Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yin Xiao | -- | 1874 | 2023-02-20 03:02:22 | | | |
| 2 | Conner Chen | Meta information modification | 1874 | 2023-02-20 03:48:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mu, Y.; Du, Z.; Xiao, L.; Gao, W.; Crawford, R.; Xiao, Y. Bone Mineral Phase and Localized Ionic Microenvironment. Encyclopedia. Available online: https://encyclopedia.pub/entry/41405 (accessed on 12 March 2026).
Mu Y, Du Z, Xiao L, Gao W, Crawford R, Xiao Y. Bone Mineral Phase and Localized Ionic Microenvironment. Encyclopedia. Available at: https://encyclopedia.pub/entry/41405. Accessed March 12, 2026.
Mu, Yuqing, Zhibin Du, Lan Xiao, Wendong Gao, Ross Crawford, Yin Xiao. "Bone Mineral Phase and Localized Ionic Microenvironment" Encyclopedia, https://encyclopedia.pub/entry/41405 (accessed March 12, 2026).
Mu, Y., Du, Z., Xiao, L., Gao, W., Crawford, R., & Xiao, Y. (2023, February 20). Bone Mineral Phase and Localized Ionic Microenvironment. In Encyclopedia. https://encyclopedia.pub/entry/41405
Mu, Yuqing, et al. "Bone Mineral Phase and Localized Ionic Microenvironment." Encyclopedia. Web. 20 February, 2023.
Copy Citation
Calcium phosphates (CaPs) based on inorganic biomaterials are one of the most extensively studied types for bone grafting. They are composed of calcium ions and phosphate groups, which are omnipresent in the bloodstream or fixed in the bone mineral phase. Considering the abundance of ions in the bone environment and the current knowledge of their modulatory roles in maintaining the bone remodeling balance, it is expected that a deeper understanding of ions in the bone environment would provide new insights to guide the future design of inorganic biomaterials for bone tissue engineering.
intrinsic osteoinductivity
inorganic biomaterials
localized ionic microenvironment
1. Introduction
A localized microenvironment in bone remodeling milieus is generated and maintained when ions and biological molecules are released during the demineralization and degradation of bone matrix by protons and proteases secreted by osteoclasts, respectively, and bone formation by osteoblasts [1]. However, the localized microenvironment will be altered at implantation sites, with biomaterials interacting with extracellular fluid and cells. Considered vehicles for localized delivery of inorganic ions and ionic groups, inorganic biomaterials are no longer merely an inert scaffold but a reservoir for bioactive cues for modulating the bone remodeling process [2][3][4].
Inspired by the abundance of elements in the biological system and the effects of nutritional deficiency or overload, therapeutic applications of bioinorganic ions have been explored for many years. For example, the platinum drug cisplatin has been used for cancer treatment, the gold drugs myocrisin and auranofin for rheumatoid arthritis treatment, silver compounds in the pharmaceutical industry for their antimicrobial properties, and lanthanides and some transition metals as radiopharmaceuticals and diagnostic agents [5][6][7]. Meanwhile, the non-scientific and unregulated usage of inorganics can sometimes also be poisonous and lead to tragic disorders or diseases. For example, grey-colored skin is caused by unsafe nasal sprays due to the precipitation of silver salts, and copper deficiency results from over-supplemented zinc for prostate problems and acne [4][5]. In the field of regenerative medicine, the roles of elements in modulating cellular activities have gradually been unraveled, either as essential cofactors of enzymes and proteins or as regulatory molecules in ion channels or secondary signaling. Uncovered biological roles of ions provided possibilities to explore the applications of inorganic biomaterials in hard and soft tissue engineering by acting as vehicles to deliver ions and ionic groups locally.
