Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Endrit Shahini | -- | 2401 | 2023-02-21 11:17:51 | | | |
| 2 | Rita Xu | Meta information modification | 2401 | 2023-02-22 03:34:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pallio, S.; Crinò, S.F.; Maida, M.; Sinagra, E.; Tripodi, V.F.; Facciorusso, A.; Ofosu, A.; Conti Bellocchi, M.C.; Shahini, E.; Melita, G. Endoscopic Ultrasound for Gastrointestinal Stromal Tumours. Encyclopedia. Available online: https://encyclopedia.pub/entry/41471 (accessed on 07 February 2026).
Pallio S, Crinò SF, Maida M, Sinagra E, Tripodi VF, Facciorusso A, et al. Endoscopic Ultrasound for Gastrointestinal Stromal Tumours. Encyclopedia. Available at: https://encyclopedia.pub/entry/41471. Accessed February 07, 2026.
Pallio, Socrate, Stefano Francesco Crinò, Marcello Maida, Emanuele Sinagra, Vincenzo Francesco Tripodi, Antonio Facciorusso, Andrew Ofosu, Maria Cristina Conti Bellocchi, Endrit Shahini, Giuseppinella Melita. "Endoscopic Ultrasound for Gastrointestinal Stromal Tumours" Encyclopedia, https://encyclopedia.pub/entry/41471 (accessed February 07, 2026).
Pallio, S., Crinò, S.F., Maida, M., Sinagra, E., Tripodi, V.F., Facciorusso, A., Ofosu, A., Conti Bellocchi, M.C., Shahini, E., & Melita, G. (2023, February 21). Endoscopic Ultrasound for Gastrointestinal Stromal Tumours. In Encyclopedia. https://encyclopedia.pub/entry/41471
Pallio, Socrate, et al. "Endoscopic Ultrasound for Gastrointestinal Stromal Tumours." Encyclopedia. Web. 21 February, 2023.
Copy Citation
Gastrointestinal Stromal Tumors (GISTs) are subepithelial lesions (SELs) that commonly develop in the gastrointestinal tract. GISTs, unlike other SELs, can exhibit malignant behavior, so differential diagnosis is critical to the decision-making process. Endoscopic ultrasound (EUS) is considered the most accurate imaging method for diagnosing and differentiating SELs in the gastrointestinal tract by assessing the lesions precisely and evaluating their malignant risk.
Gastrointestinal Stromal Tumors
endoscopic ultrasound
subepithelial lesions
1. Introduction
Gastrointestinal stromal tumours (GISTs) are the most common type of mesenchymal neoplasia that arises from the digestive tract [1]. Their histogenesis has been attributed to Cajal interstitial cells, which are thought to be the pacemaker cells of the gastrointestinal tract and are immunohistochemically positive for CD117 [2][3][4][5][6].
GISTs are more common in middle-aged (6th decade) males, with a prevalence of 14–20 cases per million, and are typically located in the gastric body (55.6%) or small intestine (31.8%) [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15]. In 6.0% and less than 1% of cases, the colorectum and oesophagus are involved, respectively [7][8][9][10][11][12][13][14].
GIST-related complications are characterized by gastrointestinal bleeding (including acute melena and hematemesis, as well as chronic bleeding with subsequent anaemia) caused by mass ulceration, abdominal pain, weakness, and organ compression symptoms [3][4][5][12]. However, up to 30% of GISTs are incidentally discovered in asymptomatic patients or during routine examinations. They are typically uncovered as small subepithelial lesions (SELs) that are not ulcerated, are slightly elevated, and are covered by normal mucosa. Their subepithelial origin and commonly small size hamper their differentiation from other SELs, which have slow growth and an indolent course [10][15][16][17][18][19].
The diagnosis of a GIST relies on typical cell morphology (spindle cells) and immunohistochemistry, with strong reactivity for receptor tyrosine kinase KIT or CD34. Additional tests include DOG1 staining or mutation search of the KIT or PDGFRA genes [16].
GISTs have a known malignant potential, ranging between 10% and 30% [7][8][9][10][11]. The assessment of malignant potential allows for patient stratification according to very low, low, intermediate, or high-risk cases, which is necessary for the selection of treatment strategies [11]. Although the prognosis for patients with GISTs is mainly associated with the tumour size (>2 cm) and mitotic index (< or >5/50 HPF) [12][13], small GISTs with a low mitotic index can also have a malignant course with metastasis. Other prognostic factors include the primary tumour location, tumour rupture, and metastasis.
When lesions are larger than 20 or 30 mm in diameter, surgical resection is the mainstay of treatment of localized GISTs [17]. Smaller tumours can be safely considered for endoscopic resection, with or without a laparoscopic control. However, despite complete resection, postoperative recurrence can occur in at least half of patients. Therefore, an early diagnosis is desirable [18][19][20][21][22].
Endoscopic Ultrasonography (EUS) is a crucial diagnostic technique for determining the potential malignancy of SELs, even though it is difficult to distinguish GISTs from other SELs using only EUS images [23][24]. The use of contrast agents or elastography in conjunction with EUS improves the latter’s diagnostic ability [25][26][27]. Furthermore, advances in artificial intelligence (AI) appear to have the potential to improve the accuracy of EUS for GIST diagnosis. However, although frequently controversial due to its associated technical difficulties and moderate diagnostic sensitivity, endoscopic biopsies or EUS-guided tissue acquisition (EUS-TA, including fine-needle aspiration (EUS-FNA) and fine-needle biopsy (EUS-FNB)) [28][29][30] continue to constitute the gold standard for making a definitive diagnosis.
2. Endoscopic and EUS-Based Findings
The majority of SELs are asymptomatic and detected incidentally during endoscopy performed for unrelated causes. In general, their endoscopic appearance is typically characterized by a rounded protuberance with normal overlying mucosa, negative cushion signs, and, occasionally, a central depression or umbilication [31]. When GISTs increase in size, ulceration may become apparent. Spontaneous bleeding or fibrin clotting is associated with an increased risk of malignant transformation.
