
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wenjing Xiong | -- | 1877 | 2023-02-16 13:31:37 | | | |
| 2 | Lindsay Dong | Meta information modification | 1877 | 2023-02-17 06:08:01 | | |
Video Upload Options
Organic/polymer light-emitting diodes (OLEDs/PLEDs) have attracted a rising number of investigations due to their promising applications for high-resolution fullcolor displays and energy-saving solid-state lightings. Near-infrared (NIR) emitting dyes have gained increasing attention for their potential applications in electroluminescence and optical imaging in optical tele-communication platforms, sensing and medical diagnosis in recent decades. And a growing number of people focus on the “heavy metal-free” NIR electroluminescent materials to gain more design freedom with cost advantage.
1. Introduction
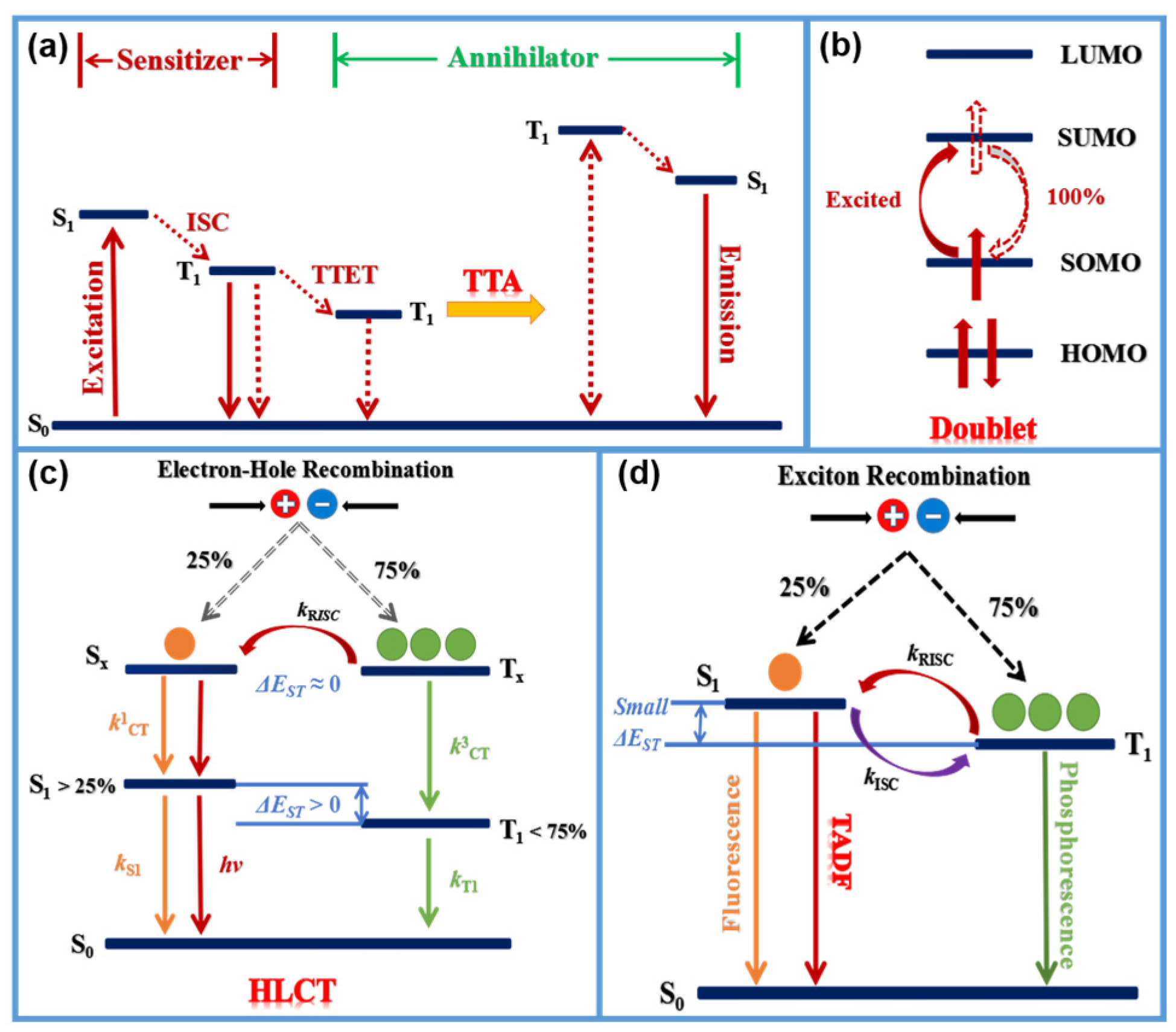
2. Tuning the Emission of Materials into NIR Region
3. NIR Fluorescent Materials Based on Polymers
4. NIR Fluorescent Materials Based on Small Molecules
Due to the parity-forbidden radiative 4f-4f transitions of the rare earth ions, the corresponding LEDs usually have a nonmeasurable or very low EQE and low power output. In contrast, the luminescence of organic molecules originates from their allowed S1–S0 transitions and thus free from the luminescence efficiency limitation. By using phosphorescent heavy metal complexes that can effectively harvest both the singlet and triplet excitons. Unfortunately, the EL quantum efficiency drops rapidly at high current densities.
Initially, attempts were made to construct NIR luminescent materials using molecules with a large area of conjugated systems. In 2006, Kageyama et al. [60] investigated that OLED using tris(8-quinolinolato)aluminum (Alq3) highly doped with N,N′ -bis(neopentyl)-3,4:9,10-perylenebis(dicarboximide) as an emitting layer exhibit near-infrared EL