OX40 and OX40L are two checkpoint molecules that bind to potentiate pro-inflammatory T-cell responses that are pivotal to atopic dermatitis pathogenesis. Two OX40-OX40L inhibitors, rocatinlimab and amlitelimab, are being developed for the treatment of atopic dermatitis. Rocatinlimab, an anti-OX40 antibody, was evaluated in phase 2b, a randomized, placebo-controlled clinical trial. At week 16, rocatinlimab groups achieved a greater reduction in the EASI percentage change from the baseline (−48.3% to −61.1%) against the placebo (−15.0%; p < 0.001), and clinical response was maintained 20 weeks after the treatment had ceased. Amlitelimab, an anti-OX40L antibody, was studied in a 12-week treatment phase 2a clinical trial, with a significant efficacy response observed within 2 weeks. At week 16, amlitelimab groups reached the EASI mean percentage change from the baseline of −69.9% and −80.1% versus the placebo (−49.4%; p = 0.072 and p = 0.009). Among the responders, 68% of amlitelimab patients were sustained 24 weeks following the last dose. Both treatments were shown to be safe and well tolerated. The evidence points to OX40-OX40L inhibitors as future options for atopic dermatitis treatment with potential disease-modifying effects.
1. Introduction
Atopic dermatitis (AD) is a chronically relapsing, inflammatory skin disease with a great impact on the patient’s quality of life, especially due to pruritus. With a prevalence of 2–5% in young adults and up to 20% in children, AD is one of the most common skin diseases
[1][2][1,2].
The currently available treatment armamentarium includes topical therapy (topical corticosteroids, calcineurin inhibitors crisaborole and ruxolitinib), phototherapy, and systemic therapy (conventional immunosuppressants and advanced target therapies). Conventional systemic immunosuppressive therapies may have limited efficacy and harbor long-term toxicity, which makes them not appropriate for continuous use
[3][4][3,4]. Fortunately, in recent years, several new therapeutic options targeting specific cytokines, cytokine receptors, or intracellular signaling pathways have been developed, showing significant clinical benefits in patients with AD and paving the way for more effective and safer therapies
[3][4][5][6][3,4,5,6]. Dupilumab, a fully human IgG4 monoclonal antibody directed against the IL-4Rα subunit of IL-4 and IL-13 receptors, changed the AD treatment paradigm as it was in 2017 as the first biological drug approved for the treatment of AD
[5][6][5,6]. Since then, other therapeutic options targeting the IL-13 and janus kinase (JAK) pathway have been approved, such as tralokinumab (IL-13 inhibitor), upadacitinib (JAK1 inhibitor), abrocitinib (JAK1 inhibitor), and baricitinib (JAK1/2 inhibitor)
[3][4][7][8][3,4,7,8], while others, such as lebrikizumab (IL-13 inhibitor) and nemolizumab (IL-31 inhibitor), are in an advanced stage of clinical development.
2. AD Pathogenesis and the Role of OX40-OX40L Pathway
A complex interplay between skin barrier dysfunction, skin inflammation, and dysbiosis contributes to AD development and chronicity
[9]. The acute phase of AD is characterized by a strong modulation of Th2 and Th22 immune responses, with variable Th17 involvement. With disease chronicity, there is also a marked Th1 activation. Anomalies in filaggrin, intercellular lipids, and tight junctions induce barrier disruption, which leads to the increased permeability of the skin to exogenous stimuli, sebostasis, and increased transepidermal water loss
[10][11][12][13][10,11,12,13]. Epidermis-derived alarmin cytokines, such as thymic stromal lymphopoietin (TSLP), interleukin (IL)-25, and IL-33, respond to environmental insults and mediate intercellular communication between the epidermal keratinocytes and immune cells. These endogenous molecules are rapidly released by keratinocytes in response to tissue damage to trigger defensive immune responses and trigger type 2 immune deviation, with a significantly higher number of T-helper (Th)2 cells expressing IL-4, IL-13, and IL-31. Consecutively, this inflammation downregulates the expression of filaggrin and loricrin in keratinocytes and exacerbates the epidermal barrier dysfunction
[3][14][3,14]. Furthermore, IL-4 and IL-13 prompt inflammation through the stimulation of IgE production from plasma cells, as well as amplifying IL-31-induced and histamine-induced pruritus
[15]. IL-22 is another important disease mediator of the disease, whose expression is increased in the skin of AD patients and mouse models, inducing keratinocyte proliferation and downregulating filaggrin expression
[16].