Even when magnifying endoscopy or chromoendoscopy are used, SELs are extremely difficult to distinguish using solely conventional endoscopy. In general, attempts to differentiate GISTs from other SELs based on endoscopic findings have been inadequate with respect to small lesions.
According to the European Society of Gastrointestinal Endoscopy (ESGE), EUS’s ability to define the morphology and features of the suspicion of malignancy render this technique the best diagnostic tool with which to characterize these lesions. EUS images show the location, size, originating layer (the fourth layer, which corresponds to the muscolaris propria), shape, internal echo pattern, heterogeneity, and vascularity of the lesion, as well as the presence of lymph nodes adjacent to or surrounding the tumour [31][32]. Several EUS features, including irregular borders, cystic spaces, ulceration, and echogenic foci, have been linked to a higher risk of malignancy (Figure 1).
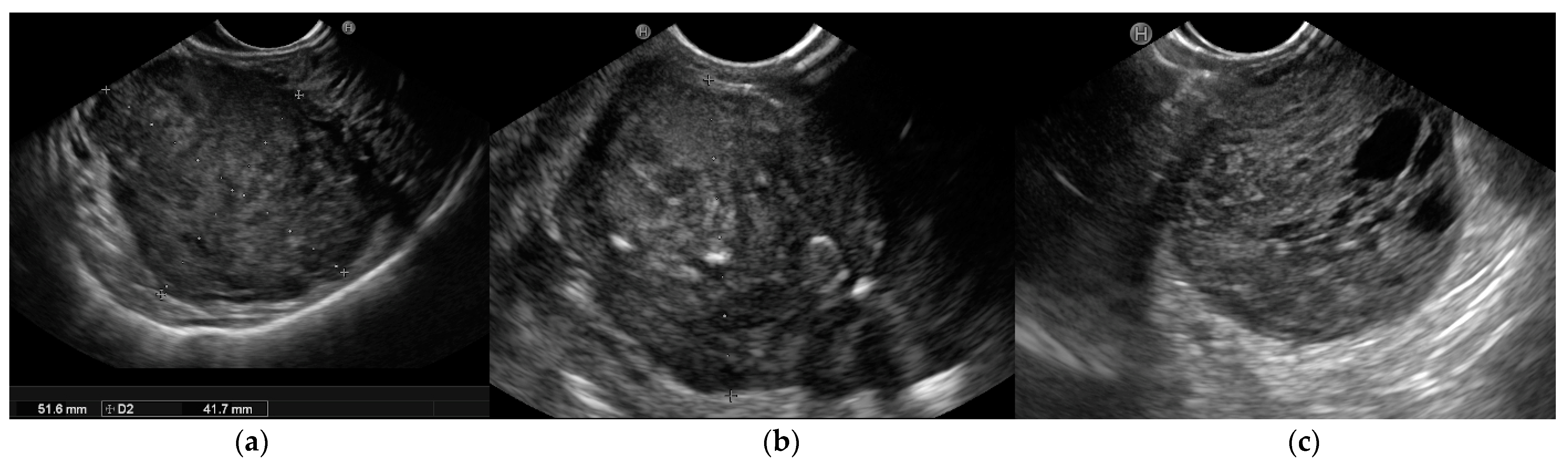
Figure 1. Endoscopic ultrasound (EUS) images of malignant gastrointestinal stromal tumors (GISTs): (a) A large submucosal lesion originating from the fourth layer of the gastric wall. Echopattern is inhomogeneous with irregular borders. (b) Another large subepithelial gastric mass with echoic foci, calcifications, and irregular profiles. (c) Cystic spaces are visible in EUS images.
Furthermore, EUS-guided techniques such as contrast enhancement, elastography, and tissue acquisition have been investigated in terms of their ability to predict diagnosis and malignant behaviour. The key issue is distinguishing GISTs from other SELs. It is especially important to guide efficient clinical therapy regarding leiomyomas because GISTs are potentially malignant, whereas leiomyomas are benign [33]. Duodenal GISTs and NETs may look similar in imaging studies, and GISTs arising from the second or third portion of the duodenum may be misdiagnosed as pancreatic NETs based solely on imaging criteria. In addition, the resection techniques differ between these two tumours. Surgical excision with regional lymph node dissection is the best treatment for pancreatic NETs. GISTs, on the other hand, are frequently treated with minimal resection and without lymph node dissection. Hence, the role of histological diagnosis is critical in determining their appropriate treatment and outcomes [31][32][33][34][35].
As SELs are located in the inner layer, with overlying normal mucosa and submucosa, the diagnostic yield of conventional endoscopic forceps-based biopsy is limited, ranging from 17% to 59%, despite the use of special devices such as the “jumbo” forceps or dedicated techniques such as the “bite-on-bite” biopsies. To address this limitation, the mucosal incision-assisted biopsy (MIAB) was developed. This technique entails lifting the mucosa that covers the SEL to make a more secure incision. The exposed lesion is sampled with biopsy forceps after an electrosurgical incision of the target mucosal and submucosal tissues with an endoscopic submucosal dissection knife [36][37][38][39][40][41].
As previously stated, EUS-based tissue acquisition, either FNA or FNB, is a viable alternative with a diagnostic rate ranging between 71% and 100%, which is strongly influenced by tumour size. The ability to perform a mitotic count, the risk of seeding, and the feasibility of the technique in specific sites were all observed to be critical issues.
Although EUS-FNB and MIAB are both recommended by ESGE guidelines, the procedure time, the size and location of the SELs, and expertise influence the choice of procedure. Tissue diagnosis is recommended for all SELs with GIST-like characteristics that are larger than 20 mm, have high-risk stigmata, or require surgical resection or oncological treatment.
