Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tonni Agustiono Kurniawan | -- | 2095 | 2023-02-20 11:07:30 | | | |
| 2 | Rita Xu | Meta information modification | 2095 | 2023-02-21 02:35:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kurniawan, T.A.; Lo, W.; Liang, X.; Goh, H.H.; Othman, M.H.D.; Chong, K.; Mohyuddin, A.; Kern, A.O.; Chew, K.W. Water Treatment Using Functional Composites. Encyclopedia. Available online: https://encyclopedia.pub/entry/41423 (accessed on 08 February 2026).
Kurniawan TA, Lo W, Liang X, Goh HH, Othman MHD, Chong K, et al. Water Treatment Using Functional Composites. Encyclopedia. Available at: https://encyclopedia.pub/entry/41423. Accessed February 08, 2026.
Kurniawan, Tonni Agustiono, Wai-Hung Lo, Xue Liang, Hui Hwang Goh, Mohd Hafiz Dzarfan Othman, Kok-Keong Chong, Ayesha Mohyuddin, Axel Olaf Kern, Kit Wayne Chew. "Water Treatment Using Functional Composites" Encyclopedia, https://encyclopedia.pub/entry/41423 (accessed February 08, 2026).
Kurniawan, T.A., Lo, W., Liang, X., Goh, H.H., Othman, M.H.D., Chong, K., Mohyuddin, A., Kern, A.O., & Chew, K.W. (2023, February 20). Water Treatment Using Functional Composites. In Encyclopedia. https://encyclopedia.pub/entry/41423
Kurniawan, Tonni Agustiono, et al. "Water Treatment Using Functional Composites." Encyclopedia. Web. 20 February, 2023.
Copy Citation
Due to its low cost, biosorption technology has been extensively carried out to treat heavy metal-laden wastewater using biosorbents. The studies on heavy metal biosorption mechanisms and the simulation of mathematical modeling on the biosorption process have enhanced scientific understanding about the binding between target metal cations and the functional group on different surfaces of biomasses as a biosorbent.
adsorption
ion exchange
water pollution
1. Introduction
Environmental sustainability has become one of the key goals in the UN’s 2030 Agenda [1]. However, as for one of its Sustainable Development Goals (SDGs), a sustainable access to clean water is not achievable due to water pollution from industrial activities that generate effluents with toxic heavy metals [2]. Consequently, this matter undermines the world’s economic growth and public health. In developing countries, serious water pollution takes place due to the limitation of appropriate water technologies that result in a low removal of heavy metals [3]. As a result, treated water does not meet the requirements of the drinking water standard. Currently, about 2 billion people worldwide still lack of access to clean water for their drinking [4]. Out of these people, only 60% of the population have basic sanitation services [5].
In spite of the increasing efficiency of water utilization, improper wastewater treatment has contributed to deteriorating water quality in the developing world, worsening water pollution [6]. This problem results not only in the scarcity of water resources, but also in the decreasing supply of clean water for public consumption [7]. This is attributed to the presence of heavy metals such as Cr, Cu, and Fe in municipal wastewater that contributes to water pollution.
Kurniawan et al. [8] reported heavy metals such as Cr(III), Cd(II), Cu(II), and Zn(II) in wastewater discharged from the electroplating industry. They seriously threaten public health and the environment [9][10]. Therefore, the industrial effluent needs to be treated thoroughly to meet the effluent limits set by legislation prior to its discharge to a water body (Table 1). Otherwise, the effluents not only contaminate the environment, but also threaten public health, limiting productive activities [11].
Table 1. Maximum contaminant level (MCL) of heavy metals in aqueous solutions.
| Heavy Metals | Potential Health Effects | MCLs (mg/L) | ||
|---|---|---|---|---|
| WHO [12] | HK EPD [13] | China’s EPA [14] | ||
| Cr(VI) | Kidney, liver damage | 0.05 | 0.05–0.1 | 0.05–0.01 |
| Cr(III) | Skin rashes | |||
| Hg(II) | Teratogenesis, liver, neural damage | 0.006 | 0.001 | 0.001 |
| Cd(II) | Anemia, emphysema, heart disease | 0.003 | 0.001 | 0.005–0.01 |
| Cu(II) | Diarrhea, kidney, liver damage | 2 | 0.05–0.1 | 1 |
| Pb(II) | Anemia, constipation, hypertension | 0.01 | 0.1 | 0.05–0.1 |
| Ni(II) | Cancer, diarrhea, insomnia | 0.07 | 0.1–0.2 | 0.05–0.1 |
Remarks: WHO: World Health Organization. EPD: Environmental Protection Department of Hong Kong SAR. EPA: Environmental Protection Agency of PR China.
