Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianxun Song | -- | 1945 | 2023-02-21 16:17:47 | | | |
| 2 | Conner Chen | Meta information modification | 1945 | 2023-02-22 03:55:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, L.; Peng, H.; Pham, A.; Villazana, E.; Ballard, D.J.; Das, J.K.; Kumar, A.; Xiong, X.; Song, J. T Cell Response to SARS-CoV-2 Coinfection and Comorbidities. Encyclopedia. Available online: https://encyclopedia.pub/entry/41489 (accessed on 08 February 2026).
Wang L, Peng H, Pham A, Villazana E, Ballard DJ, Das JK, et al. T Cell Response to SARS-CoV-2 Coinfection and Comorbidities. Encyclopedia. Available at: https://encyclopedia.pub/entry/41489. Accessed February 08, 2026.
Wang, Liqing, Hao-Yun Peng, Aspen Pham, Eber Villazana, Darby J. Ballard, Jugal Kishore Das, Anil Kumar, Xiaofang Xiong, Jianxun Song. "T Cell Response to SARS-CoV-2 Coinfection and Comorbidities" Encyclopedia, https://encyclopedia.pub/entry/41489 (accessed February 08, 2026).
Wang, L., Peng, H., Pham, A., Villazana, E., Ballard, D.J., Das, J.K., Kumar, A., Xiong, X., & Song, J. (2023, February 21). T Cell Response to SARS-CoV-2 Coinfection and Comorbidities. In Encyclopedia. https://encyclopedia.pub/entry/41489
Wang, Liqing, et al. "T Cell Response to SARS-CoV-2 Coinfection and Comorbidities." Encyclopedia. Web. 21 February, 2023.
Copy Citation
COVID-19 has become an increasing global health issue. Adaptive immune cells, especially T cells, have been extensively investigated in regard to SARS-CoV-2 infection. However, human health and T cell responses are also impacted by many other pathogens and chronic diseases.
SARS-CoV-2
T cells
coinfection
1. Introduction
Since its start at the end of 2019, the COVID-19 outbreak has posed significant health risks to the human population, surpassing more than 6.5 million deaths across the world (WHO cumulative data up to Oct 2022). Over the past 3 years, publications regarding coinfection with SARS-CoV-2 and other pathogens, such as viruses, bacteria, and parasites, have been reported. Tissue damage resulting from SARS-CoV-2 includes lung failure [1], brain damage [2], intestinal mucosal damage [3], and cardiovascular disease [4]. Furthermore, in cases when a cytokine storm is present in SARS-CoV-2 infection, coinfection may exacerbate the outcomes of other types of diseases, such as allergy, diabetes, and hypertension.
T cells play a pivotal role during viral infection and immune-related diseases. Due to SARS-CoV-2’s ability to alter the T cell response, it may affect the outcomes of several common pathogenic infections and chronic diseases. In a reported case of HIV coinfection with SARS-CoV-2, depletion of CD4+ T cells was observed to affect the patient’s clinical outcome [5]. In other conditions such as TB infection and diabetes, there is an increased risk of severe SARS-CoV-2 due to exacerbation of symptoms [6]. In diabetic patients, the increased clinical risk is positively correlated with SARS-CoV-2 infection [7].
2. SARS-CoV-2 Infection and T Cell Response
Within lethal contagious respiratory viruses, the most prevalent transmission route is an airborne infection [8]. In Figure 1, when the SARS-CoV-2 reaches the lung’s epithelial layer, the spike protein on the viral surface will bind with the ACE2 receptor, accelerating the entry of the virus [9][10]. Fusion of the virus and host cell membrane results in the release of the viral genome [11]. Essential components (including N, S, M, and E proteins) and sub-genomic viral RNA are subsequently generated and assembled to form an intact virus [12]. The newly generated virus is released from the cytoplasm through exocytosis [12] and SARS-CoV-2 will be captured by antigen-presenting cells (APC). Viral antigens are then processed and presented to the T cells [13][14]. Meanwhile, the defense mechanism mediated by T cells with SARS-CoV-2 is different from the antibody-dependent response due to their ability to recognize a broad range of viral epitopes [13][15]. Compared with the common cold, SARS-CoV-2 infection generates a diverse epitope pool and increases the frequency of both viral-epitope-specific CD4+ and CD8+ T cells among convalescent SARS-CoV-2 patients [13]. In terms of infection, T cells secrete IFNγ, Granzyme B, and TNFα to help eliminate SARS-CoV-2 infected cells [16]. In mild COVID-19 patients, the effector CD4+ or CD8+ T cells will proliferate and form a defense mechanism during the acute phase [17]. In moderate-symptom patients, the natural killer T cell (NKT) CD160 population responds quickly by direct cytotoxicity [18]. Furthermore, the SARS-CoV-2-specific memory T cells are beneficial for providing essential protection against future reinfection [15][19][20][21].