3. Contrast-Enhanced Harmonic EUS
The use of contrast agents has improved the diagnostic performance of EUS, particularly with respect to differentiating GISTs from other gastrointestinal SELs [42].
Conventional EUS B-mode analysis is performed to assess the size and shape of SELs, their origin wall layer, and ultrasonographic characteristics (tissue echogenicity, calcifications, vascularization, or the presence of avascular areas using Power Doppler or hi-flow). Contrast-enhanced harmonic EUS (CH-EUS) can then visualize the microvascularization of SELs, enhancing their characterization, with hyperenhancement specific to GIST and hypo-enhancement specific to benign SELs.
When exposed to an ultrasonic wave, the contrast agents oscillate or break [42]. SonoVue (Bracco SpA, Milan, Italy) and Sonozaid (Daiichin-Sankyo, Tokyo, Japan) are contrast media that contain safe microbubbles covered by a protective lipophilic shell that carries carbon dioxide gas. In response to acoustic stimuli, these bubbles oscillate, thereby increasing the echo levels in the target tissue. During CH-EUS, the optimal amount of contrast medium is injected intravenously while the ultrasound machine is in contrast-harmonic mode. When performing CH-EUS with the SonoVue® contrast agent, a 4.8 mL bolus of SonoVue® is injected through a peripheral intravenous cannula, followed by a 10 mL saline flush [43]. Each patient’s contrast study usually lasts 90 s after the intravenous bolus injection and is documented by a video clip that includes B-mode examination and the arterial, portal, and late phases.
Contrast enhancement is typically evaluated in the early (after a few seconds) and late phases (after more than 30 s), and the enhancement patterns are then classified (as hyper-, iso-, or hypo-enhancement, and as homogeneous or inhomogeneous) along with the features (the presence or absence of regular or irregular intratumoral vessels, and the presence or absence of an unenhanced area) that can be observed after the injection of the contrast medium [44].
Pancreatic diseases are the primary application field for CH-EUS [45]. The pooled sensitivity and specificity of CE-EUS with respect to distinguishing pancreatic cancers from solid inflammatory masses were reported as 93% and 88%, respectively, in a 2017 meta-analysis [45]. Moreover, CH-EUS is also recommended for investigating pancreatic cysts, gallbladder and biliary tract lesions, lymph nodes, and SELs [46][47][48].
Several studies [31][44][49][50][51][52][53][54][55] have found CH-EUS to be useful for the characterization of GISTs. The pooled sensitivity and specificity were 89% (95%CI 82–93%) and 82% (95%CI 66–92%), respectively, in a meta-analysis [44] published in 2019 that included seven studies [49][50][51][52][53][54][55] with a total of 187 patients and assessed the value of CH-EUS towards distinguishing between GISTs and other benign SELs. One limitation of this meta-analysis was the inclusion of only two prospective studies [49][50]. The first [50], an international multicentre study, compared GISTs with leiomyoma using the CH-EUS-based characterization of 62 SELs in different locations in the upper gastrointestinal tract. Despite the small number of benign SELs discovered (5 leiomyomas vs. 57 GISTs), CH-EUS revealed hyperenhancement and avascular areas in a high percentage of GISTs but not in leiomyomas. However, the lesion size was not uniform (mean size 62.6 ± 42.1 with a range from 16 to 200), and there was a trend toward a smaller size for GISTs without avascular areas (65.8 ± 43 (16–200) vs. 39.6 ± 26.9 (22–90) p = 0.062). Moreover, there was no attempt to stratify malignant potential. In the second study, Sakamoto et al. used microvasculature evaluation with intratumoral vessel quantification (regular pattern, irregular pattern, or absence of vessels) to characterize 29 GISTs, and compared the results to histological or surgical specimen diagnosis and malignancy assessment. Similarly, many studies reported sensitivity and specificity ranging from 75% to 100% and 63% to 100%, respectively [44][49][52]. Sakamoto et al. demonstrated that an irregular intratumoral vessel pattern was an 83% accurate predictor of high-grade malignant GISTs [49] (Figure 2).
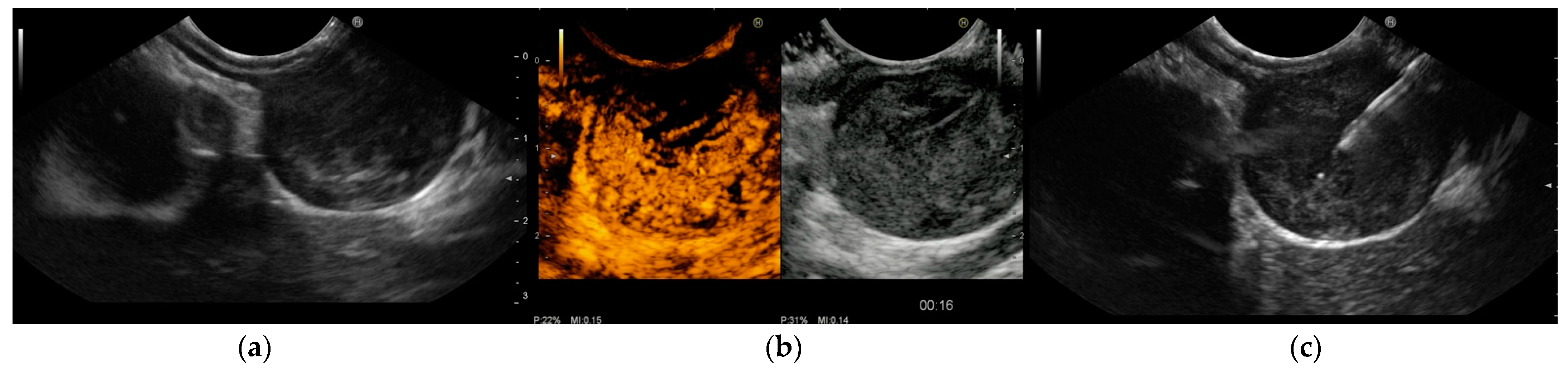
Figure 2. Endoscopic ultrasound (EUS) images of gastrointestinal stromal tumor (GIST) of the stomach: (a) The originating layer is visible when the ultrasound transducer is placed at the peripheral portion of the lesion. (b) Contrast-enhanced harmonic EUS (CH-EUS) demonstrated a hypervascular pattern. Moreover, CH-EUS allowed for the identification of irregular large vessels and avascular areas inside the tumor. (c) EUS-guided fine-needle biopsy was performed using a 22-gauge end-cutting needle while trying to avoid avascular areas previously defined using CH-EUS. Histology confirmed a GIST with a high replicative index.