To comply with the effluent limits set by national legislation, a variety of water technologies such as chemical precipitation and ion exchange have been applied to eliminate heavy metals from contaminated water [15][16][17]. However, chemical precipitation possesses bottlenecks such as the excessive generation of sludge that needs to be disposed of in local landfills. This adds to the operational cost for sludge disposal [18].
To overcome the limitations, the search for novel technologies has intensified recently [19]. Recently, due to its low cost, heavy metals removal has been extensively tested by using biomass through biosorption. This process binds a target metal from aqueous solutions using certain biomaterials [20]. The sorption can be described as ‘active’ or ‘passive’. Active sorption such as phytoremediation involves metabolic activities of living biomass, while passive sorption is non-metabolic, as metal binding is undertaken using functional groups and surface characteristics of cell walls on dead biomass [21]. Phytoremediation is widely used to accumulate target pollutants in constructed wetlands [22]. For this treatment, metallothioneins and phytochelatins have been identified as the active units that contribute to heavy metals’ binding by plants [23]. The active metal binding is more complex than passive sorption in terms of removal mechanisms by biomaterials [24].
Recently, passive sorption using algae, bacteria, and fungi biomasses has been employed to sequester metals [25][26][27]. Their works have been carried out at a laboratory scale for bath studies. In a few cases, the investigations were scaled up using column operations [28]. Although biosorbents have demonstrated high affinity for metals [29][30], not many studies have reviewed heavy metal-binding mechanisms by biosorption technology [31].
Preliminary works have dealt with biosorption using plant-derived biomass [32][33][34][35]. In spite of their novelty, their reviews did not provide a complete mechanistic study of biosorption for heavy metal removal. In addition, how to optimize biosorption performance using mathematical modelings and the elucidation on metal-binding mechanisms were not elaborated. Despite the widespread applications of the Langmuir and Freundlich isotherm models for wastewater treatment, only a few studies have employed the surface complexation model (SCM) to study the effects of pH changes on heavy metal removal using certain functional groups [36][37][38]. Therefore, there is a growing need to simulate mathematical modeling for scaling up the application of biosorption in water treatment.
2. Advances in Water Treatment Using Functional Composites
2.1. Nanomaterials for Water Treatment
The need for water technologies, which do not lead to the production of toxic by-products, has resulted in the applications of nanoscale materials as adsorbents for environmental remediation. In recent years, cutting-edge research has been undertaken to synthesize and develop cost-effective and environmentally benign nanomaterials for environmental remediation [39]. When the nanomaterials are manufactured at atomic scales, they show better properties than starting materials due to enhanced structures and additional functionalities relevant for degradation or for sequestration [40]. In the next few years, adsorbents will not only play leading roles in the new generation of environmental technology but also revolutionize the world in which we live [41]. It is expected that low-cost and eco-friendly nanomaterials capable of detecting contaminants at an atomic level may substantially help improve public health and protect the environment.
Since existing treatment options are unable to maximize the removal of target contaminants in aqueous solutions, there is a growing consensus to develop other cost-effective technologies to enhance their treatment performance. The diverse applications of nanomaterials across a number of disciplines in recent years have responded to the need for an efficient and effective water treatment [42]. According to [43], any material can be categorized as nanoscale if it can meet any one of the following criteria: (1) Development at an atomic scale with length from 1 to 100 nm, (2) the technology creates and uses structures and/or systems with certain characteristics and functions because of their small sizes, and (3) the technology has the capability of being controlled or manipulated on a nanoscale level. The requirements underlie the principle that the properties of matter change at small scales (Figure 1).