Among all, calcium phosphates (CaPs) based on inorganic biomaterials are one of the most extensively studied types for bone grafting. They are composed of calcium ions and phosphate groups, which are omnipresent in the bloodstream or fixed in the bone mineral phase [8][9][10]. These synthetic bone substitutes can bind with natural bones by forming a solid biomaterial-bone interface, lacking osteoinductive and angiogenic properties [3][11][12][13][14]. Significant progress has been made in designing functional CaPs-based biomaterials with: (a) optimized geometry, roughness, and appropriate porosity for entrapping and concentrating growth factors or osteoprogenitor cells via proteins that could enhance cell adhesion, (b) incorporated growth factors or proteins that could modulate cellular activity, (c) doped trace elements that enhance osteogenesis in vitro [15][16]. However, the clinical performances of current CaP-based biomaterials are still unsatisfactory and incomparable to autologous bone grafts due to low bioactivities [8][10]. Nevertheless, the optimization of CaPs-based biomaterials significantly boosted the understanding of the modulatory effects of ions in the biological system [3][4]. Considering the abundance of ions in the bone environment and the current knowledge of their modulatory roles in maintaining the bone remodeling balance, it is expected that a deeper understanding of ions in the bone environment would provide new insights to guide the future design of inorganic biomaterials for bone tissue engineering [3][17].
2. Bone Mineral Phase and Localized Ionic Microenvironment
Bone homeostasis is maintained in a series of highly complicated events orchestrated by: (a) interactions among different types of cells, mainly mesenchymal stem cells (MSCs), osteoprogenitor cells, osteoblasts, osteoclasts, and osteocytes, and (b) interactions of cells with extracellular matrix in a localized microenvironment, and (c) interactions of cells with components in surrounding biological milieus, such as organics (amino acids, enzymes, hormones, fatty acids, neurotransmitters, sugars, vitamins, etc.), inorganics (inorganic ions or groups, such as calcium, phosphate, potassium, sodium, carbonate, etc.), as well as waste products from cell metabolism (Figure 1) [9]. Naturally, the localized ionic microenvironment is maintained by the balance between bone-forming cells, osteoblasts, and bone-resorbing cells, osteoclasts, during the bone remodeling process [18] (Figure 2). Specifically, osteoclasts firmly attaching to the bone surface could achieve a pH fall to a limit value of pH 3.0 or less for dissolving the bone mineral and favor collagen degradation by secreting lysosomal proteinases [19]. Organics in this microenvironment have been extensively studied since the last century, especially cell-secreted growth factors that play roles in bone formation, such as TGF-β (transforming growth factor-beta), FGF (fibroblast growth factor), BMP (bone morphogenetic proteins), IGF-I (insulin-like growth factors I), etc. [20]. Inorganics in this microenvironment have also been extensively studied because they are essential for the bone mineral formation, and quality of the mineralized tissue, either liberated from bone or circulating in body fluid. Moreover, ions from the localized microenvironment are now considered to consist of crystal components and molecular modulators in many biological processes in bone remodeling, i.e., bone formation and resorption [3][9][21][22]. The list of inorganic ions and ionic groups that affect bone metabolism and homeostasis as signaling molecules has dramatically increased in the past decades. More previously less-studied elements in the periodic table have been surprisingly found to play a role in the etiology and pathogenesis of some bone diseases or the modulation of cellular activities, especially metallic elements, because they are prone to lose electrons to form positively charged ions and tend to dissolve in biological fluids or be attracted by negatively charged biological molecules, proteins, or DNAs, to form active metal complexes [3][4][23].