According to Tamura T and Kitano M’s 2019 review, CH-EUS can distinguish between GISTs and other gastrointestinal SELs with sensitivity and specificity ranging from 78–100% and 60–100%, respectively [43].
Even though CH-EUS improves the accuracy of EUS towards SEL characterization, it cannot replace tissue acquisition for differentiating GISTs from other spindle cell neoplasms (leiomyomas), which share a “regular” vessel pattern. As a result, histology must be used to assess the malignant potential of GISTs.
4. EUS-Elastography
EUS-E is a real-time imaging technique that analyses tissue elasticity and displays this information graphically as a colour spectrum of shades [56]. While green represents average stiffness, blue represents harder tissue and red represents softer tissue. Each colour is associated with a specific value of tissue elasticity in a defined region of interest, ranging from 1 to 255 kPa [56] (Figure 3).
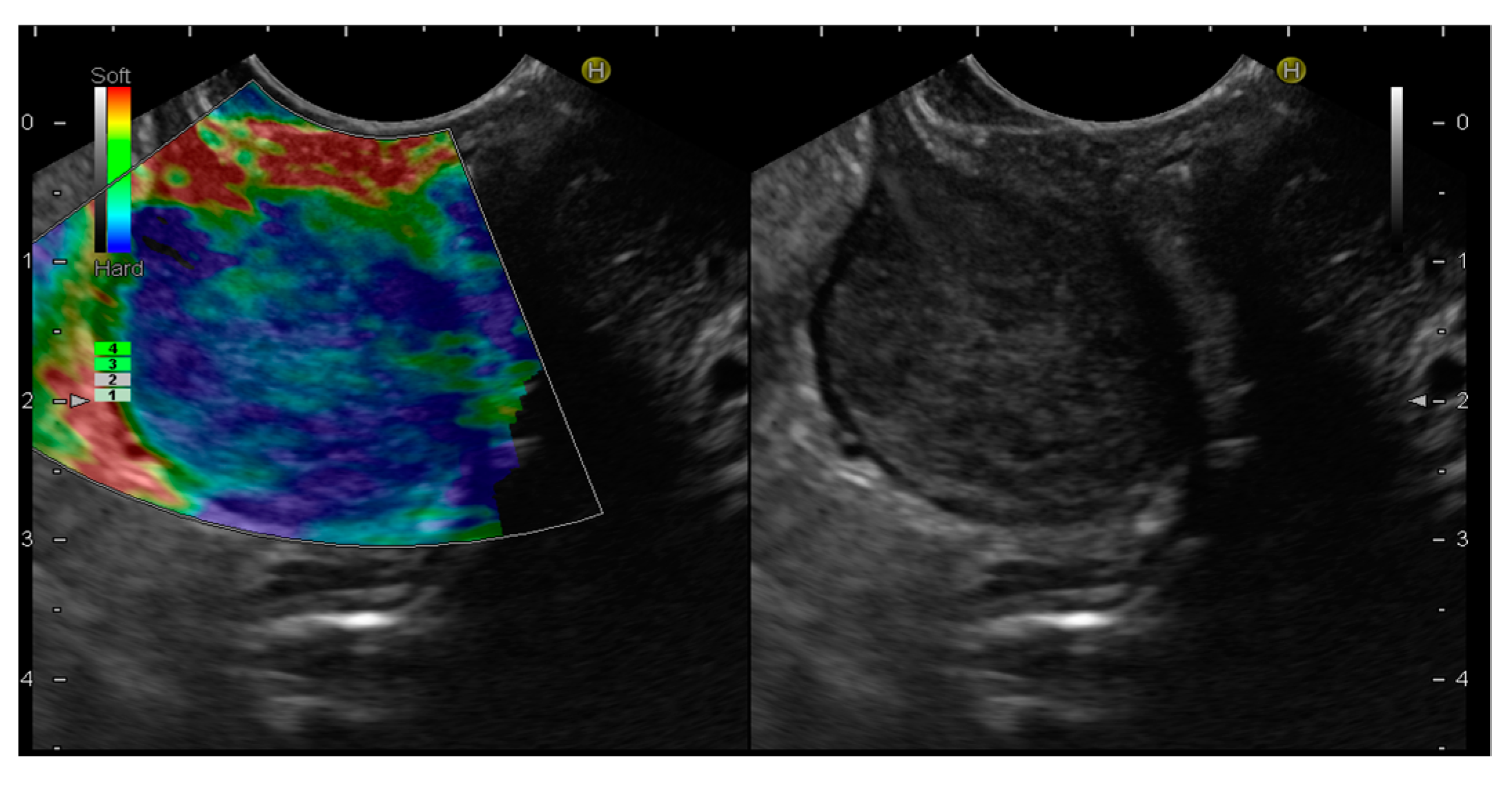
Figure 3. Endoscopic ultrasound elastography (EUS-E) images of gastrointestinal stromal tumor (GIST) of the stomach. The lesion shows a blue color, indicative of a hard tissue, compared to the red color of the gastric wall.
