Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yin Xiao and Version 2 by Conner Chen.
Calcium phosphates (CaPs) based on inorganic biomaterials are one of the most extensively studied types for bone grafting. They are composed of calcium ions and phosphate groups, which are omnipresent in the bloodstream or fixed in the bone mineral phase. Considering the abundance of ions in the bone environment and the current knowledge of their modulatory roles in maintaining the bone remodeling balance, it is expected that a deeper understanding of ions in the bone environment would provide new insights to guide the future design of inorganic biomaterials for bone tissue engineering.
- intrinsic osteoinductivity
- inorganic biomaterials
- localized ionic microenvironment
1. Introduction
A localized microenvironment in bone remodeling milieus is generated and maintained when ions and biological molecules are released during the demineralization and degradation of bone matrix by protons and proteases secreted by osteoclasts, respectively, and bone formation by osteoblasts [1]. However, the localized microenvironment will be altered at implantation sites, with biomaterials interacting with extracellular fluid and cells. Considered vehicles for localized delivery of inorganic ions and ionic groups, inorganic biomaterials are no longer merely an inert scaffold but a reservoir for bioactive cues for modulating the bone remodeling process [2][3][4][2,3,4].
Inspired by the abundance of elements in the biological system and the effects of nutritional deficiency or overload, therapeutic applications of bioinorganic ions have been explored for many years. For example, the platinum drug cisplatin has been used for cancer treatment, the gold drugs myocrisin and auranofin for rheumatoid arthritis treatment, silver compounds in the pharmaceutical industry for their antimicrobial properties, and lanthanides and some transition metals as radiopharmaceuticals and diagnostic agents [5][6][7][5,6,7]. Meanwhile, the non-scientific and unregulated usage of inorganics can sometimes also be poisonous and lead to tragic disorders or diseases. For example, grey-colored skin is caused by unsafe nasal sprays due to the precipitation of silver salts, and copper deficiency results from over-supplemented zinc for prostate problems and acne [4][5][4,5]. In the field of regenerative medicine, the roles of elements in modulating cellular activities have gradually been unraveled, either as essential cofactors of enzymes and proteins or as regulatory molecules in ion channels or secondary signaling. Uncovered biological roles of ions provided possibilities to explore the applications of inorganic biomaterials in hard and soft tissue engineering by acting as vehicles to deliver ions and ionic groups locally.
Among all, calcium phosphates (CaPs) based on inorganic biomaterials are one of the most extensively studied types for bone grafting. They are composed of calcium ions and phosphate groups, which are omnipresent in the bloodstream or fixed in the bone mineral phase [8][9][10][8,9,10]. These synthetic bone substitutes can bind with natural bones by forming a solid biomaterial-bone interface, lacking osteoinductive and angiogenic properties [3][11][12][13][14][3,11,12,13,14]. Significant progress has been made in designing functional CaPs-based biomaterials with: (a) optimized geometry, roughness, and appropriate porosity for entrapping and concentrating growth factors or osteoprogenitor cells via proteins that could enhance cell adhesion, (b) incorporated growth factors or proteins that could modulate cellular activity, (c) doped trace elements that enhance osteogenesis in vitro [15][16][15,16]. However, the clinical performances of current CaP-based biomaterials are still unsatisfactory and incomparable to autologous bone grafts due to low bioactivities [8][10][8,10]. Nevertheless, the optimization of CaPs-based biomaterials significantly boosted the understanding of the modulatory effects of ions in the biological system [3][4][3,4]. Considering the abundance of ions in the bone environment and the current knowledge of their modulatory roles in maintaining the bone remodeling balance, it is expected that a deeper understanding of ions in the bone environment would provide new insights to guide the future design of inorganic biomaterials for bone tissue engineering [3][17][3,17].