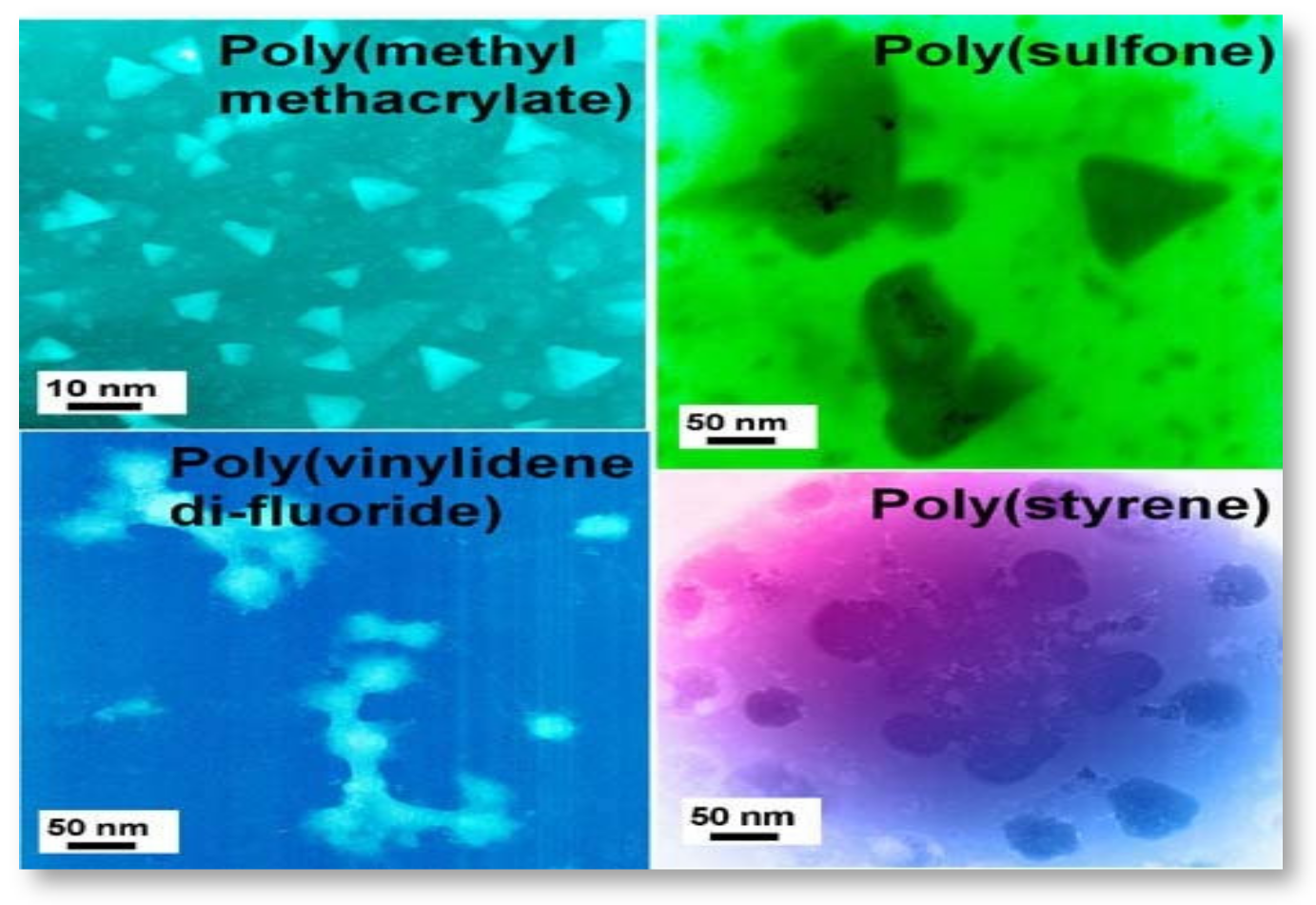
Figure 1. Functional nanocomposites for wastewater treatment.
2.2. Technological Strengths of Nanomaterials
Due to their high porosity and active surface, not only can nanomaterials sequester target pollutants with different molecular sizes, but they also facilitate the manufacturing process to utilize raw materials efficiently [44]. Nanomaterials work rapidly and have high metal-binding adsorption capacities, and are regenerated after being saturated [45].
Currently, the design of environmentally friendly materials, products, and processes with tailor-made properties is based on the enhanced understanding of their synthesis, physico-chemical properties, and structure–property relationships. This understanding is essential at a molecular and nanoscale level. For this reason, nanomaterials can be manufactured by generating small structures from larger pieces of materials (‘top down’ techniques) such as etching to create circuits on the surface of a silicon microchip. They may be constructed by the arrangement of atom-wise additions into a nanostructure through physico-chemical interactions between the units (‘bottom up’ techniques). This technique results in nanomaterials with properties which vary from one to another [46].
To manufacture nanomaterials with various levels of quality, different methods can be applied to exploit certain properties of the matter at atomic levels. Because of their tiny framework, the manipulation techniques under certain pH and doses enable the self-assembly of molecules to form fibrous nanostructures [47]. Because of their nano size, ranging from 1 to 100 nm, the nanoparticles have a substantial surface area to mass ratio, unlike bulk particles such as activated carbon [48]. Structures such as core/shell nanoparticles and multi-components, resulting from the proximity of nano-structured components, can demonstrate enhanced properties and new functionality. These properties allow the nanoparticles to stay suspended for a period of time, allowing them to eliminate target pollutants effectively. For example, a carbon nanotube was found to effectively eliminate inorganic contaminants [49].
Apart from their large surface area, due to the electronic density, certain functional groups can be easily attached to nanoparticles to improve their reactivity toward inorganic pollutants in contaminated water [50]. The key properties enable nanomaterials to be a powerful precursor to improve environmental sustainability through environmental remediation. Nanoscale materials provide indirect strategies for water pollution control through enhanced catalytic processes that not only generate minimum waste, but also produce improved sensors for effective separations.