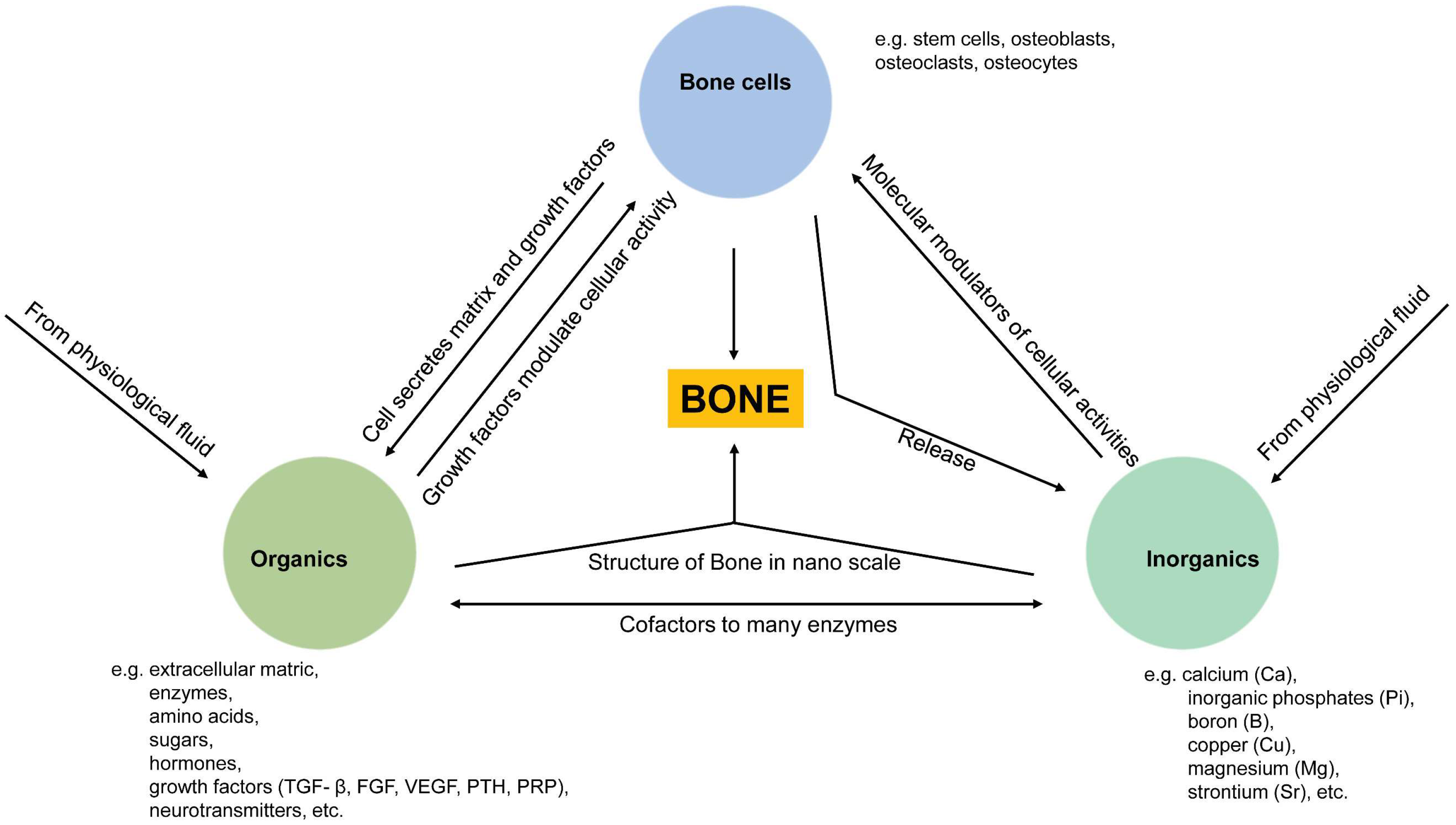
Figure 1. Schematic illustration of relationships between three essential components (cells, organics, inorganics) in maintaining bone homeostasis. Cell-derived organic molecules, such as growth factors and enzymes, modulate cellular activities; Osteoclasts release ions from the bone matrix during bone resorption, and in turn, ions act as molecular modulators of cellular activities and as components of apatite crystals being deposited into the bone matrix with the modulation of cells; Ions are co-factors to many enzymes, and ions are immobilized as apatite crystals into collagen fibrils from the bone structure at the nanoscale.