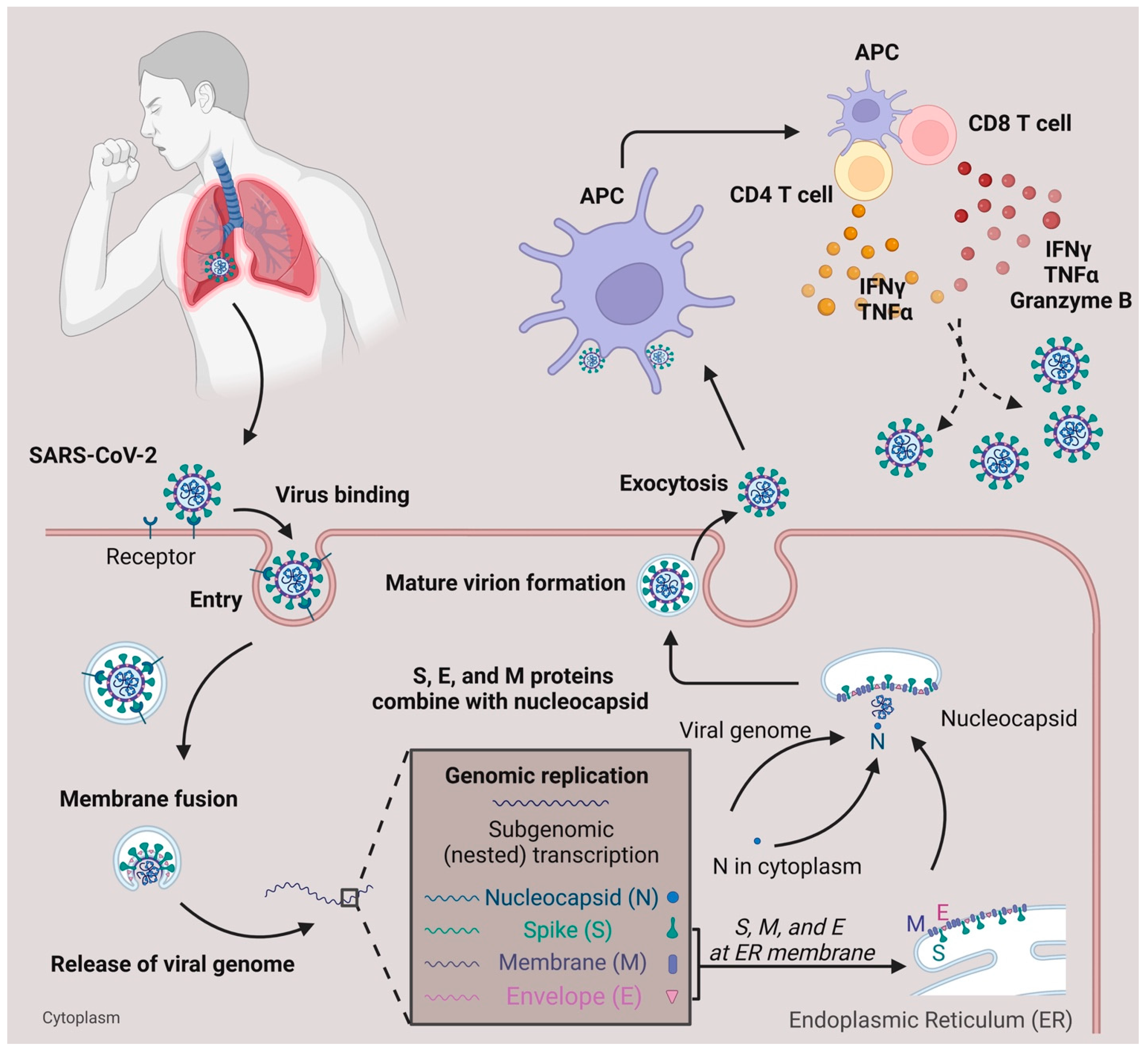
Figure 1. SARS-CoV-2 infection and T cell response. SARS-CoV-2 infects the respiratory tract by binding to the ACE2 surface receptor. Following entry via membrane fusion, it releases its viral genome in the cytoplasm. Using the host cell machinery, viral genome replication, and subgenomic transcription generate essential components needed for the virus to pack together. After the maturation of the virion, it is released from the host cell through exocytosis. When the viruses are captured and processed by antigen-presenting cells (APC), the T cells, either CD4+ or CD8+, are activated and secrete cytokines (IFNγ, TNFα, Granzyme B) to defend against viruses.