2.3. Types of Nanomaterials
2.3.1. nZVI
One of the nanomaterials that is commercially available in market is the nZVI particle. With its diameters ranging from 1 to 200 nm, nZVI particles represent the novelty of nanoiron technology for wastewater treatment. Several studies have reported that iron-based nanoparticles can effectively reduce a large number of recalcitrant contaminants such as heavy metals [51]. By reacting NaBH4 with FeCl3·6H2O, Fe(III) is reduced to Fe(0) according to the equation below:
2 Fe(H2O)63+ + 6 BH4− + 6 H2O → 2 Fe0 + 6 B(OH)3 + 21 H2
Quinn [51] reported that nZVI particles had a larger surface area (19 m2/g) than Fe(0). It was reported that the presence of boron and the shell thickness enhanced the reactivity of the particles toward target compounds. For this reason, nZVI could dechlorinate polychlorobiphenyl (PCB) at a removal rate higher than that of Fe(0) powder.
2.3.2. Dendrimer
Advanced progress in material sciences has also enabled the manipulation of tiny structures called dendrimers to address water quality problems due to aquatic pollutants. Dendrimers are core-shell nanostructures with a certain composition and an architecture consisting of nanoscale features [52]. The particle comprises three covalently bonded elements, a core, and terminal branch cells. Due to its reactivity, this polymer has the ability to serve as a ligand for inorganic pollutants. Metal–dendrimer complexes can be removed from wastewater by pH adjustment. This enables the particles to be reused and allows metal recovery for polymer-supported ultrafiltration.
2.3.3. Metal Organic Framework (MOF)
Metal-organic frameworks (MOFs), widely known as porous coordination polymers, as a new class of porous hybrid materials with reticular structure, have demonstrated promising applications for water treatment such as adsorption, catalysis, and detection. However, there are still technical barriers to their practical application such as blockage of pipes, difficulty in recovery, high cost, and potential environmental toxicity [53].
2.3.4. Nanoporous Materials
Nanoporous materials are another emerging class of nanomaterials used in environmental applications. The materials were classified according to pore size (diameter) under microporous (less than 2 nm), mesoporous (between 2 and 50 nm), and macroporous materials (more than 50 nm), based on the IUPAC convention [54]. The materials with a diameter less than 100 nm could be categorized as nanoporous materials, useful in environmental applications. Zeolites are used in catalytic converters in car exhaust systems, which can filter and degrade harmful exhaust gases [55]. Energy efficiency and energy saving could be achieved with the application of zeolite, metallic foam, and activated carbon in a heat pump [55].
Spherical, well-defined core-shell PEI/PMMA (polyethyleimine/polymethyl methacrylate) nanoparticles represent a specific molecular type of particles that can be developed for wastewater treatment. In a preliminary work, nanoparticles were developed from PEI and PMMA, as well as PMMA and chitosan through graft copolymerization [47] (Figure 2). This method is not directly employed to immobilize monomers with amine chelating groups. Instead, a two-stage modification was conducted by involving the grafting of an epoxide-containing glycidyl methacrylate followed by the immobilization of PEI (Figure 3). The PEI not only improved the dispersion of the nanoparticles, but also brought new properties to the nanocomposite. Grafted PMMA provided support for the PEI chains in the adsorbent [56]. Further variations in the structure and functionality of the polymers can be achieved by altering the properties and synthesis techniques for the precursor chains.