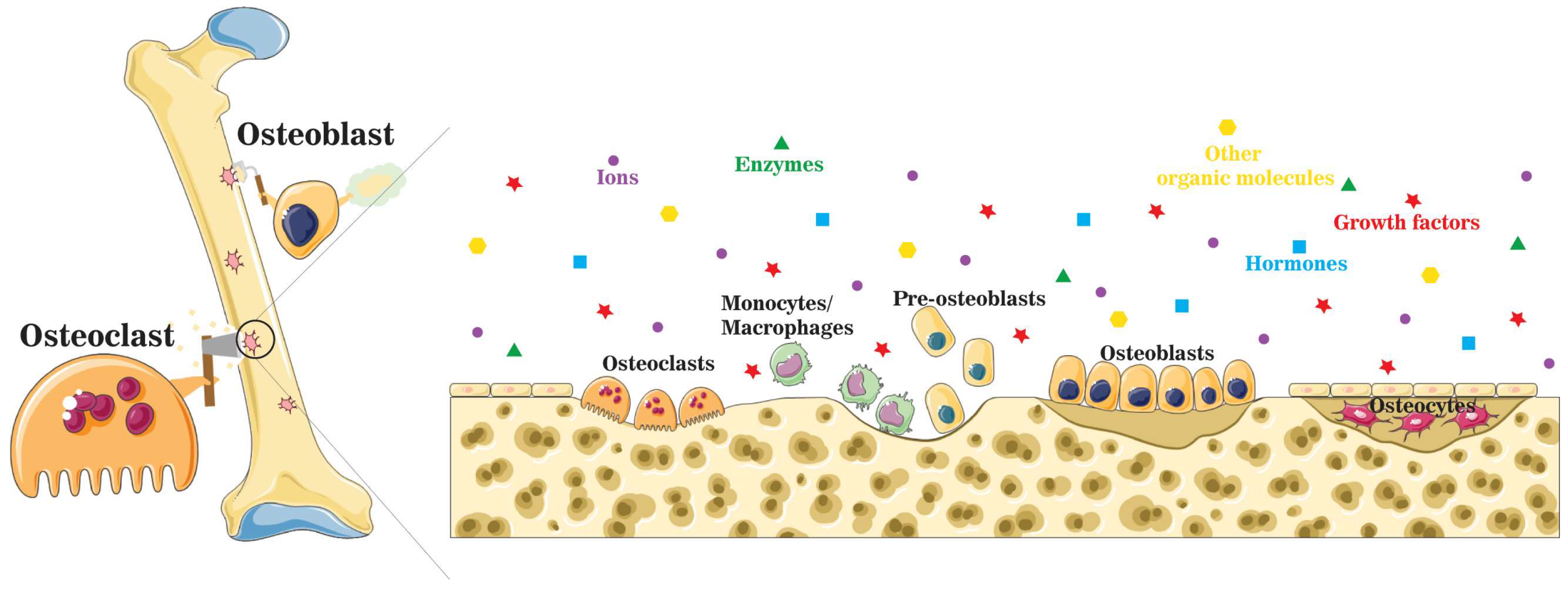
Figure 2. A schematic illustration of the localized microenvironment at the bone remodeling site. Bone homeostasis is maintained by the balance between bone formation by osteoblasts and bone resorption by osteoclasts. During the bone remodeling process, organic molecules, such as enzymes, growth factors, and hormones, are released into the localized microenvironment, together with a mixture of inorganic components.
Bone mineral, known as biological apatite, is incorporated in collagen fibrils, arranged with a c-axis parallel to the direction of fibrils, with lengths of 30–50 nm, widths of 15–30 nm, and thicknesses of 2–10 nm [24]. Biological apatite has been modeled as hexagonal carbonated hydroxyapatite based on X-ray diffraction (XRD) results, with the lattice parameters of a = b = 9.432 Å, c = 6.881 Å, and γ = 120°. Hydroxyl ions (OH−), parallel to the c-axis, are positioned on the screw axes at every one-half of the unit cell, pointing in opposite directions to neighboring OH−s. Tetrahedral phosphate ions (PO43−), immobilized by calcium ions (Ca2+) interspersed among them, as well as marginal calcium ions (Ca2+), shared with neighbor unit cell. Notably, steric interference between adjacent OH−s in hexagonal hydroxyapatite unit cells could be overcome by vacancy or replacement of an OH− by impurity ions, the most likely event in organisms, or by conversion of hexagonal to monoclinic space group at high temperature, rearranging adjacent OH−s to a uniform direction [25][26].