2. Bone Mineral Phase and Localized Ionic Microenvironment
Bone homeostasis is maintained in a series of highly complicated events orchestrated by: (a) interactions among different types of cells, mainly mesenchymal stem cells (MSCs), osteoprogenitor cells, osteoblasts, osteoclasts, and osteocytes, and (b) interactions of cells with extracellular matrix in a localized microenvironment, and (c) interactions of cells with components in surrounding biological milieus, such as organics (amino acids, enzymes, hormones, fatty acids, neurotransmitters, sugars, vitamins, etc.), inorganics (inorganic ions or groups, such as calcium, phosphate, potassium, sodium, carbonate, etc.), as well as waste products from cell metabolism (Figure 1) [9]. Naturally, the localized ionic microenvironment is maintained by the balance between bone-forming cells, osteoblasts, and bone-resorbing cells, osteoclasts, during the bone remodeling process [18] (Figure 2). Specifically, osteoclasts firmly attaching to the bone surface could achieve a pH fall to a limit value of pH 3.0 or less for dissolving the bone mineral and favor collagen degradation by secreting lysosomal proteinases [19]. Organics in this microenvironment have been extensively studied since the last century, especially cell-secreted growth factors that play roles in bone formation, such as TGF-β (transforming growth factor-beta), FGF (fibroblast growth factor), BMP (bone morphogenetic proteins), IGF-I (insulin-like growth factors I), etc. [20]. Inorganics in this microenvironment have also been extensively studied because they are essential for the bone mineral formation, and quality of the mineralized tissue, either liberated from bone or circulating in body fluid. Moreover, ions from the localized microenvironment are now considered to consist of crystal components and molecular modulators in many biological processes in bone remodeling, i.e., bone formation and resorption [3][9][21][22][3,9,21,22]. The list of inorganic ions and ionic groups that affect bone metabolism and homeostasis as signaling molecules has dramatically increased in the past decades. More previously less-studied elements in the periodic table have been surprisingly found to play a role in the etiology and pathogenesis of some bone diseases or the modulation of cellular activities, especially metallic elements, because they are prone to lose electrons to form positively charged ions and tend to dissolve in biological fluids or be attracted by negatively charged biological molecules, proteins, or DNAs, to form active metal complexes [3][4][23][3,4,23].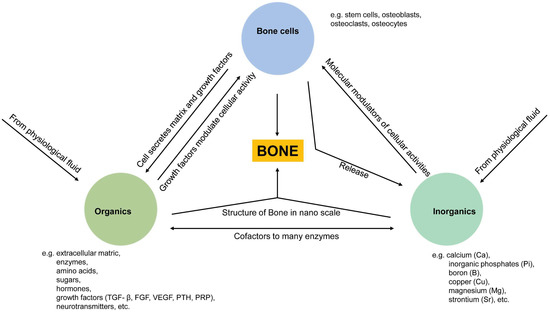
Figure 1. Schematic illustration of relationships between three essential components (cells, organics, inorganics) in maintaining bone homeostasis. Cell-derived organic molecules, such as growth factors and enzymes, modulate cellular activities; Osteoclasts release ions from the bone matrix during bone resorption, and in turn, ions act as molecular modulators of cellular activities and as components of apatite crystals being deposited into the bone matrix with the modulation of cells; Ions are co-factors to many enzymes, and ions are immobilized as apatite crystals into collagen fibrils from the bone structure at the nanoscale.
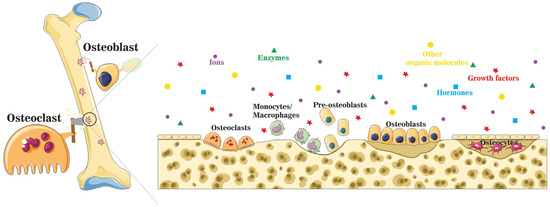
Figure 2. A schematic illustration of the localized microenvironment at the bone remodeling site. Bone homeostasis is maintained by the balance between bone formation by osteoblasts and bone resorption by osteoclasts. During the bone remodeling process, organic molecules, such as enzymes, growth factors, and hormones, are released into the localized microenvironment, together with a mixture of inorganic components.
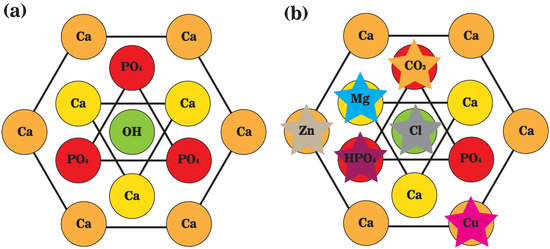
Figure 3. A schematic representation of the top view of unit cells of (a) stoichiometric hydroxyapatite; and (b) biological apatite crystals. Hydroxyl ions (OH−) are positioned on the screw axes at every one-half of the unit cell, paralleling the c-axis. Calcium ions (Ca2+) are interspersed among tetrahedral phosphate ions (PO43−), and the marginal ones are shared with neighbor unit cells.
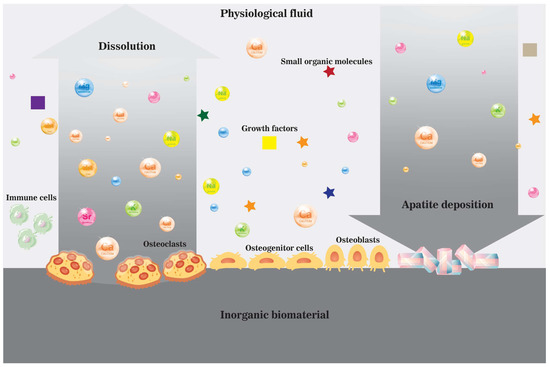
Figure 4. A schematic illustration of the dissolution and precipitation process near the surface of an inorganic biomaterial in vivo in the ionic microenvironment created by cells and physiological fluid enriched by dissolved biomaterial.
