
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tiago Torres | -- | 1885 | 2023-02-16 13:36:36 | | | |
| 2 | Lindsay Dong | Meta information modification | 1885 | 2023-02-17 06:10:27 | | |
Video Upload Options
OX40 and OX40L are two checkpoint molecules that bind to potentiate pro-inflammatory T-cell responses that are pivotal to atopic dermatitis pathogenesis. Two OX40-OX40L inhibitors, rocatinlimab and amlitelimab, are being developed for the treatment of atopic dermatitis. Rocatinlimab, an anti-OX40 antibody, was evaluated in phase 2b, a randomized, placebo-controlled clinical trial. At week 16, rocatinlimab groups achieved a greater reduction in the EASI percentage change from the baseline (−48.3% to −61.1%) against the placebo (−15.0%; p < 0.001), and clinical response was maintained 20 weeks after the treatment had ceased. Amlitelimab, an anti-OX40L antibody, was studied in a 12-week treatment phase 2a clinical trial, with a significant efficacy response observed within 2 weeks. At week 16, amlitelimab groups reached the EASI mean percentage change from the baseline of −69.9% and −80.1% versus the placebo (−49.4%; p = 0.072 and p = 0.009). Among the responders, 68% of amlitelimab patients were sustained 24 weeks following the last dose. Both treatments were shown to be safe and well tolerated. The evidence points to OX40-OX40L inhibitors as future options for atopic dermatitis treatment with potential disease-modifying effects.
1. Introduction
2. AD Pathogenesis and the Role of OX40-OX40L Pathway
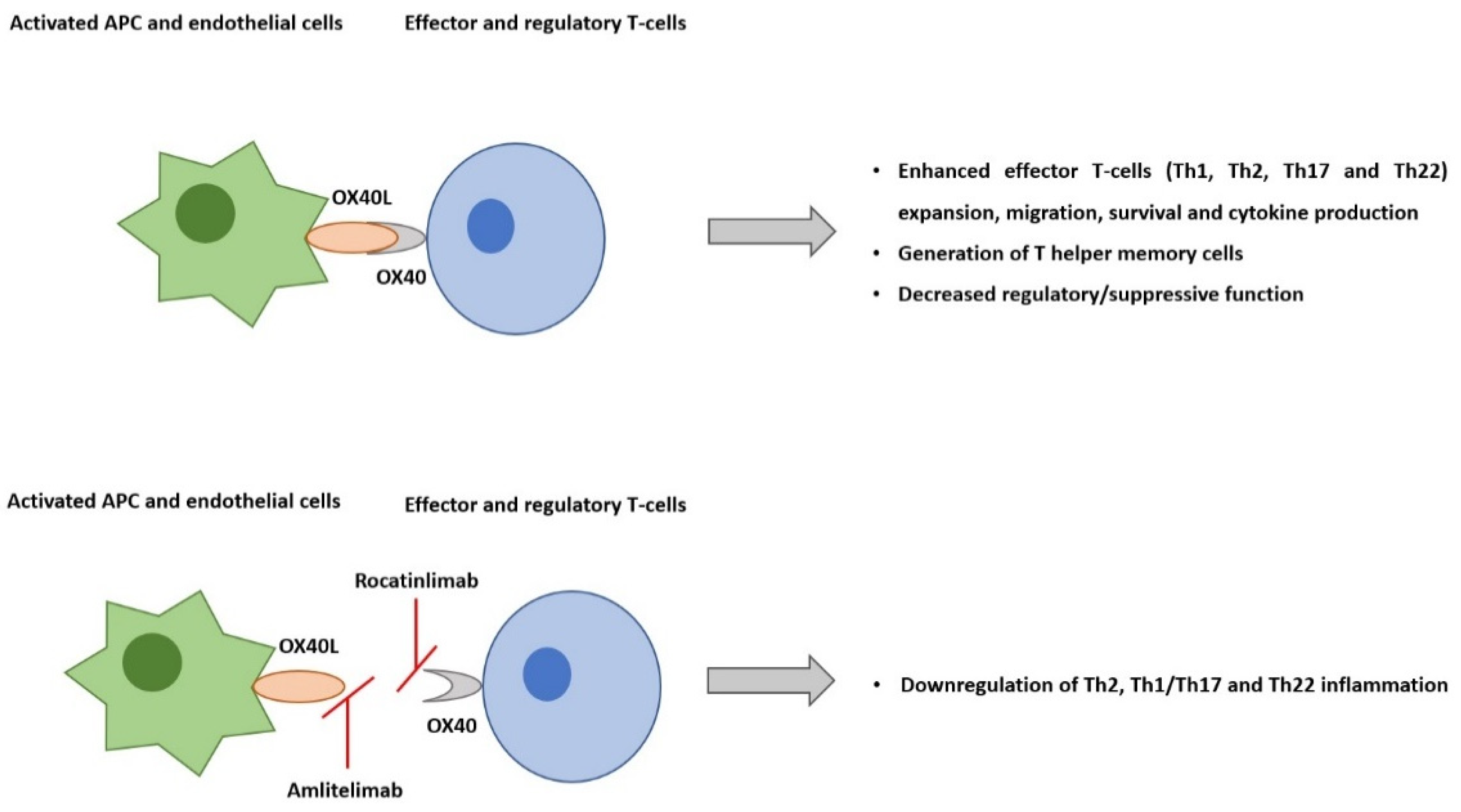
3. OX40-OX40L Inhibition
3.1. GBR 830
3.2. Rocatinlimab
3.3. Amlitelimab
4 . Summary
References
- Kido-Nakahara, M.; Furue, M.; Ulzii, D.; Nakahara, T. Itch in Atopic Dermatitis. Immunol. Allergy Clin. N. Am. 2017, 37, 113–122.
- Wollenberg, A.; Oranje, A.; Deleuran, M.; Simon, D.; Szalai, Z.; Kunz, B.; Svensson, A.; Barbarot, S.; Von Kobyletzki, L.; Taieb, A.; et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 729–747.