3. T Cell Subsets and Surface Markers Change during SARS-CoV-2 Infection
As studied, SARS-CoV-2 patients with severe symptoms often displayed progressive lymphopenia, while patients with mild symptoms showed normal absolute lymphocyte counts [22]. Within the CD8+ T cell populations in these patients, an increased population of CD8+ effector memory T cells was observed [22]. On the other hand, lower CD4+ or CD8+ frequencies were detected in severe COVID-19 patients compared to mild COVID-19 patients [23]. A study performed by Neidleman et al. indicated that CD27+CD28+CD8+ T effector memory cells re-expressing CD45RA (TEMRA) were predominant in the recovery period of SARS-CoV-2 patients, contradicting the expression of the CD27 marker [24]. Moreover, there was an increase in both central memory CD8+ T cells (CD45RA−CD27+CCR7+) and effector memory CD8+ T cells (CD45RA−CD27+CCR7−) [25][26][27]. In regards to Ki67+ and HLA-DR markers co-expressed on CD8+ T cells, SARS-CoV-2 infection led to an increase in Ki67+HLA-DR+CD8+ T cells in the patients [26]. In addition, there was an increase in the expression of activation markers, including HLA-DR and CD45RO, on CD8 T cells in severe SARS-CoV-2 patients compared to mild patients [28]. An increased proportion of CD38hi+ CD8+ T cells was also observed in severe SARS-CoV-2 infected patients [26][29][30]. In addition, a reduction in CD27+CD8+ T cells and an increase in CD127+CD8+ T cells were found in SARS-CoV-2 patients [24]. When compared to healthy blood donors, CTLA-4, LAG-3, and Tim-3 were significantly expressed in memory CD8+ T cells from patients with severe SARS-CoV-2 infection, while there was no difference for the inhibitory immune checkpoint PD-1 marker [26][30].
Cords detected that LAG-3 and TIGIT expression were upregulated in SARS-CoV-2-specific CD4+ T cells when compared to CD4+ T cells in healthy patients [31]. A higher percentage of cells expressing HLA-DR and CD45RO were also observed within the effector CD4+ T cell population in severe patients compared to mild patients [32]. On the other hand, a reduction in CD28+CD4+ T cells was detected in severe patients compared to mild patients [32]. Cytokines, including IL-2R, IL-6, IL-8, and IL-10, experienced an increase in production from effector CD4+ T cells in severe SARS-CoV-2 patients [33][34]. The decreased expression of CD45RA and CCR7 indicated that T central memory cells are more dominant than T effector memory cells [24]. It was also reported that there were high populations of memory CD4+ T cells expressing CD38, CD69, Ki-67, and PD-1 in memory CD4+ T cells from patients with severe SARS-CoV-2 infection compared to healthy blood donors [26].
Among CD4+ T cell subsets, CD4+ Teff cells differentiate into functionally diverse subsets such as Th1, Th2, Tfh, Th17, and Treg cells. T helper 1 (Th1) cells protect against intracellular parasites by the secretion of IFNγ [35][36]. Studies have indicated that Th1 cells predominate during SARS-CoV-2 infection through upregulation of IFNγ but not IL-4 or IL-17. T helper 2 (Th2) cells help to eliminate extracellular pathogens [36]. Studies also indicate that Th2 and Th17 cells play a role in SARS-CoV-2 infection in which Th2 cells were claimed to be unfavorable for recovery [24][30][37]. Lower levels of Th2 populations were observed in mild patients compared to healthy donors, but no difference was indicated between mild and severe patients. The disproportionate ratio of Th2/Th1 cells revealed a lead in severe infection, with abnormal secretion of associated cytokines such as IL-4, IL-5, IL-10, and IL-13 [30][38][39][40]. T follicular helper cells (Tfh), a particular subset of CD4+ T cells that regulates antibody responses with B cells, have been noted to play a role in SARS-CoV-2 infection [22]. Mathew et al. indicated that there was a decreased population of CD45RA−CXCR5+PD-1+ circulating Tfh (cTfh) cells post-SARS-CoV-2 infection with an upward trend of Tfh cell population in recovered patients within six months [27]. Among cTfh cells, there are multiple subsets including Tfh1, Tfh2, and Tfh17 that are categorized based on differences in the secretion of cytokines. A lower population of cTfh17 (CXCR5+CXCR3−CCR6+) cells, which secrete IL-21 and IL-17A, was discovered in a common variable immunodeficiency disease (CVID) patient with SARS-CoV-2 infection compared to the immunocompetent donor [22][41]. However, for PD-1 marker expression, there were multiple contradictory studies indicating that there were no changes in PD-1+ Tfh cells in SARS-CoV-2 patients [42]. A high expression of ICOS was also found in SARS-CoV-2-specific Tfh cells [24]. In regard to Treg cells, which act as suppressors in the immune system, there are conflicting studies. An increased percentage of regulatory T cells (CD3+CD4+CD25+CD127low) and a higher secretion of IL-10, TGF-b, IL-6, and IL-18 were detected during COVID-19 infection, specifically with N peptide stimulation [43][44]. Increased populations of Treg cells were present in mildly symptomatic patients compared to healthy controls [43].