The accumulation of most inorganics in the body can be attributed to the formation of apatite crystals in bone, with distinguished content and composition among species and individuals, resulting from differed preferences on elements in different species, variations in diet, and relative abundance in the environment [27][28]. During bone resorption, ions and ionic groups will be liberated from CaP based network into the local microenvironment in acidic conditions, participating in local bone remodeling or being carried away by physiological fluid. Therefore, ions and ionic groups entering the localized microenvironment are determined by the composition of bone minerals and vice versa. Specifically, the content and level of ions and ionic groups in the localized biological milieu affect the formation of the bone mineral through ionic substitution and, consequently, the properties of the final crystalline product in the mineral phase [29][30][31][32]. For example, OH− (minor site) or PO43− (primary site) sites could be replaced by CO32−, forming type A and B carbonated hydroxyapatite, respectively. PO43− site could also be replaced by hydrolyzed phosphate (HPO42−) structure in mature bone, while OH− could be substituted with florin (F−), chlorin (Cl−) ion, or orthosilicic acid (SiO44−) structure [29][30][32]. Moreover, ionic substitutions also happen where Ca2+ is replaced by other metals, such as sodium (Na+), potassium (K+), magnesium (Mg2+), zinc (Zn2+), manganese (Mn2+), cobalt (Co2+), strontium (Sr2+), iron (Fe2+), copper (Cu2+). Ionic exchange in biological apatite alters crystalline structures, resulting in modified crystal size, growth rate, and properties. Compared with stoichiometric or geological apatite crystals, biological apatite crystals have smaller crystallite sizes, less ordered crystal structure, lower crystallinity, and higher solubility (Figure 3) [3][33]. The influences of different ions or ionic groups on biological apatite crystals are balanced by each other. For example, substitutions of PO43− by CO32− and of Ca2+ by Zn2+ or Mg2+ inhibit crystal growth, increase crystal disorder and solubility, and lower the crystallinity [29][30][34]. Replacements of Ca2+ by Al3+, La2+, or Fe2+ accelerate crystal growth, and replacement of OH− by F− on the lattice reduces the solubility [29][30][34]. Additionally, replacing OH− with SiO44− causes a contraction on the a-axis and an expansion on the c-axis of the crystal lattice [35]; replacing Ca2+ with Sr2+ causes an expansion on both the a- and c-axes [4].