- Qi, H.-J.; Li, L.-F. New Biologics for the Treatment of Atopic Dermatitis: Analysis of Efficacy, Safety, and Paradoxical Atopic Dermatitis Acceleration. BioMed Res. Int. 2021, 2021, 5528372.
- Newsom, M.; Bashyam, A.M.; Balogh, E.A.; Feldman, S.R.; Strowd, L.C. New and Emerging Systemic Treatments for Atopic Dermatitis. Drugs 2020, 80, 1041–1052.
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014, 371, 130–139.
- Simpson, E.L.; Bieber, T.; Guttman-Yassky, E.; Beck, L.A.; Blauvelt, A.; Cork, M.J.; Silverberg, J.I.; Deleuran, M.; Kataoka, Y.; Lacour, J.-P.; et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N. Engl. J. Med. 2016, 375, 2335–2348.
- Rodrigues, M.A.; Torres, T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J. Dermatol. Treat. 2020, 31, 33–40.
- Ferreira, S.; Guttman-Yassky, E.; Torres, T. Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: Focus on Upadacitinib and Abrocitinib. Am. J. Clin. Dermatol. 2020, 21, 783–798.
- Yosipovitch, G.; Berger, T.; Fassett, M. Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 239–250.
- Grobe, W.; Bieber, T.; Novak, N. Pathophysiology of atopic dermatitis. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 433–440.
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Med. Port. 2019, 32, 606–613.
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192.
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis endotypes and implications for targeted therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11.
- Nakahara, T.; Kido-Nakahara, M.; Tsuji, G.; Furue, M. Basics and recent advances in the pathophysiology of atopic dermatitis. J. Dermatol. 2021, 48, 130–139.
- Furue, M.; Ulzii, D.; Vu, Y.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran J. Immunol. 2019, 16, 97–107.
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555.
- Furue, M. OX40L–OX40 Signaling in Atopic Dermatitis. J. Clin. Med. 2021, 10, 2578.