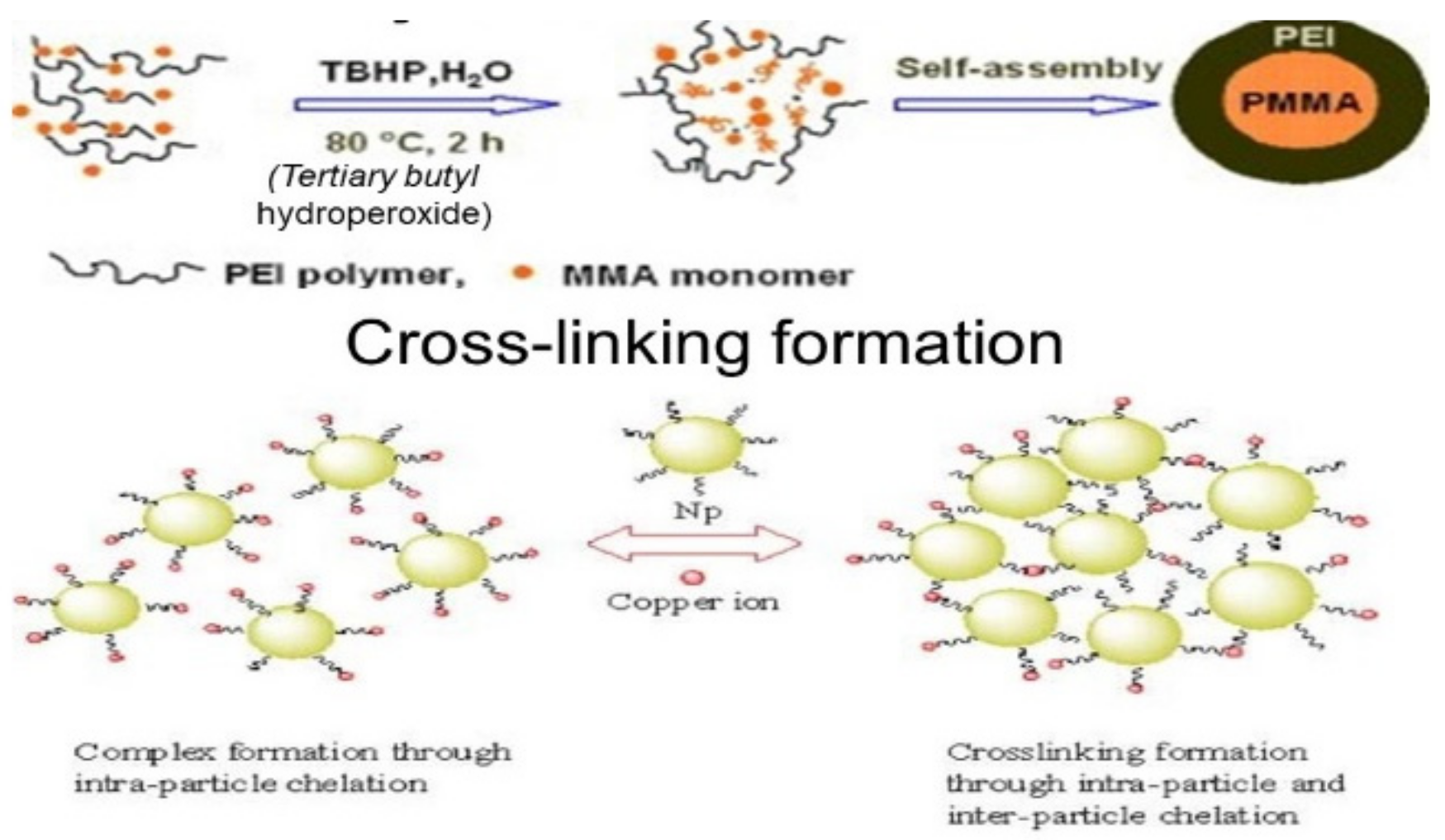
Figure 2. Synthesis route to PEI/PMMA composites.
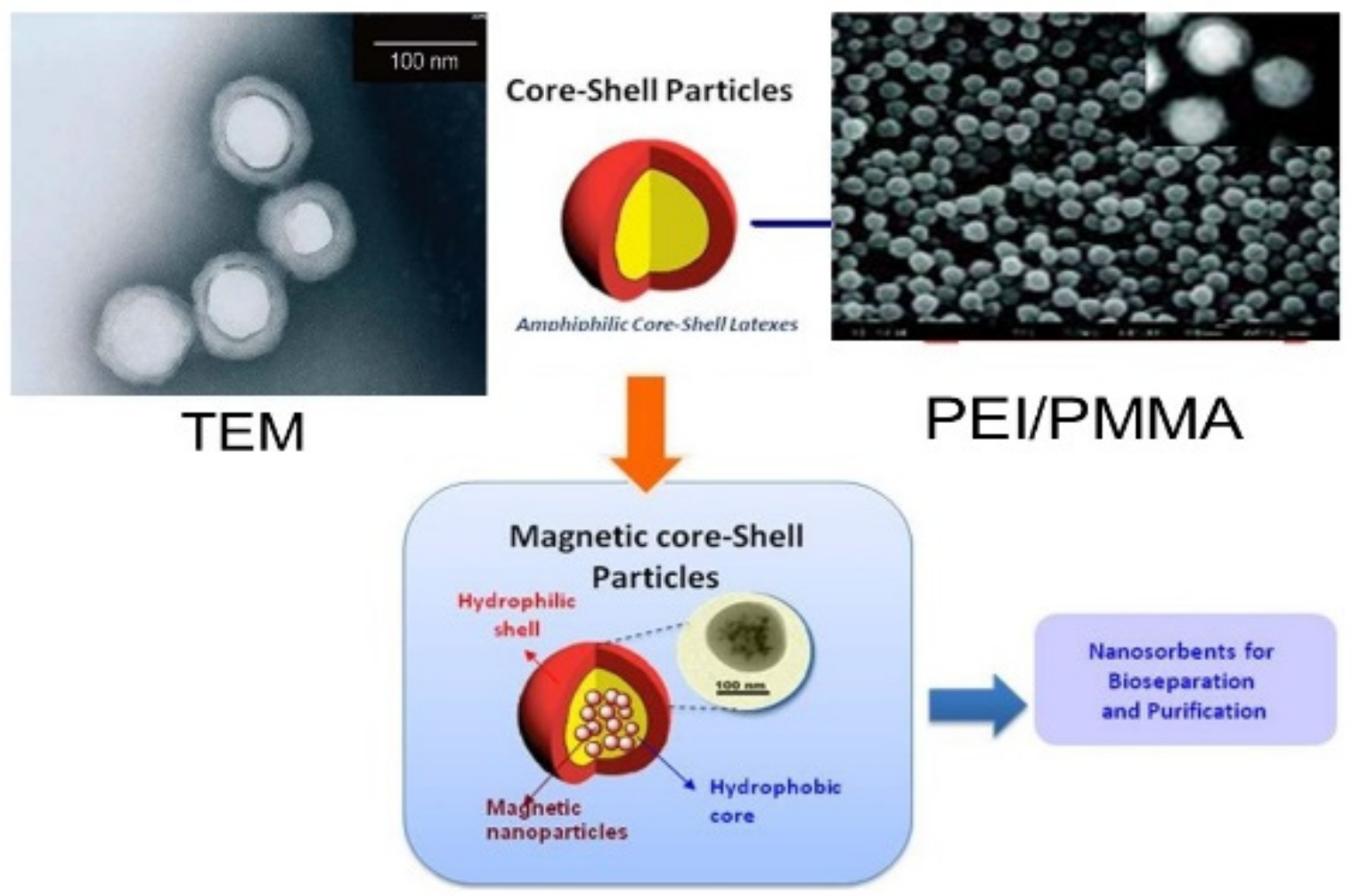
Figure 3. PEI/PMMA adsorbents for wastewater treatment.
The applications of PEI/PMMA for water treatment may revolutionize the ways in which we deal with environmental pollution sustainably. Industries may shift their paradigm in dealing with contaminated water, using less costly and more eco-friendly products while improving resource efficiency. As nano-adsorbents facilitate a powerful removal of target pollutants from contaminated water, it is anticipated that they may no longer apply physico-chemical treatments that cost half of the total treatment cost.
Overall, the applications of the TiO2 nanocatalyst, nZVI, or polymeric nanoparticles for treatment of contaminated wastewater are only the starting point of nanomaterial applications in water pollution control [57]. To advance their development for water treatment, research collaborations and scientific exchanges among interdisciplinary researchers need to be undertaken not only to foster mutual partnership across industries and borders, but also to enable the sharing of tools, knowledge, and techniques [58].
References
- Zhu, M.; Kurniawan, T.A.; Duan, L.; Song, Y.; Hermanowicz, S.W.; Othman, M.H.D. Advances in BiOX-based ternary photocatalysts for water technology and energy storage applications: Research trends, challenges, solutions, and ways forward. Rev. Environ. Sci. Bio/Technol. 2022, 21, 331–370.