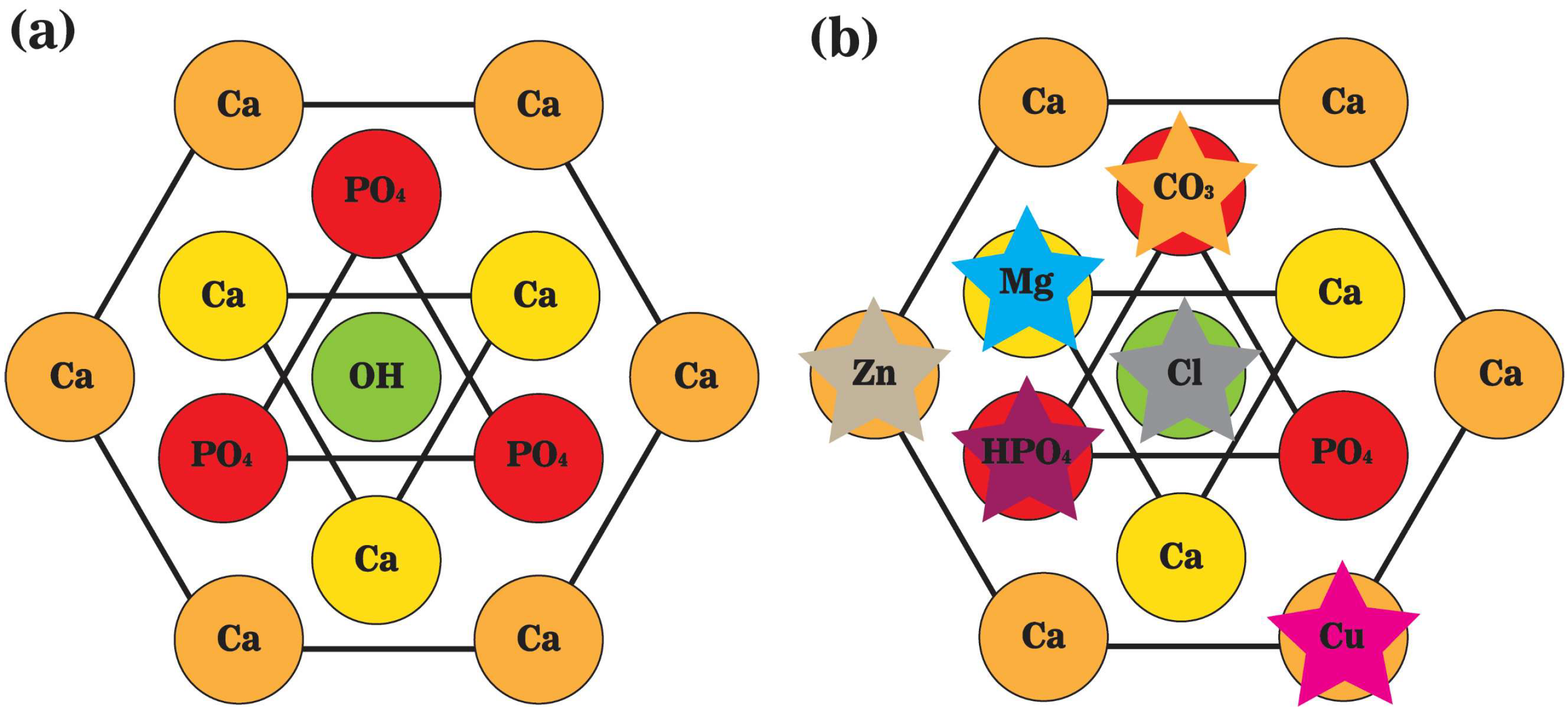
Figure 3. A schematic representation of the top view of unit cells of (a) stoichiometric hydroxyapatite; and (b) biological apatite crystals. Hydroxyl ions (OH−) are positioned on the screw axes at every one-half of the unit cell, paralleling the c-axis. Calcium ions (Ca2+) are interspersed among tetrahedral phosphate ions (PO43−), and the marginal ones are shared with neighbor unit cells.
During bone trauma, such as a fracture, bone healing starts with the invasion of blood into the traumatic space. A microenvironment is formed along with blood clots and calluses, where cells interact with components in the extracellular matrix and extracellular fluid. However, the localized microenvironment will be altered at the implantation site with the involvement of inorganic biomaterials due to extensive interactions between biomaterials and the microenvironment [16]. The contribution of inorganic biomaterial at the implantation site to the healing process can never be underestimated because many biomaterial intrinsic features, including parameters (composition, structure, topography), and properties (crystallinity, dissolution profile, surface charge), can make a difference in the localized microenvironment and cellular interactions, as well as cellular activities, and consequently the bone formation process (Figure 4) [16]. Therefore, understanding the influence of biomaterials on components in the localized ionic microenvironment shall guide the design of future inorganic biomaterials for bone grafting. Active roles of ions as molecular modulators upon many cellular activities during bone remodeling provide the material with more possibilities other than structural support and protein/cell entrapping.