4. Role of T Cells during Coinfection with Viruses, Bacteria, and Parasites
During the three-year pandemic, researchers have noticed cases of coinfection with SARS-CoV-2 and other viruses (e.g., HBV, HIV, HCV, and influenza), bacteria (e.g., Mtb), and parasites (e.g., protozoa and helminths) (Figure 2).
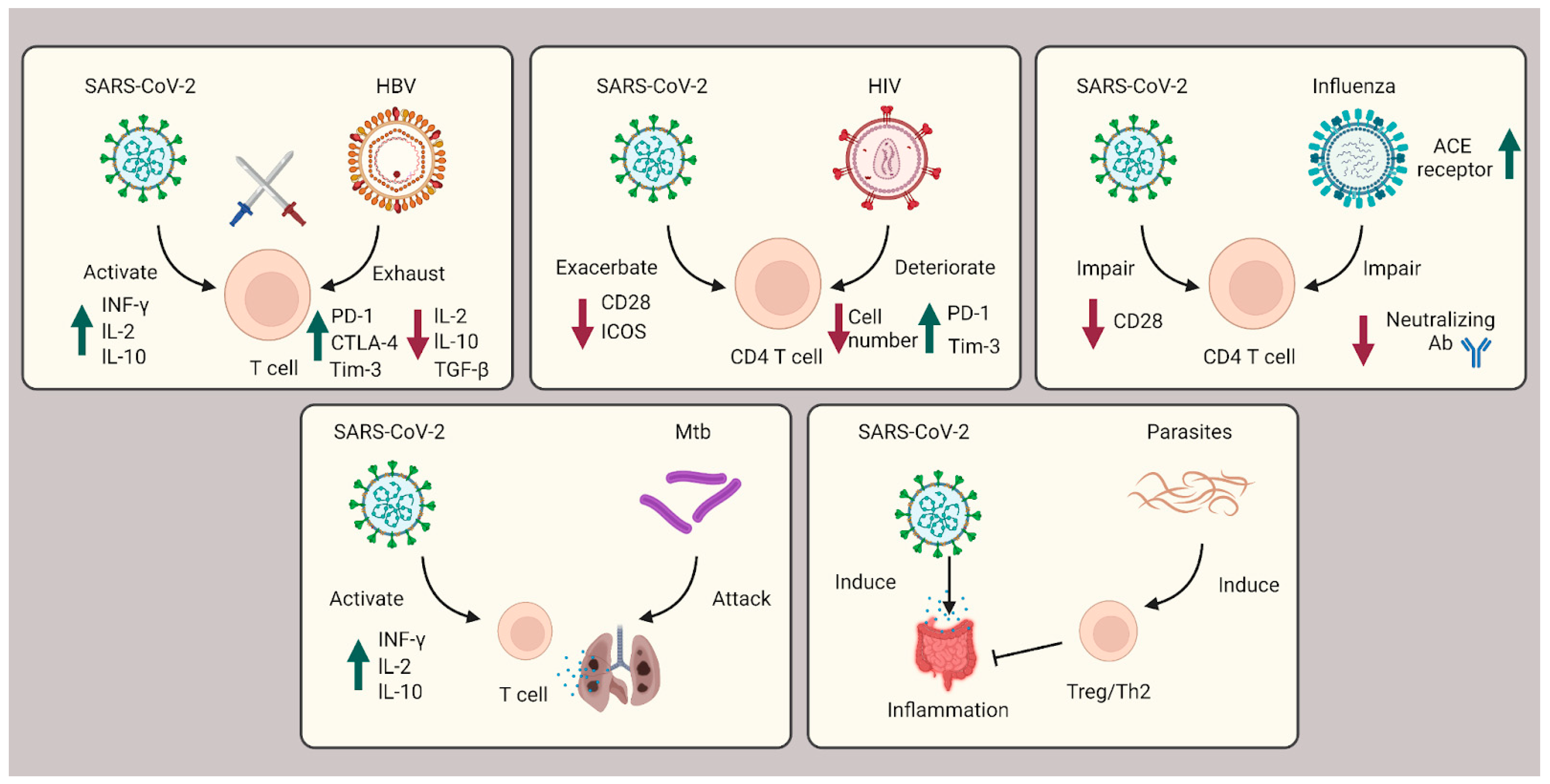
Figure 2. Role of T cells during coinfection with viruses, bacteria, and parasites. In SARS-CoV-2 coinfection with HBV, exhausted T cells are caused by HBV infection and subsequently countered with SARS-CoV-2 infection cytokine storms. In HIV coinfection, CD4+ T cells are corrupted, leading to exacerbated patient outcomes. Influenza coinfection decreases neutralization antibody efficacy and increases ACE2 receptors, overall boosting SARS-CoV-2 infection. For Mtb coinfection, both infections will affect the lung tissue, resulting in respiratory failure. In coinfection with parasites, Th2 and Treg cells are induced to suppress the immune system and ameliorate the intestinal inflammation severity of SARS-CoV-2.