In addition, EUS-E compares the strain between the target and other reference areas, delivering a semi-quantitative analysis of tissue stiffness [57]. In more detail, strain ratio (SR) is a value derived from the ratio of the stiffness of two user-defined areas within an elastogram that provides an objective estimation of the lesion’s hardness [58].
Due to its lack of invasiveness, EUS-E was initially used for the differential diagnosis of SELs by providing a qualitative/semi-quantitative stiffness analysis [59][60][61][62][63][64][65][66][67][68]. EUS-E is an imaging technique that detects diseased and normal tissue elasticity changes on conventional B-mode ultrasound images [68]. The fundamental principle of EUS-E imaging is that tissues have varying elasticity, thus causing different strains when compressed by an external force or when compressed by normal breathing and blood circulation. An ultrasonic system’s software program can then characterize and visualize these strain values in real-time [69]. The elasticity values are then visually characterized in different colours on the elastography images based on tissue deformation [50][62]. Elastography is commonly used in clinical practice to diagnose diseases of the liver, thyroid, kidney, lymph nodes, prostate, mammary glands, and pancreas [34][67][70].
However, to date, only a few studies have examined the role of EUS-E in the diagnosis of SELs in the gastrointestinal tract, and the results are still debatable. The first pilot study on the efficacy of EUS-E for differentiating 25 consecutive gastric SELs demonstrated that GISTs were qualitatively harder than other SELs by rating the degree of stiffness based on the majority and colour distribution [60]. As proven by a prior study, GISTs tend to have a blue colour (61/62, 98%), which was confirmed by a subsequent study. This tendency, however, resembles that of leiomyoma (4/5, 80%) [50]. The feasibility of quantitative EUS-E based on SR with respect to the differential diagnosis of SELs has been investigated since its introduction. A preliminary retrospective study of 30 patients found that EUS-E with SR may be promising in terms of differentiating GI SELs, wherein a cut-off of 11.18 can distinguish between GISTs and leiomyomas with a sensitivity of 81.8% and a specificity of 85.7% [71]. In a prospective study by Kim et al., the SR of 41 gastric SELs was compared with the histopathologic diagnosis. GISTs presented an elevated SR (mean 51.1 ± 11.1) that enabled them to be distinguished from leiomyoma, whose SR was considerably lower (6.0 ± 6.9), with a favourable sensitivity of 100% and a specificity of 94.1% when using an SR cut-off of 22.7 [61]. The distinction of GISTs from schwannoma, a mesenchymal tumour with a similar appearance to a GIST, appears to be challenging, given that the mean SR of the only schwannoma in the series was 62.0 [32]. In a recent study, Guo et al. utilized hue histograms to quantify EUS-E images but did not find adequate evidence to support the utility of EUS-E for differentiating between GISTs and gastrointestinal leiomyomas using EUS-E [64].
References
- Joensuu, H.; Hohenberger, P.; Corless, C.L. Gastrointestinal stromal tumour. Lancet 2013, 382, 973–983.
- Sircar, K.; Hewlett, B.R.; Huizinga, J.D.; Chorneyko, K.; Berezin, I.; Riddel, R.H. Interstizial cells of Cajal as precursor of gastrointestinal stromal tumors. Am. J. Surg. Pathol. 1999, 23, 377–389.
- van Roggen, J.F.G.; van Velthunysen, M.L.; Hogendoorn, P.C. The Histopathological differential diagnosis of gastrointestinal stromal tumors. J. Clin. Pathol. 2001, 54, 96–102.
- Wong, D.W.; Lupton, S.C.; Bhatt, L.; Gross, L.; Tanière, P.; Peake, D.R.; Spooner, D.; Geh, J.I. Use in imatinib mesylate in gastrointestinal stromal tumors: Pan-Birmingham Cancer Network experience. Clin. Oncol. 2008, 20, 517–522.
- Maleshwari, V.; Alam, K.; Varshney, M.; Jain, A.; Siddiqui, F.A.; Bhargava, S. Fine needle aspiration diagnosis of Gist: A diagnostic dilemma. Diagn. Cytopathol. 2012, 40, 834–838.
- Todaro, P.; Crinò, S.F.; Pallio, S.; Fazzari, C.; Consolo, P.; Tuccari, G. Gastrointestinal stromal tumours of the stomach: Cytological and immunocytochemical diagnostic features of two cases diagnosed by endoscopic ultrasound-guided fine needle aspiration. Oncol. Lett. 2013, 5, 1862–1866.
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143.
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46.
- Miettinen, M.; Lasota, J. Gastrointestinal stromal tumour.Review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch. Pathol. Lab. Med. 2006, 130, 1466–1478.
- Miettinen, M.; Sobin, L.H.; Lasota, J. Gastrointestinal stromal tumours of the stomach: A clinicopathologic, immunoihistochemical, and molecular genetic study of 1765 cases with long-term follow up. Am. J. Surg. Pathol. 2005, 29, 52–68.
- Joensuu, H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum. Pathol. 2008, 39, 1411–1419.
- Tran, T.; Davila, J.A.; El-Serag, H.B. The epidemiology of malignant gastrointestinal stromal tumors: An anlysis of 1458 cases from 1992 to 2000. Am. J. Gastroenterol. 2005, 100, 162–168.
- Parab, T.M.; DeRogatis, M.J.; Boaz, A.M.; Grasso, S.A.; Issack, P.S.; Duarte, D.A.; Urayeneza, O.; Vahdat, S.; Qiao, J.H.; Hinika, G.S. Gastrointestinal stromal tumors: A comprehensive review. J. Gastrointest. Oncol. 2019, 10, 144–154.