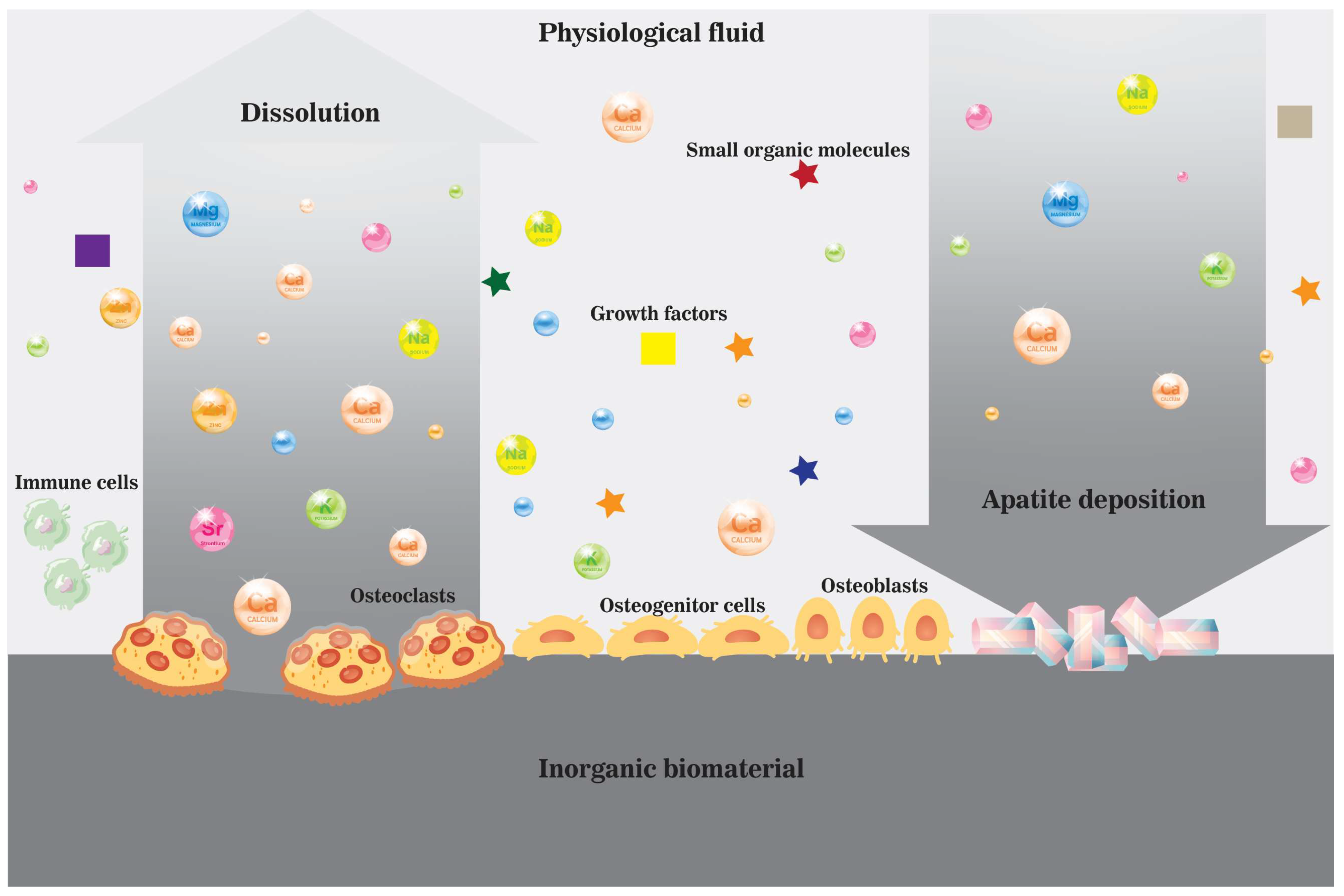
Figure 4. A schematic illustration of the dissolution and precipitation process near the surface of an inorganic biomaterial in vivo in the ionic microenvironment created by cells and physiological fluid enriched by dissolved biomaterial.