- Elsner, J.S.; Carlsson, M.; Stougaard, J.K.; Nygaard, U.; Buchner, M.; Fölster-Holst, R.; Hvid, M.; Vestergaard, C.; Deleuran, M.; Deleuran, B. The OX40 Axis is Associated with Both Systemic and Local Involvement in Atopic Dermatitis. Acta Dermato-Venereol. 2020, 100, adv00099–5.
- Jember, A.G.; Zuberi, R.; Liu, F.T.; Croft, M. Development of Allergic Inflammation in a Murine Model of Asthma Is Dependent on the Costimulatory Receptor Ox40. J. Exp. Med. 2001, 193, 387–392.
- Seshasayee, D.; Lee, W.P.; Zhou, M.; Shu, J.; Suto, E.; Zhang, J.; Diehl, L.; Austin, C.D.; Meng, Y.G.; Tan, M.; et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Investig. 2007, 117, 3868–3878.
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332.
- Zhang, Z.; Zhong, W.; Hinrichs, D.; Wu, X.; Weinberg, A.; Hall, M.; Spencer, D.; Wegmann, K.; Rosenbaum, J.T. Activation of OX40 Augments Th17 Cytokine Expression and Antigen-Specific Uveitis. Am. J. Pathol. 2010, 177, 2912–2920.
- Zhou, S.; Qi, F.; Gong, Y.; Zhang, J.; Zhu, B. Biological Therapies for Atopic Dermatitis: A Systematic Review. Dermatology 2021, 237, 542–552.
- Guttman-Yassky, E.; Pavel, A.B.; Zhou, L.; Estrada, Y.D.; Zhang, N.; Xu, H.; Peng, X.; Wen, H.-C.; Govas, P.; Gudi, G.; et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 144, 482–493.
- Wang, Y.-H.; Liu, Y.-J. OX40-OX40L interactions: A promising therapeutic target for allergic diseases? J. Clin. Investig. 2007, 117, 3655–3657.
- Saghari, M.; Gal, P.; Gilbert, S.; Yateman, M.; Porter-Brown, B.; Brennan, N.; Quaratino, S.; Wilson, R.; Grievink, H.W.; Klaassen, E.S.; et al. OX40L Inhibition Suppresses KLH-driven Immune Responses in Healthy Volunteers: A Randomized Controlled Trial Demonstrating Proof-of-Pharmacology for KY1005. Clin. Pharmacol. Ther. 2022, 111, 1121–1132.
- Weidinger, S.; Cork, M.; Reich, A.; Bieber, T.; Gilber, S.; Brennan, N.; Wilson, R.; Lucchesi, D.; Rynkiewicz, N.; Stebegg, M.; et al. Treatment with Amlitelimab–A Novel Non-Depleting, Non-Cytotoxic antiOX40Ligand Monoclonal Antibody–Reduces IL-22 Serum Levels in a Phase 2a Randomized, Placebo-Controlled Trial in Patients with Moderate-to-Severe Atopic Dermatitis. In Proceedings of the 31st European Academy of Dermatology and Venereology (EADV) Congress 2022, Milan, Italy, 7–10 September 2022.
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020, 9, 1–7.
- Blauvelt, A.; Teixeira, H.D.; Simpson, E.L.; Costanzo, A.; De Bruin-Weller, M.; Barbarot, S.; Prajapati, V.H.; Lio, P.; Hu, X.; Wu, T.; et al. Efficacy and Safety of Upadacitinib vs. Dupilumab in Adults with Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2021, 157, 1047–1055.
- Rodrigues, A.M.; Nogueira, M.; Torres, T. Dupilumab for atopic dermatitis: Evidence to date. G. Ital. Dermatol. Venereol. 2019, 154, 696–713.
- Guttman-Yassky, E.; Esfandiari, E.; Chong, C.; Matsui, T.; Mano, H. Rocatinlimab* (AMG 451/KHK4083) Demonstrates Improvements in Head and Neck Atopic Dermatitis in Patients with Moderate-Severe Disease in A Phase 2 Trial. In Proceedings of the 31st European Academy of Dermatology and Venereology (EADV) Congress, Virtual Meeting, Milan, Italy, 7–10 September 2022.