- Peng, C.Y.; Xu, G.F. Evaluation of preoperative endoscopic ultrasonography for diagnosis and invasive risk assessment of gastric gastrointestinal stromal tumors: A single center retrospective study. Chin. J. Digest. Endosc. 2015, 32, 361–366.
- Nilsson, B.; Bumming, P.; Meis-Kindblom, J.M.; Odén, A.; Dortok, A.; Gustavsson, B.; Sablinska, K.; Kindblom, L.G. Gastrointestinal Stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era-a population-based study in western Sweden. Cancer 2005, 103, 821–829.
- West, R.B.; Corless, C.L.; Chen, X.; Rubin, B.P.; Subramanian, S.; Montgomery, K.; Zhu, S.; Ball, C.A.; Nielsen, T.O.; Patel, R.; et al. The novel marker, DOGI, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of Kit or PDGFRA mutation status. Am. J. Pathol. 2004, 165, 107–113.
- Nishida, T.; Sakai, Y.; Takagi, M.; Ozaka, M.; Kitagawa, Y.; Kurokawa, Y.; Masuzawa, T.; Naito, Y.; Kagimura, T.; Hirota, S.; et al. Adherence to the guidelines and the pathological diagnosis of high-risk gastrointestinal stromal tumors in the real world. Gastric. Cancer 2020, 23, 118–125.
- Sepe, P.S.; Brugge, W.R. A guide for the diagnosis and management of gastrointestinal stromal tumors. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 363–371.
- Akahoshi, K.; Oya, M. Gastrointestinal Stromal tumor of the stomach: How to manage? World. J. Gastrointest. Endosc. 2010, 2, 271–277.
- Rubin, B.P.; Heinrich, M.C.; Corless, C.L. Gastrointestinal Stromal Tumour. Lancet 2007, 369, 1731–1741.
- Plaat, B.E.; Hollema, H.; Molenar, W.M.; Torn Broers, G.H.; Pijpe, J.; Mastik, M.F.; Hoekstra, H.J.; van den Berg, E.; Scheper, R.J.; van der Graaf, W.T. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: Differences in clinical outcome and expression of multidrug resistance proteins. J. Clin. Oncol. 2000, 18, 3220–3222.
- Facciorusso, A.; Di Maso, M.; Serviddio, G.; Vendemiale, G.; Spada, C.; Costamagna, G.; Muscatiello, N. Factors Associated with Recurrence of Advanced Colorectal Adenoma after Endoscopic Resection. Clin. Gastroenterol. Hepatol. 2016, 14, 1148–1154.
- Standards of Practice Committee; Faulx, A.L.; Kothari, S.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Gurudu, S.R.; et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest. Endosc. 2017, 85, 1117–1132.
- Yoshinaga, S.; Hilmi, I.N.; Kwek, B.E.; Hara, K.; Goda, K. Current status of endoscopic ultrasound for the upper gastrointestinal tract in Asia. Dig. Endosc. 2015, 27, 2–10.
- Lisotti, A.; Napoleon, B.; Facciorusso, A.; Cominardi, A.; Crinò, S.F.; Brighi, N.; Gincul, R.; Kitano, M.; Yamashita, Y.; Marchegiani, G.; et al. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: Systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 881–889.
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 821–828.
- Crinó, S.F.; Brandolese, A.; Vieceli, F.; Paiella, S.; Bellocchi, M.C.C.; Manfrin, E.; Bernardoni, L.; Sina, S.; D‘Onofrio, M.; Marchegiani, G.; et al. Endoscopic Ultrasound Features Associated with Malignancy and Aggressiveness of Nonhypovascular Solid Pancreatic Lesions: Results from a Prospective Observational Study. Ultraschall. Med. 2021, 42, 167–177.
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Ofosu, A.; Muscatiello, N.; Mangiavillano, B.; Lamonaca, L.; Lisotti, A.; Fusaroli, P.; Gkolfakis, P.; et al. Comparison between endoscopic ultrasound-guided fine-needle biopsy and bite-on-bite jumbo biopsy for sampling of subepithelial lesions. Dig. Liver Dis. 2022, 54, 676–683.
- Gkolfakis, P.; Crinò, S.F.; Tziatzios, G.; Ramai, D.; Papaefthymiou, A.; Papanikolaou, I.S.; Triantafyllou, K.; Arvanitakis, M.; Lisotti, A.; Fusaroli, P.; et al. Comparative diagnostic performance of end-cutting fine-needle biopsy needles for EUS tissue sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2022, 95, 1067–1077.
- Crinò, S.F.; Bernardoni, L.; Manfrin, E.; Parisi, A.; Gabbrielli, A. Endoscopic ultrasound features of pancreatic schwannoma. Endosc. Ultrasound. 2016, 5, 396–398.
- Deprez, P.; Moons, L.; O’Toole, D.; Gincul, R.; Seicean, A.; Pimentel-Nunes, P.; Fernández-Esparrach, G.; Polkowski, M.; Vieth, M.; Borbath, I.; et al. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2022, 54, 412–429.
- Goto, O.; Kaise, M.; Iwakiri, K. Advancements in the Diagnosis of Gastric subepithelial Tumors. Gut Liver 2022, 16, 321–330.
- Moon, K.S.; Young, K.E.; Woong, C.J. Predictive factors for differentiating gastrointestinal stromal tumors from leiomyomas on endoscopic ultrasonography finding in patients with Gastric subepitelial tumors: A Multicenter Retrospective Study. Clin. Endosc. 2021, 54, 872–880.
- Melita, G.; Pallio, S.; Tortora, A.; Crinò, S.F.; Macrì, A.; Dionigi, G. Diagnostic and Interventional Role of Endoscopic Ultrasonography for the Management of Pancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 2638.