- Kurniawan, T.A.; Sillanpää, M. Nanoadsorbents for remediation of aquatic environment: Local and practical solutions for global water pollution problems. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1233–1295.
- Liang, X.; Kurniawan, T.A.; Goh, H.H.; Zhang, D.; Dai, W.; Liu, H.; Goh, K.C.; Othman, M.H.D. Conversion of landfilled waste-to-electricity (WTE) for energy efficiency improvement in Shenzhen (China): A strategy to contribute to resource recovery of unused methane for generating renewable energy on-site. J. Clean. Prod. 2022, 369, 133078.
- Liang, X.; Goh, H.H.; Kurniawan, T.A.; Zhang, D.; Dai, W.; Liu, H.; Liu, J.; Goh, K.C. Utilizing landfill gas (LFG) to electrify digital data centers in China for accelerating energy transition in Industry 4.0 era. J. Clean. Prod. 2022, 369, 133297.
- Sniatala, B.; Kurniawan, T.A.; Sobotka, D.; Makinia, J.; Othman, M.H.D. Macro-nutrients recovery from liquid waste as a sustainable resource for production of recovered mineral fertilizer: Uncovering alternative options to sustain global food security cost-effectively. Sci. Total Environ. 2023, 856, 159283.
- Maiurova, A.; Kurniawan, T.A.; Kustikova, M.; Bykovskaia, E.; Othman, M.H.D.; Singh, D.; Goh, H.H. Promoting digital transformation in waste collection service and waste recycling in Moscow (Russia): Applying a circular economy paradigm to mitigate climate change impacts on the environment. J. Clean. Prod. 2022, 354, 131604.