OX40–OX40L interaction (two co-stimulatory immune checkpoint molecules) plays a central role in the pathogenesis of AD. Immune checkpoint molecules have co-stimulatory and co-inhibitory roles in adaptative immune responses. They are primarily classified into two groups: (1) the immunoglobulin superfamily and the (2) the tumor necrosis factor superfamily (TNFSF) and its receptors (TNFRSFs). OX40 (TNFRSF4, CD134) and its ligand OX40L (TNFSF4, CD252) are two of the TNFSF/TNFRSF co-stimulatory immune checkpoint molecules
[17].
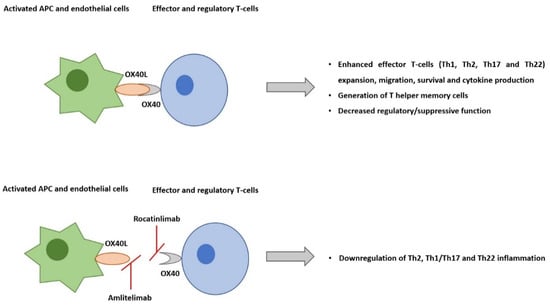
The co-stimulatory T-cell receptor OX40 is expressed predominantly on effector and regulatory T-cells. Its ligand, OX40L, is expressed on activated antigen-presenting cells, including dendritic cells (DCs), endothelial cells, macrophages, and activated B-cells. OX40–OX40L engagement is key to potentiating the expansion of effector T-cells and the prolongation of their survival by suppressing apoptosis, enhancing T-cell effector functions, such as cytokine production, and generating T helper memory cells. Naïve T-cells are activated by antigen-presenting cells through co-stimulatory molecule interaction, such as CD80/CD86 and CD28. The activated effector Th1 and Th2 T-cell expansion are sustained by OX40-OX40L ligation. Even though resting memory T-cells do not express OX40, upon reactivation, they become effector memory T-cells, and they start expressing it. OX40-OX40L ligation promotes the expansion of these cells
[17][18][17,18].
Preclinical studies of skin inflammation and asthma models have supported that OX40-OX40L signaling interactions are pivotal to the efficiency of the responses that are regulated by memory Th2 cells
[19][20][19,20]. TSLP and IL-25 activate DCs to express OX40L. OX40L-positive DCs induce OX40-positive T-cell differentiation, including Th2 cells, which promote IL-4 and IL-13 production from T-cells and signature cytokines of type 2 inflammatory response
[14][21][14,21]. Il-33 is produced by barrier-disrupted epidermic keratinocytes and stimulates type 2 innate lymphoid cells and dendritic cells to express OX40L. Preclinical evidence suggests that OX40-OX40L signaling also modulates IL-22 production from T-cells (
Figure 1)
[22].
Figure 1. The co-stimulatory T-cell receptor OX40 is expressed predominantly on effector and regulatory T-cells. Its ligand, OX40L, is expressed on activated antigen-presenting cells and endothelial cells. Their interaction plays a central role in pro-inflammatory T-cell responses, including in atopic dermatitis. Rocatinlimab is a fully human anti-OX40 monoclonal antibody, which selectively depletes sOX40+ activated T-cells. On the other hand, amlitelimab, is a non-depleting IgG4 human anti-OX40L monoclonal antibody that binds OX40L and blocks interaction with OX40. Besides blocking antigens, the presenting T-cell activation, amlitelimab also blocks T-cell independent APC pro-inflammatory activity via the inhibition of OX40L back signaling.
3. OX40-OX40L Inhibition
3.1. GBR 830
GBR 830 was the first-in-class, humanized, a monoclonal antibody against OX40 to enter a phase 2a trial investigating the efficacy, safety, and tissue effects in AD patients (NCT02683928)
[23][24][24,25]. Sixty-two moderate-to-severe AD patients were randomized to 3:1 to 10 mg/kg GBR830 or placebo on day 1 and day 29. On day 71, the proportion of patients achieving a 50% or greater improvement in the Eczema Area and Severity Index (EASI) score was greater with GBR 830 (76.9% [20/26]) versus the placebo (37.5% [3/8]). Biopsy specimens from lesioned skin were obtained before the first dose (baseline) at days 29 and day 71. Significant decreases from the baseline in OX401 T-cell and OX40L1 DC staining in the lesioned skin were found with GBR 830 treatment at days 29 (
p < 0.05) and 71 (
p < 0.001). A significant reduction in mRNA cytokines such as IL-31, CCL11, CCL17, and S100 was also demonstrated. GBR 830 was well tolerated, with an equal treatment-emergent adverse events (TEAE) distribution (GBR 830, 63.0% [29/46] and placebo, 63.0% [10/16]).