- Futo, Y.; Saito, S.; Miyato, H.; Sadatomo, A.; Kaneko, Y.; Kono, Y.; Matsubara, D.; Horie, H.; Lefor, A.K.; Sata, N. Duodenal gastrointestinal stromal tumors appear similar to pancreatic neuroendocrine tumors: A case report. Int. J. Surg. Case Rep. 2018, 53, 358–361.
- Buscaglia, J.M.; Nagula, S.; Jayaraman, V.; Robbins, D.H.; Vadada, D.; Gross, S.A.; DiMaio, C.J.; Pais, S.; Patel, K.; Sejpal, D.V.; et al. Diagnostic yield and safety of jumbo biopsy forceps in patients with subepithelial lesions of the upper and lower GI tract. Gastrointest. Endosc. 2012, 75, 1147–1152.
- Cantor, M.J.; Davila, R.E.; Faigel, D.O. Yield of tissue sampling for subepithelial lesions evaluated by EUS: A comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest. Endosc. 2006, 64, 29–34.
- Minoda, Y.; Chinen, T.; Osoegawa, T.; Itaba, S.; Haraguchi, K.; Akiho, H.; Aso, A.; Sumida, Y.; Komori, K.; Ogino, H.; et al. Superiority of mucosal incision-assisted biopsy over ultrasound-guided fine needle aspiration biopsy in diagnosing small gastric subepithelial lesions: A propensity score matching analysis. BMC Gastroenterol. 2020, 20, 19.
- Tacelli, M.; Bina, N.; Crinò, S.F.; Facciorusso, A.; Celsa, C.; Vanni, A.S.; Fantin, A.; Antonini, F.; Falconi, M.; Monica, F.; et al. Reliability of grading preoperative pancreatic neuroendocrine tumors on EUS specimens: A systematic review with meta-analysis of aggregate and individual data. Gastrointest. Endosc. 2022, 96, 898–908.
- Crinò, S.F.; Bellocchi, M.C.C.; Di Mitri, R.; Inzani, F.; Rimbaș, M.; Lisotti, A.; Manfredi, G.; Teoh, A.Y.B.; Mangiavillano, B.; Sendino, O.; et al. Wet-suction versus slow-pull technique for endoscopic ultrasound-guided fine-needle biopsy: A multicenter, randomized, crossover trial. Endoscopy 2022, 27.
- Mangiavillano, B.; Crinò, S.F.; Facciorusso, A.; Di Matteo, F.; Barbera, C.; Larghi, A.; Rizzatti, G.; Carrara, S.; Spadaccini, M.; Auriemma, F.; et al. Endoscopic ultrasound-guided fine-needle biopsy with or without macroscopic on-site evaluation: A randomized controlled noninferiority trial. Endoscopy 2022, 55, 129–137.
- Meng, F.S.; Zhang, Z.H.; Ji, F. New endoscopic ultrasound techniques for digestive tract diseases: A comprehensive review. World. J. Gastroenterol. 2015, 21, 4809–4816.
- Tamura, T.; Kitano, M. Contrast Enhanced Endoscopic Ultrasound Imaging for Gastroin-testinal Subepithelial Tumors. Clin. Endosc. 2019, 52, 306–313.
- Tang, J.Y.; Tao, K.G.; Zhang, L.Y.; Wu, K.M.; Shi, J.; Zeng, X.; Lin, Y. Value of contrast-enhanced harmonic endoscopic ultrasonography in differentiating between gastrointestinal stromal tumors: A meta-analysis. J. Dig. Dis. 2019, 20, 127–134.
- He, X.K.; Ding, Y.; Sun, L.M. Contrast-enhanced endoscopic ultrasound for differential di-agnosis of pancreatic cancer: An updated meta-analysis. Oncotarget 2017, 8, 66392–66401.
- Pesenti, C.; Bories, E.; Caillol, F.; Ratone, J.P.; Godat, S.; Monges, G.; Poizat, F.; Raoul, J.L.; Ries, P.; Giovannini, M. Characterization of subepithelial lesions of the stomach and esophagus by contrast-enhanced EUS: A retrospective study. Endosc. Ultrasound. 2019, 8, 43–49.
- Choi, J.H.; Seo, D.W. The Expanding Role of Contrast-Enhanced Endoscopic Ultrasound in Pancreatobiliary Disease. Gut Liver 2015, 9, 707–713.
- Lisotti, A.; Ricci, C.; Serrani, M.; Calvanese, C.; Sferrazza, S.; Brighi, N.; Casadei, R.; Fusaroli, P. Contrast-enhanced endoscopic ultrasound for the differential diagnosis between benign and malignant lymph nodes: A meta-analysis. Endosc. Int. Open 2019, 7, E504–E513.
- Sakamoto, H.; Kitano, M.; Matsui, S.; Kamata, K.; Komaki, T.; Imai, H.; Dote, K.; Kudo, M. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos). Gastrointest. Endosc. 2011, 73, 227–237.
- Ignee, A.; Jenssen, C.; Hocke, M.; Dong, Y.; Wang, W.P.; Cui, X.W.; Woenckhaus, M.; Iordache, S.; Saftoiu, A.; Schuessler, G.; et al. Contrast-enhanced (endoscopic) ultrasound and endoscopic ultrasound elastography in gastrointestinal stromal tumors. Endosc. Ultrasound. 2017, 6, 55–60.
- Kannengiesser, K.; Mahlke, R.; Petersen, F.; Peters, A.; Ross, M.; Kucharzik, T.; Maaser, C. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand. J. Gastroenterol. 2012, 47, 1515–1520.
- Yamashita, Y.; Kato, J.; Ueda, K.; Nakamura, Y.; Abe, H.; Tamura, T.; Itonaga, M.; Yoshida, T.; Maeda, H.; Moribata, K.; et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J. Clin. Ultrasound 2015, 43, 89–97.
