung, P.; Jamkrajang, E.; Sunphorka, S.; Kuchonthara, P.; Mekasut, L. NiO/dolomite catalyzed steam/O2 gasification of different plastics and their mixtures. Ind. Eng. Chem. Res. 2014, 53, 1909–1915.
- Rutberg, P.G.; Kuznetsov, V.A.; Serba, E.O.; Popov, S.D.; Surov, A.V.; Nakonechny, G.V.; Nikonov, A.V. Novel three-phase steam-air plasma torch for gasification of high-caloric waste. Appl. Energy 2013, 108, 505–514.
- Park, Y.; Namioka, T.; Sakamoto, S.; Min, T.J.; Roh, S.A.; Yoshikawa, K. Optimum operating conditions for a two-stage gasification process fueled by polypropylene by means of continuous reactor over ruthenium catalyst. Fuel Process. Technol. 2010, 91, 951–957.
- Barbarias, I.; Lopez, G.; Artetxe, M.; Arregi, A.; Santamaria, L.; Bilbao, J.; Olazar, M. Pyrolysis and in-line catalytic steam reforming of polystyrene through a two-step reaction system. J. Anal. Appl. Pyrolysis 2016, 122, 502–510.
- Wu, C.; Williams, P.T. Pyrolysis-gasification of plastics, mixed plastics and real-world plastic waste with and without Ni-Mg-Al catalyst. Fuel 2010, 89, 3022–3032.
- Hori, T.; Kobayashi, K.; Teranishi, S.; Nagao, M.; Hibino, T. Fuel cell and electrolyzer using plastic waste directly as fuel. Waste Manag. 2020, 102, 30–39.
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023.