- Kurniawan, T.A.; Lo, W.; Sillanpää, M. Treatment of contaminated water laden with 4-chlorophenol using coconut shell waste-based activated carbon modified with chemical agents. Sep. Sci. Technol. 2011, 46, 460–472.
- Kurniawan, T.A.; Liang, X.; O’Callaghan, E.; Goh, H.; Othman, M.H.D.; Avtar, R.; Kusworo, T.D. Transformation of solid waste management in China: Moving towards sustainability through digitalization-based circular economy. Sustainability 2022, 14, 2374.
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967.
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243.
- Kurniawan, T.A.; Avtar, R.; Singh, D.; Xue, W.; Dzarfan Othman, M.H.; Hwang, G.H.; Iswanto, I.; Albadarin, A.B.; Kern, A.O. Reforming MSWM in Sukunan (Yogjakarta, Indonesia): A case-study of applying a zero-waste approach based on circular economy paradigm. J. Clean. Prod. 2020, 284, 124775.
- World Health Organization, Europe, Water Sanitation and Health (WSH). 2008. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/en/index.html (accessed on 12 January 2023).
- Hong Kong Environmental Protection Department (HK EPD). Technical Memorandum Standards for Effluents Discharged into Drainage and Sewerage Systems, Inland and Coastal Water. 2008. Available online: http://www.legislation.gov.hk/eng/home.htm (accessed on 12 January 2023).
- China Environmental Protection Agency (China EPA). Available online: http://english.mee.gov.cn/Resources/laws/ (accessed on 12 January 2023).
- Kurniawan, T.A.; Chan, G.; Lo, W.H.; Babel, S. Physico-chemical treatment techniques for wateswater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98.
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.H. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426.
- Kurniawan, T.A.; Lo, W.H.; Chan, G. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, 29, 80–100.
- Fu, D.; Kurniawan, T.A.; Gui, H.; Li, H.; Wang, Y.; Li, Q. Role of CuxO-anchored pyrolyzed hydrochars on H2O2-activated degradation of tetracycline: Effects of pyrolysis temperature and pH. Ind. Eng. Chem. Res. 2022, 61, 8847–8857.
- Kurniawan, T.A.; Singh, D.; Avtar, R.; Dzarfan Othman, M.H.; Hwang, G.H.; Albadarin, A.B.; Rezakazemi, M.; Setiadi, T.; Shirazian, S. Resource recovery from landfill leachate: An experimental investigation and perspectives. Chemosphere 2021, 274, 129986.
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029.
- Kurniawan, T.A.; Liang, X.; Singh, D.; Othman, M.H.D.; Goh, H.H.; Gikas, P.; Kern, A.O.; Kusworo, T.D.; Shoqeir, J.A. Harnessing landfill gas (LFG) for electricity: A strategy to mitigate greenhouse gas emissions in Jakarta (Indonesia). J. Environ. Manag. 2022, 301, 113882.
- Fu, D.; Kurniawan, T.A.; Li, Q.; Gui, H.; Othman, M.H.D. Treatment of As(III)-contaminated water using iron-coated carbon fiber. Materials 2022, 15, 4365.
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 122, 825–832.
- Kurniawan, T.A.; Othman, M.H.D.; Singh, D.; Avtar, R.; Goh, H.H.; Setiadi, T.; Lo, W.H. Technological solutions for long-term management of partially used nuclear fuel: A critical review. Ann. Nucl. Energy 2022, 166, 108736.
- Volesky, B.; Holan, Z.R. Biosorption of heavy metal. Biotechnol. Prog. 1995, 11, 235–250.
- Kratochvil, D.; Volesky, B. Advances in the biosorption of heavy metals. Trends Biotechnol. 1998, 16, 291–300.
- Premakumara, D.G.J.; Canete, A.L.M.L.; Nagaishi, M.; Kurniawan, T.A. Policy implementation of the Republic Act (RA) No. 9003 in the Philippines on MSW management: A case study of Cebu City. Waste Manag. 2014, 34, 971–979.
- Muraleedharan, T.R.; Philip, L.; Iyengar, L.; Venkobachar, C. Application studies of biosorption for monazite processing industry effluents. Bioresour. Technol. 1994, 49, 179–186.
- Wang, L.; Chua, H.; Wong, P.K.; Lo, W.; Yu, P.H.F.; Zhao, Y.G. An optimal magnetite immobilized Pseudomonas putida 5-x cell system for Cu2+ removal from industrial waste effluent. Water Sci. Technol. 2000, 41, 241–248.
- Sud, D.; Mahajan, G.; Kaur, M.P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solution—A review. Bioresour. Technol. 2008, 99, 6017–6027.
- Kurniawan, T.A.; Othman, M.H.D.; Hwang, G.H.; Gikas, P. Unlocking digital technology in waste recycling industry in Industry 4.0 era: A transformation towards digitalization-based circular economy in Indonesia. J. Clean. Prod. 2022, 357, 131911.