with a peak at 805 nm originating from N,N′ -bis(neopentyl)-3,4:9,10-perylenebis(dicarboximide) aggregates. Phthalocyanines are known to be organic semiconductors and have attracted much attention because of their high chemical stability, various synthetic modifications, epitaxial growth of thin films by organic molecular beam epitaxy and unique absorption bands extending from the ultraviolet region to infrared region [61][62]. Cheng et al. [63] reported the OLED device used purple phthalocyanine single crystal as an active light-emitting layer with the emission of 936 nm.
5. NIR Phosphorescent Materials Based on Small Molecules
6. Conclusions
References
- Tang, C.W.; Van Slyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915.
- Kim, J.-H.; Park, J.-W. Intrinsically stretchable organic light-emitting diodes. Sci. Adv. 2021, 7, 9715.
- Su, R.; Park, S.H.; Ouyang, X.; Ahn, S.I.; McAlpine, M.C. 3D-printed flexible organic light-emitting diode displays. Sci. Adv. 2022, 8, 8798.
- Song, H.; Song, Y.J.; Hong, J.; Kang, K.S.; Yu, S.; Cho, H.; Kim, J.; Lee, S. Water stable and matrix addressable OLED fiber textiles for wearable displays with large emission area. NPJ Flex Electron. 2022, 6, 66.
- Choi, S.; Kang, C.; Byun, C.-W.; Cho, H.; Kwon, B.-H.; Han, J.-H.; Yang, J.-H.; Shin, J.-W.; Hwang, C.-S.; Cho, N.S.; et al. Thin-film transistor-driven vertically stacked full-color organic light-emitting diodes for high-resolution active-matrix displays. Nat. Commun. 2020, 11, 2732.
- Zhang, H.; Su, Q.; Chen, S. Quantum-dot and organic hybrid tandem light-emitting diodes with multi-functionality of full-color-tunability and white-light-emission. Nat. Commun. 2020, 11, 2826.
- Hu, Y.X.; Miao, J.; Hua, T.; Huang, Z.; Qi, Y.; Zou, Y.; Qiu, Y.; Xia, H.; Liu, H.; Cao, X.; et al. Efficient selenium-integrated TADF OLEDs with reduced roll-off. Nat. Photon. 2022, 16, 803–810.