References
- Itzstein, C.; Coxon, F.P.; Rogers, M.J. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2011, 2, 117–130.
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87.
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247.
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026.
- van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097.
- Ahmad, S.; Isab, A.A.; Ali, S.; Al-Arfaj, A.R. Perspectives in bioinorganic chemistry of some metal based therapeutic agents. Polyhedron 2006, 25, 1633–1645.
- Cohen, S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 2007, 11, 115–120.
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater 2017, 53, 1–12.
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332.
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421.
- Wahl, D.A.; Czernuszka, J.T. Collagen-hydroxyapatite composites for hard tissue repair. Eur. Cells Mater. 2006, 11, 43–56.
- Xie, J.; Baumann, M.J.; McCabe, L.R. Osteoblasts respond to hydroxyapatite surfaces with immediate changes in gene expression. J. Biomed. Mater. Res. Part A 2004, 71, 108–117.
- Hu, C.; Zilm, M.; Wei, M. Fabrication of intrafibrillar and extrafibrillar mineralized collagen/apatite scaffolds with a hierarchical structure. J. Biomed. Mater. Res. Part A 2016, 104, 1153–1161.
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand Clin. 2012, 28, 457–468.
- Ripamonti, U. Soluble osteogenic molecular signals and the induction of bone formation. Biomaterials 2006, 27, 807–822.
- Legeros, R.Z. Calcium phosphate-based osteoinductive materials. Chem. Rev. 2008, 108, 4742.
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605.
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873.
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276.
- Solheim, E. Growth factors in bone. Int. Orthop. 1998, 22, 410–416.
- Ash, C.; Stone, R. A question of dose-Introduction. Science 2003, 300, 925.
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants: A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34.
- Thompson, K.; Orvig, C. Boon and bane of metal ions in medicine. Science 2003, 300, 936–939.
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58, 77–116.
- Ma, G.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994.
- Morgan, H.; Wilson, R.M.; Elliott, J.C.; Dowker, S.E.P.; Anderson, P. Preparation and characterisation of monoclinic hydroxyapatite and its precipitated carbonate apatite intermediate. Biomaterials 2000, 21, 617–627.
- Buddhachat, K.; Klinhom, S.; Siengdee, P.; Brown, J.L.; Nomsiri, R.; Kaewmong, P.; Thitaram, C.; Mahakkanukrauh, P.; Nganvongpanit, K. Elemental Analysis of Bone, Teeth, Horn and Antler in Different Animal Species Using Non-Invasive Handheld X-Ray Fluorescence. PLoS ONE 2016, 11, e0155458.
- Castro, W.; Hoogewerff, J.; Latkoczy, C.; Almirall, J.R. Application of laser ablation (LA-ICP-SF-MS) for the elemental analysis of bone and teeth samples for discrimination purposes. Forensic Sci. Int. 2010, 195, 17–27.
- Medvecký, Ľ.; Štulajterová, R.; Parilák, Ľ.; Trpčevská, J.; Ďurišin, J.; Barinov, S.M. Influence of manganese on stability and particle growth of hydroxyapatite in simulated body fluid. Colloids Surf. A Physicochem. Eng. Asp. 2006, 281, 221–229.
- Zhang, J.; Dai, C.; Wei, J.; Wen, Z.; Zhang, S.; Lin, L. Calcium phosphate/chitosan composite coating: Effect of different concentrations of Mg2+ in the m-SBF on its bioactivity. Appl. Surf. Sci. 2013, 280, 256–262.
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021.
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763.
- Omelon, S.J.; Grynpas, M.D. Relationships between Polyphosphate Chemistry, Biochemistry and Apatite Biomineralization. Chem. Rev. 2008, 108, 4694–4715.
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669.
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
801
Revisions:
2 times
(View History)
Update Date:
20 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