- Bonavina, L.; Ariani, A.; Ficano, L.; Iannuzziello, D.; Pasquale, L.; Aragona, S.E.; Drago, L.; Ciprandi, G.; On Digestive Disorders ISG. Lactobacillus plantarum LP01, Lactobacillus lactis subspecies cremoris LLC02, and Lactobacillus delbrueckii LDD01 in patients undergoing bowel preparation. Acta Biomed. 2019, 90, 13–17.
- Kamata, K.; Takenaka, M.; Kitano, M.; Omoto, S.; Miyata, T.; Minaga, K.; Yamao, K.; Imai, H.; Sakurai, T.; Watanabe, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of submucosal tumors of the upper gastrointestinal tract. J. Gastroenterol. Hepatol. 2017, 32, 1686–1692.
- Park, H.Y.; Jeon, S.W.; Lee, H.S.; Cho, C.M.; Bae, H.I.; Seo, A.N.; Kweon, O.K. Can contrast-enhanced harmonic endosonography predict malignancy risk in gastrointestinal subepithelial tumors? Endosc. Ultrasound 2016, 5, 384–389.
- Chantarojanasiri, T.; Kongkam, P. Endoscopic ultrasound elastography for solid pancreatic lesions. World. J. Gastrointest. Endosc. 2017, 9, 506–513.
- Itokawa, F.; Itoi, T.; Sofuni, A.; Kurihara, T.; Tsuchiya, T.; Ishii, K.; Tsuji, S.; Ikeuchi, N.; Umeda, J.; Tanaka, R.; et al. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J. Gastroenterol. 2011, 46, 843–853.
- Nesje, L.B. Real-Time Elastography: Strain Ratio Measurements Are Influenced by the Position of the Reference Area. Echtzeit-Elastografie: Strainraten-Messungen sind ab-hängig von der Position des Referenzareals. Ultraschall. Med. 2012, 33, 559–568.
- Lefort, C.; Gupta, V.; Lisotti, A.; Palazzo, L.; Fusaroli, P.; Pujol, B.; Gincul, R.; Fumex, F.; Palazzo, M.; Napoléon, B. Diagnosis of gastric submucosal tumors and estimation of malignant risk of GIST by endoscopic ultrasound. Comparison between B mode and contrast-harmonic mode. Dig. Liver Dis. 2021, 53, 1486–1491.
- Tsuji, Y.; Kusano, C.; Gotoda, T.; Itokawa, F.; Fukuzawa, M.; Sofuni, A.; Matsubayashi, J.; Nagao, T.; Itoi, T.; Moriyasu, F. Diagnostic potential of endoscopic ultrasonography-elastography for gastric submucosal tumors: A pilot study. Dig. Endosc. 2016, 28, 173–178.
- Kim, S.H.; Yoo, I.K.; Kwon, C.I.; Hong, S.P.; Cho, J.Y. Utility of EUS elastography in the diagnosis of gastric subepithelial tumors: A pilot study (with video). Gastrointest. Endosc. 2020, 91, 172–177.
- Dietrich, C.F.; Săftoiu, A.; Jenssen, C. Real time elastography endoscopic ultrasound (rte-eus), a comprehensive review. Eur. J. Radiol. 2014, 83, 405–414.
- Kwon, S.J.; Jeong, M.K. Advances in ultrasound elasticity imaging. Biomed. Eng. Lett. 2017, 7, 71–79.
- Guo, J.; Bai, T.; Ding, Z.; Du, F.; Liu, S. Efficacy of Endoscopic Ultrasound Elastography in Differential Diagnosis of Gastrointestinal Stromal Tumor Versus Gastrointestinal Leiomyoma. Med. Sci. Monit. 2021, 27, 927619.
- Sigris, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applicatios. Theranostics 2017, 7, 1303–1329.
- Itoh, Y.; Itoh, A.; Kawashima, H.; Ohno, E.; Nakamura, Y.; Hiramatsu, T.; Sugimoto, H.; Sumi, H.; Hayashi, D.; Kuwahara, T.; et al. Quantitative analysis of diagnosing pancreatic fibrosis using eus-elastography (comparison with surgical specimens). J. Gastroenterol. 2014, 49, 1183–1192.
- Săftoiu, A.; Vilmann, P.; Ciurea, T.; Popescu, G.L.; Iordache, A.; Hassan, H.; Gorunescu, F.; Iordache, S. Dynamic analysis of eus used for the differentiation of benign and malignant lymph nodes. Gastrointest. Endosc. 2007, 66, 291–300.
- Schrader, H.; Wiese, M.; Ellrichmann, M.; Belyaev, O.; Uhl, W.; Tannapfel, A.; Schmidt, W.; Meier, J. Diagnostic value of quantitative eus elastography for malignant pancreatic tumors: Relationship with pancreatic fibrosis. Ultraschall. Med. 2012, 33, 196–201.
- Xue, Y.; Yao, S.; Li, X.; Zhang, H. Value of shear wave elastography in discriminating malignant and benign breast lesions. Medicine 2017, 96, 7412.
- Armellini, E.; Manfrin, E.; Trisolini, E.; Andorno, S.; Ballarè, M.; Bernardoni, L.; Boldorini, R.L.; Gabbrielli, A.; Frulloni, L.; Larghi, A.; et al. Histologic retrieval rate of a newly designed side-bevelled 20G needle for EUS-guided tissue acquisition of solid pancreatic lesions. United Eur. Gastroenterol. J. 2019, 7, 96–104.
- Antonini, F.; Fusaroli, P.; Frazzoni, L.; Belfiori, V.; Auriemma, F.; Rahal, D.; Serrani, M.; Lisotti, A.; Giorgini, S.; Fuccio, L.; et al. EUS elastography strain ratio in the differential diagnosis of gastrointestinal subepithelial lesions: Preliminary results of a multicenter study. Endoscopy 2018, 50, OP170.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
22 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