4.1. T Cells’ Role during SARS-CoV-2 Coinfection with HBV, HIV, HCV, and Influenza
Hepatitis B virus (HBV) is a contagious virus that causes chronic liver diseases such as fibrosis and hepatocellular carcinoma (HCC) [45]. Currently, the coinfection outcome of SARS-CoV-2 and HBV is still ambiguous. A report displayed that 3% of SARS-CoV-2 patients will suffer from chronic liver disease [46]. Some researchers reported cases where coinfection resulted in severe liver injury and other severe outcomes [47][48][49]. Clinical studies show that coinfected patients experience a high severity and poor prognosis, both of which should be taken seriously [50]. However, other publications indicate that no significant difference has been observed in the clinical outcome of chronic HBV carriers who were SARS-CoV-2 coinfected [51][52]. On one hand, T cells are important for a host to defend against the invasion of SARS viruses. On the other hand, it is well known that SARS-CoV-2 will induce excessive cytokine storms from T cells, causing increased severity [53]. In terms of immune suppression by HBV infection, T cell exhaustion is accelerated, and partial cytokine secreted T or NK cells become dysfunctional [54][55]. These counterparts of coinfection may explain the lack of significant difference observed for HBV patients coinfected with SARS-CoV-2 [56].
4.2. T Cells’ Role during SARS-CoV-2 Coinfection with Mycobacterium tuberculosis (Mtb)
Mycobacterium tuberculosis (Mtb) is an aerobic bacterium that attacks human lung cells [57]. Although the Bacille Calmette–Guérin (BCG) vaccine is available and provides sufficient protection for infants and children, adults are still vulnerable to infection [58]. Mtb infection affects the immune system by causing delays in the initial activation of both Mtb-specific CD4+ and CD8+ T cells [59]. In a reported study, one article suggests that CD4+ T cells may be capable of preventing prevent CD8+ T cell exhaustion during Mtb infection [60]. Since both Mtb and SARS-CoV-2 infections cause damage to lung tissue, coinfection has been associated with a higher death rate compared with SARS-CoV-2 infection only [6]. In coinfection, researchers have discovered that SARS-CoV-2 patients display a decreased frequency of Mtb-specific CD4+ T cells [61]. Furthermore, SARS-CoV-2 patients infected with either TB or latent TB showed a lower response to SARS-CoV-2 and had a decreased Mtb-specific immune response [62].
4.3. T Cells’ Role during SARS-CoV-2 Coinfection with Parasite
Parasite infection is a long-standing area of concern in the field of public health. In response to common parasitic infections, such as protozoa and helminths, the body will induce regulatory T cells to suppress the host’s antiparasitic immune system [63]. Multiple researchers have discovered that intestinal parasite coinfection with SARS-CoV-2 decreases SARS-CoV-2-mediated intestinal inflammation risk [64]. In addition, parasite-driven Th2 and Treg cell responses were able to counterbalance SARS-CoV-2-induced cytokine storms [64][65]. This discovery may explain why parasite coinfection with SARS-CoV-2 may ameliorate the severity of SARS-CoV-2 patients.
References
- Upadhya, S.; Rehman, J.; Malik, A.B.; Chen, S. Mechanisms of Lung Injury Induced by SARS-CoV-2 Infection. Physiology 2022, 37, 88–100.
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 2022, 604, 697–707.
- Vanella, G.; Capurso, G.; Burti, C.; Fanti, L.; Ricciardiello, L.; Lino, A.S.; Boskoski, I.; Bronswijk, M.; Tyberg, A.; Krishna Kumar Nair, G.; et al. Gastrointestinal mucosal damage in patients with COVID-19 undergoing endoscopy: An international multicentre study. BMJ Open Gastroenterol. 2021, 8, e000578.