- Wang, L.; Chua, H.; Wong, P.K.; Lo, W.H.; Yu, P.H.F. Ni2+ removal and recovery from electroplating effluent by Pseudomonas putida 5-x cell biomass. J. Environ. Sci. Health Part A 2003, 38, 521–531.
- Congeevaram, S.; Dhanarani, S.; Park, J.; Dexilin, M.; Thamaraiselvi, K. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J. Hazard. Mater. 2007, 146, 270–277.
- Ahluwalia, S.S.; Goyal, D. Micobial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257.
- Morales-Barrera, L.; Cristiani-Urbina, E. Hexavalent chromium removal by a Trichoderma inhamatum fungal strain isolated from tannery effluent. Water Air Soil Pollut. 2008, 187, 327–336.
- Leung, W.C.; Wong, M.F.; Chua, H.; Lo, W.; Yu, P.H.F.; Leung, C.K. Removal and recovery of heavy metals by bacteria isolated from activated sludge treating industrial effluents and municipal wastewater. Water Sci. Technol. 2000, 41, 233–240.
- Liu, Y.; Liu, Y.J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242.
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291.
- Guo, M.; Weng, X.; Wang, T.; Chen, Z. Biosynthesized iron-based nanoparticles used as a heterogeneous catalyst for the removal of 2, 4-dichlorophenol. Sep. Purif. Technol. 2017, 175, 222–228.
- Schulte, J.; Dutta, J. Nanotechnology in environmental protection and pollution. Sci. Technol. Adv. Mater. 2005, 6, 219–220.
- Masciangioli, T.; Zhang, W.X. Environmental technologies at nanoscale. Environ. Sci. Technol. 2003, 37, 102A–108A.
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Gharanjig, K. Photocatalytic degradation of agricultural N-heterocyclic organic pollutants using immobilized nanoparticles of titania. J. Hazard. Mater. 2007, 145, 65–71.
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laîné, J.M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl. Catal. B Environ. 2004, 50, 259–269.
- Fernández-Pacheco, R.; Arruebo, M.; Marquina, C.; Ibarra, R.; Arbiol, J.; Santamaría, J. Highly magnetic silica-coated iron nanoparticles prepared by the arc-discharge method. Nanotechnology 2006, 17, 1188.
- Yang, K.; Xing, B. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ. Pollut. 2007, 145, 529–537.
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931.
- Li, G.; Fudickar, W.; Skupin, M.; Klyszcz, A.; Draeger, C.; Lauer, M.; Fuhrhop, J.H. Rigid lipid membranes and nanometer clefts: Motifs for the creation of molecular landscapes. Angew. Chem. Int. 2002, 41, 1828–1852.
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588.
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic carbon-nanotube–TiO2 composites. Adv. Mater. 2009, 21, 2233–2239.
- DeMarco, M.J.; SenGupta, A.K.; Greenleaf, J.E. Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res. 2003, 37, 164–176.
- Quinn, J.; Geiger, C.; Clausen, C.; Brooks, K.; Coon, C.; O’Hara, S.; Krug, T.; Major, D.; Yoon, W.S.; Gavaskar, A.; et al. Field demonstration of DNAPL dehalogenation using emulsified zero-valent iron. Environ. Sci. Technol. 2005, 39, 1309–1318.
- Jia, Z.; Srinivasan, M.P. Langmuir–Blodgett film fabricated with dendrimer modified polyimide. Coll. Surfaces A Physicochem. Eng. Asp. 2005, 257, 183–190.
- Rouquerol, J.; Avnir, D.; Everett, D.H.; Fairbridge, C.; Haynes, M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Guidelines for the characterization of porous solids. Stud. Surface Sci. Catal. 1994, 87, 1–9.
- Farrauto, R.J.; Heck, R.M. Catalytic converters: State of the art and perspectives. Catal. Today 1999, 51, 351–360.
- Wongsuwan, W.; Kumar, S.; Neveu, P.; Meunier, F. A review of chemical heat pump technology and applications. Appl. Therm. Eng. 2001, 21, 1489–1519.
- Wang, Y.P.; Pei, X.W.; He, X.Y.; Yuan, K. Synthesis of well-defined, polymer-grafted silica nanoparticles via reverse ATRP. Eur. Polym. J. 2005, 41, 1326–1332.
- Sharma, V.; Sharma, A. Nanotechnology: An emerging future trend in wastewater treatment with its innovative products and processes. Nanotechnology 2012, 1, 1–8.
- Roco, M.C. Nanoscale science and engineering education activities in the United States (2001–2002). J. Nanopart. Res. 2002, 4, 271.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
889
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
21 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