3.2. Rocatinlimab
Rocatinlimab, formerly known as AMG 451/KHK4083, is a fully human, non-fucosylated, immunoglobulin G1 (IgG1) anti-OX40 monoclonal antibody currently under investigation for the treatment of moderate-to-severe AD. It has been shown to selectively deplete OX40+ activated T-cells and suppress clonal T-cells and is expected to control Th2-driven conditions
[25][26].
3.3. Amlitelimab
Amlitelimab, also known as KY1005/SAR445229, is another OX40-OX40L pathway inhibitor but with a different mechanism of action. It is a non-depleting IgG4 human anti-OX40L monoclonal antibody that binds OX40L and blocks interactions with OX40. By targeting OX40L, amlitelimab aims to restore immune homeostasis between pro-inflammatory and anti-inflammatory T-cells. In addition to blocking antigen-presenting T-cell activation, amlitelimab also blocks T-cell independent antigens that present the cells’ pro-inflammatory activity via the inhibition of OX40L back signaling, thus blocking both type 2 and Th1/17/22 inflammation
[26][27][31,32].
4 . Summary
Atopic dermatitis poses a therapeutic challenge for clinicians, particularly in moderate-to-severe forms of the disease. Systemic corticosteroids and classic immunosuppressants, such as cyclosporine, methotrexate, mycophenolate mofetil, and azathioprine, have their prolonged use limited by safety concerns, variable efficacy response, and the need for frequent laboratory monitoring. Increased knowledge of atopic dermatitis complex pathophysiology has allowed the consideration of more immunological pathways as potential therapeutic targets. The currently available targeted options include biologic therapy, dupilumab, tralokinumab, and oral JAK inhibitors. Dupilumab, an IL-4 and IL-13 pathway inhibitor, was the first available biologic agent for the treatment of atopic dermatitis, and it is currently approved by FDA for adults and children aged 6 months and older with moderate to severe diseases that are not adequately controlled with topical therapies. Tralokinumab is an anti-IL13 antibody recently approved for adult patients. JAK inhibitors, such as baricitinib, upadacitinib and abrocitinib, are efficient new oral small molecules but with a less specific mechanism of action, raising more safety concerns, especially in high-risk patients
[28][29][30][34,35,36]. OX40-OX40L interaction is an essential part of the immune cascade that allows T-cell functioning and Th1, Th2, and Th22-mediated pathways, which have all been implicated in AD.
GBR 830 was the first-in-class monoclonal antibody against OX40 to present good efficacy and safety results, but its investigation has not been pursued. Rocatinlimab, an anti-OX40 monoclonal antibody, recently completed a phase 2b trial in which four different doses were evaluated, all of them with good efficacy results. The patients kept improving until week 36 of treatment. A post hoc head and neck analysis revealed that the therapeutic effect seemed to persist for 5 months after the treatment had ceased, which was supported by skin transcriptomic analysis. This durability of the response with rocatinlimab potentially reflects how this mechanism of action may induce disease modification
[31][30]. Rocatinlimab 300 mg Q2W was the dosage scheme that obtained greater improvements in all efficacy endpoints. However, administrations every 4 weeks may be more convenient and improve compliance. A phase 3 trial is currently in progress, aiming to evaluate two different doses every 2 weeks for 24 weeks, followed by every 4 weeks for 28 weeks. It will provide further information on which dose and periodicity are more adequate and whether extended dosing is feasible.
Amlitelimab is another OX40-OX40L pathway inhibitor that binds OX40L and blocks the interaction with OX40. It presented a rapid and marked clinical improvement in patients with moderate-to-severe AD, with a good safety profile. These efficacy results were maintained for 6 months after the treatment ceased, which also could suggest a disease-modifying effect. This could indicate a long and sustained response following the last dose, opening up the opportunity for extended dosing. The sustained reduction in IL-22 serum levels in amlitelimab-treated patients strongly indicates that amlitelimab effectively targets immune dysregulation in AD. It supports the hypothesis that targeting OX40L on antigen-presenting cells modulates not only type 2 response but also other T-cell pathways, including Th22
[27][32].
Both rocatinlimab and amlitelimab presented no major safety issues. Pyrexia and chills, after the first administration of rocatinlimab, were frequent, but they were not reported in subsequent administrations. No hypersensitivity or tolerability events were reported with amlitelimab. However, long-term studies will be essential to determine the potential risks. Animal OX40 deficient models seem to have impaired interferon-γ production and Th1 differentiation. On the other hand, in the viral infection model, virus-specific antibody production and the virus-specific cytotoxic T-cell response were not affected.