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590.
- Tesoriero, J.M.; Swain, C.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw Open 2021, 4, e2037069.
- Mollalign, H.; Chala, D.; Beyene, D. Clinical Features and Treatment Outcome of Coronavirus and Tuberculosis Co-Infected Patients: A Systematic Review of Case Reports. Infect. Drug. Resist. 2022, 15, 4037–4046.
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30.
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149.
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422.
- Li, W.; Wong, S.K.; Li, F.; Kuhn, J.H.; Huang, I.C.; Choe, H.; Farzan, M. Animal origins of the severe acute respiratory syndrome coronavirus: Insight from ACE2-S-protein interactions. J. Virol. 2006, 80, 4211–4219.
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20.
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170.
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.
- Gutierrez, L.; Beckford, J.; Alachkar, H. Deciphering the TCR Repertoire to Solve the COVID-19 Mystery. Trends Pharmacol. Sci. 2020, 41, 518–530.
- Vardhana, S.; Baldo, L.; Morice, W.G., 2nd; Wherry, E.J. Understanding T cell responses to COVID-19 is essential for informing public health strategies. Sci. Immunol. 2022, 7, eabo1303.
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020, 5, eabd6260.
- Niessl, J.; Sekine, T.; Buggert, M. T cell immunity to SARS-CoV-2. Semin. Immunol. 2021, 55, 101505.
- Zhang, J.Y.; Wang, X.M.; Xing, X.; Xu, Z.; Zhang, C.; Song, J.W.; Fan, X.; Xia, P.; Fu, J.L.; Wang, S.Y.; et al. Single-cell landscape of immunological responses in patients with COVID-19. Nat. Immunol. 2020, 21, 1107–1118.
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Fernandez, N.D.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-reactive memory T cells associate with protection against SARS-CoV-2 infection in COVID-19 contacts. Nat. Commun. 2022, 13, 80.
- Wang, Z.; Yang, X.; Zhong, J.; Zhou, Y.; Tang, Z.; Zhou, H.; He, J.; Mei, X.; Tang, Y.; Lin, B.; et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat. Commun. 2021, 12, 1724.
- Wang, L.; Kumar, A.; Das, J.K.; Ren, Y.; Peng, H.Y.; Ballard, D.J.; Xiong, X.; Davis, J.R.; Ren, X.; Yang, J.M.; et al. Expression of NAC1 Restrains the Memory Formation of CD8(+) T Cells during Viral Infection. Viruses 2022, 14, 1713.
- Gupta, S.; Su, H.; Narsai, T.; Agrawal, S. SARS-CoV-2-Associated T-Cell Responses in the Presence of Humoral Immunodeficiency. Int. Arch. Allergy Immunol. 2021, 182, 195–209.
- Popescu, I.; Snyder, M.E.; Iasella, C.J.; Hannan, S.J.; Koshy, R.; Burke, R.; Das, A.; Brown, M.J.; Lyons, E.J.; Lieber, S.C.; et al. CD4(+) T-Cell Dysfunction in Severe COVID-19 Disease Is Tumor Necrosis Factor-α/Tumor Necrosis Factor Receptor 1-Dependent. Am. J. Respir. Crit. Care Med. 2022, 205, 1403–1418.
- Neidleman, J.; Luo, X.; Frouard, J.; Xie, G.; Gill, G.; Stein, E.S.; McGregor, M.; Ma, T.; George, A.F.; Kosters, A.; et al. SARS-CoV-2-Specific T Cells Exhibit Phenotypic Features of Helper Function, Lack of Terminal Differentiation, and High Proliferation Potential. Cell Rep. Med. 2020, 1, 100081.
- Zhang, F.; Gan, R.; Zhen, Z.; Hu, X.; Li, X.; Zhou, F.; Liu, Y.; Chen, C.; Xie, S.; Zhang, B.; et al. Adaptive immune responses to SARS-CoV-2 infection in severe versus mild individuals. Signal. Transduct Target Ther. 2020, 5, 156.
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Stralin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511.
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 2020, 5, e137799.
- Du, J.; Wei, L.; Li, G.; Hua, M.; Sun, Y.; Wang, D.; Han, K.; Yan, Y.; Song, C.; Song, R.; et al. Persistent High Percentage of HLA-DR(+)CD38(high) CD8(+) T Cells Associated With Immune Disorder and Disease Severity of COVID-19. Front Immunol. 2021, 12, 735125.
- Song, J.W.; Zhang, C.; Fan, X.; Meng, F.P.; Xu, Z.; Xia, P.; Cao, W.J.; Yang, T.; Dai, X.P.; Wang, S.Y.; et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020, 11, 3410.
- Cords, L.; Knapp, M.; Woost, R.; Schulte, S.; Kummer, S.; Ackermann, C.; Beisel, C.; Peine, S.; Johansson, A.M.; Kwok, W.W.; et al. High and Sustained Ex Vivo Frequency but Altered Phenotype of SARS-CoV-2-Specific CD4(+) T-Cells in an Anti-CD20-Treated Patient with Prolonged COVID-19. Viruses 2022, 14, 1265.
- Kaaijk, P.; Pimentel, V.O.; Emmelot, M.E.; Poelen, M.C.M.; Cevirgel, A.; Schepp, R.M.; den Hartog, G.; Reukers, D.F.M.; Beckers, L.; van Beek, J.; et al. Children and Adults With Mild COVID-19: Dynamics of the Memory T Cell Response up to 10 Months. Front Immunol. 2022, 13, 817876.
- Li, J.; Rong, L.; Cui, R.; Feng, J.; Jin, Y.; Chen, X.; Xu, R. Dynamic changes in serum IL-6, IL-8, and IL-10 predict the outcome of ICU patients with severe COVID-19. Ann. Palliat. Med. 2021, 10, 3706–3714.
- Jing, X.; Xu, M.; Song, D.; Yue, T.; Wang, Y.; Zhang, P.; Zhong, Y.; Zhang, M.; Lam, T.T.; Faria, N.R.; et al. Association between inflammatory cytokines and anti-SARS-CoV-2 antibodies in hospitalized patients with COVID-19. Immun. Ageing 2022, 19, 12.
- Wang, L.; Das, J.K.; Kumar, A.; Peng, H.Y.; Ren, Y.; Xiong, X.; Yang, J.M.; Song, J. Autophagy in T-cell differentiation, survival and memory. Immunol. Cell Biol. 2021, 99, 351–360.
- Peng, H.-Y.; Lucavs, J.; Ballard, D.; Das, J.K.; Kumar, A.; Wang, L.; Ren, Y.; Xiong, X.; Song, J. Metabolic reprogramming and reactive oxygen species in T cell immunity. Front. Immunol. 2021, 12, 652687.
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071.
- Wong, C.K.; Lam, C.W.; Wu, A.K.; Ip, W.K.; Lee, N.L.; Chan, I.H.; Lit, L.C.; Hui, D.S.; Chan, M.H.; Chung, S.S.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Pavel, A.B.; Glickman, J.W.; Michels, J.R.; Kim-Schulze, S.; Miller, R.L.; Guttman-Yassky, E. Th2/Th1 Cytokine Imbalance Is Associated With Higher COVID-19 Risk Mortality. Front Genet. 2021, 12, 706902.
- Kasahara, T.M.; Bento, C.A.M.; Gupta, S. Phenotypic and Functional Analysis of T Follicular Cells in Common Variable Immunodeficiency. Int. Arch. Allergy Immunol. 2020, 181, 635–647.
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Oldridge, D.A.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals patient heterogeneity and distinct immunotypes with implications for therapeutic interventions. bioRxiv 2020.
- Seepathomnarong, P.; Ongarj, J.; Sophonmanee, R.; Seeyankem, B.; Chusri, S.; Surasombatpattana, S.; Pinpathomrat, N. Regulatory T Cells Decreased during Recovery from Mild COVID-19. Viruses 2022, 14, 1688.
- Galvan-Pena, S.; Leon, J.; Chowdhary, K.; Michelson, D.A.; Vijaykumar, B.; Yang, L.; Magnuson, A.M.; Chen, F.; Manickas-Hill, Z.; Piechocka-Trocha, A.; et al. Profound Treg perturbations correlate with COVID-19 severity. Proc. Natl. Acad. Sci. USA 2021, 118, e2111315118.
- Hong, X.; Kawasawa, Y.I.; Menne, S.; Hu, J. Host cell-dependent late entry step as determinant of hepatitis B virus infection. PLoS Pathog 2022, 18, e1010633.
- Mantovani, A.; Beatrice, G.; Dalbeni, A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020, 40, 1316–1320.
- Chen, X.; Jiang, Q.; Ma, Z.; Ling, J.; Hu, W.; Cao, Q.; Mo, P.; Yao, L.; Yang, R.; Gao, S.; et al. Clinical Characteristics of Hospitalized Patients with SARS-CoV-2 and Hepatitis B Virus Co-infection. Virol. Sin. 2020, 35, 842–845.
- Wu, J.; Yu, J.; Shi, X.; Li, W.; Song, S.; Zhao, L.; Zhao, X.; Liu, J.; Wang, D.; Liu, C.; et al. Epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: A multicentre descriptive study. J. Viral. Hepat. 2021, 28, 80–88.
- Lin, Y.; Yuan, J.; Long, Q.; Hu, J.; Deng, H.; Zhao, Z.; Chen, J.; Lu, M.; Huang, A. Patients with SARS-CoV-2 and HBV co-infection are at risk of greater liver injury. Genes Dis. 2021, 8, 484–492.
- Zou, X.; Fang, M.; Li, S.; Wu, L.; Gao, B.; Gao, H.; Ran, X.; Bian, Y.; Li, R.; Yu, S.; et al. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin. Gastroenterol. Hepatol. 2021, 19, 597–603.
- Yu, R.; Tan, S.; Dan, Y.; Lu, Y.; Zhang, J.; Tan, Z.; He, X.; Xiang, X.; Zhou, Y.; Guo, Y.; et al. Effect of SARS-CoV-2 coinfection was not apparent on the dynamics of chronic hepatitis B infection. Virology 2021, 553, 131–134.
- Liu, R.; Zhao, L.; Cheng, X.; Han, H.; Li, C.; Li, D.; Liu, A.; Gao, G.; Zhou, F.; Liu, F.; et al. Clinical characteristics of COVID-19 patients with hepatitis B virus infection-a retrospective study. Liver Int. 2021, 41, 720–730.
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256.
- Boni, C.; Fisicaro, P.; Valdatta, C.; Amadei, B.; Di Vincenzo, P.; Giuberti, T.; Laccabue, D.; Zerbini, A.; Cavalli, A.; Missale, G.; et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007, 81, 4215–4225.
- Rehermann, B. Pathogenesis of chronic viral hepatitis: Differential roles of T cells and NK cells. Nat. Med. 2013, 19, 859–868.
- Xiang, T.D.; Zheng, X. Interaction between hepatitis B virus and SARS-CoV-2 infections. World J. Gastroenterol. 2021, 27, 782–793.
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Prim. 2016, 2, 16076.
- Martinez, L.; Cords, O.; Liu, Q.; Acuna-Villaorduna, C.; Bonnet, M.; Fox, G.J.; Carvalho, A.C.C.; Chan, P.C.; Croda, J.; Hill, P.C.; et al. Infant BCG vaccination and risk of pulmonary and extrapulmonary tuberculosis throughout the life course: A systematic review and individual participant data meta-analysis. Lancet Glob. Health 2022, 10, e1307–e1316.
- Urdahl, K.B.; Shafiani, S.; Ernst, J.D. Initiation and regulation of T-cell responses in tuberculosis. Mucosal. Immunol. 2011, 4, 288–293.
- Lu, Y.J.; Barreira-Silva, P.; Boyce, S.; Powers, J.; Cavallo, K.; Behar, S.M. CD4 T cell help prevents CD8 T cell exhaustion and promotes control of Mycobacterium tuberculosis infection. Cell Rep. 2021, 36, 109696.
- Riou, C.; du Bruyn, E.; Stek, C.; Daroowala, R.; Goliath, R.T.; Abrahams, F.; Said-Hartley, Q.; Allwood, B.W.; Hsiao, N.Y.; Wilkinson, K.A.; et al. Relationship of SARS-CoV-2-specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J. Clin. Investig. 2021, 131, e149125.
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Gualano, G.; Vittozzi, P.; Nicastri, E.; Maffongelli, G.; Grifoni, A.; Sette, A.; et al. Coinfection of tuberculosis and COVID-19 limits the ability to in vitro respond to SARS-CoV-2. Int. J. Infect Dis. 2021, 113 (Suppl. 1), S82–S87.
- Velavan, T.P.; Ojurongbe, O. Regulatory T cells and parasites. J. Biomed Biotechnol. 2011, 2011, 520940.
- Wolday, D.; Gebrecherkos, T.; Arefaine, Z.G.; Kiros, Y.K.; Gebreegzabher, A.; Tasew, G.; Abdulkader, M.; Abraha, H.E.; Desta, A.A.; Hailu, A.; et al. Effect of co-infection with intestinal parasites on COVID-19 severity: A prospective observational cohort study. EClinicalMedicine 2021, 39, 101054.
- Wolday, D.; Tasew, G.; Amogne, W.; Urban, B.; Schallig, H.D.; Harris, V.; de Wit, T.F.R. Interrogating the Impact of Intestinal Parasite-Microbiome on Pathogenesis of COVID-19 in Sub-Saharan Africa. Front Microbiol. 2021, 12, 614522.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
524
Revisions:
2 times
(View History)
Update Date:
22 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