- Liu, H.; Fu, Y.; Tang, B.Z.; Zhao, Z. All-fluorescence white organic light-emitting diodes with record-beating power efficiencies over 130 lm W−1 and small roll-offs. Nat. Commun. 2022, 13, 5154.
- Chen, H.; Liu, L.; Qian, K.; Liu, H.; Wang, Z.; Gao, F.; Qu, C.; Dai, W.; Lin, D.; Chen, K.; et al. Bioinspired large Stokes shift small molecular dyes for biomedical fluorescence imaging. Sci. Adv. 2022, 8, 3289.
- Zhang, Y.; Zhao, W.; Chen, Y.; Yuan, H.; Fang, H.; Yao, S.; Zhang, C.; Xu, H.; Li, N.; Liu, Z.; et al. Rational construction of a reversible arylazo-based NIR probe for cycling hypoxia imaging in vivo. Nat. Commun. 2021, 12, 2772.
- Sisak, M.A.A.; Louis, F.; Aoki, I.; Lee, S.H.; Chang, Y.-T.; Matsusaki, M. A near-infrared organic fluorescent probe for broad applications for blood vessels imaging by high-throughput screening via 3D-blood vessel models. Small Methods 2021, 5, 2100338.
- Wang, L.G.; Barth, C.W.; Kitts, C.H.; Mebrat, M.D.; Montaño, A.R.; House, B.J.; McCoy, M.E.; Antaris, A.L.; Galvis, S.N.; McDowall, I.; et al. Near-infrared nerve-binding fluorophores for buried nerve tissue imaging. Sci. Transl. Med. 2021, 12, 0712.
- Salem, D.P.; Gong, X.; Liu, A.T.; Akombi, K.; Strano, M.S. Immobilization and function of NIR-fluorescent carbon nanotube sensors on paper substrates for fluidic manipulation. Anal. Chem. 2020, 92, 916–923.
- Nißler, R.; Bader, O.; Dohmen, M.; Walter, S.G.; Noll, C.; Selvaggio, G.; Groß, U.; Kruss, S. Remote near infrared identification of pathogens with multiplexed nanosensors. Nat. Commun. 2020, 11, 5995.
- Lan, Z.; Lei, Y.; Chan, W.K.E.; Chen, S.; Luo, D.; Zhu, F. Near-infrared and visible light dual-mode organic photodetectors. Sci. Adv. 2020, 6, 8065.
- Chen, Y.; Pei, P.; Lei, Z.; Zhang, X.; Yin, D.; Zhang, F. A Promising NIR-II Fluorescent Sensor for Peptide-Mediated LongTerm Monitoring of Kidney Dysfunction. Angew. Chem. Int. Ed. 2021, 60, 15809–15815.
- Bideh, B.N.; Shahroosvand, H.; Sousaraei, A.; Cabanillas-Gonzalez, J. A near infrared light emitting electrochemical cell with a 2.3 V turn-on voltage. Sci. Rep. 2019, 9, 228.
- Liu, Q.; Kanahashi, K.; Matsuki, K.; Manzhos, S.; Feron, K.; Bottle, S.E.; Tanaka, K.; Nanseki, T.; Takenobu, T.; Tanaka, H.; et al. Triethylene Glycol Substituted Diketopyrrolopyrroleand Isoindigo-Dye Based Donor–Acceptor Copolymers for Organic Light-Emitting Electrochemical Cells and Transistors. Adv. Electron. Mater. 2020, 6, 1901414.
- Mone, M.; Tang, S.; Genene, Z.; Murto, P.; Jevric, M.; Zou, X.; Ràfols-Ribé, J.; Abdulahi, B.A.; Wang, J.; Mammo, W.; et al. Near-Infrared Emission by Tuned Aggregation of a Porphyrin Compound in a Host–Guest Light-Emitting Electrochemical Cell. Adv. Opt. Mater. 2021, 9, 2001701.
- Xiong, W.; Tang, S.; Murto, P.; Zhu, W.; Edman, L.; Wang, E. Combining Benzotriazole and Benzodithiophene Host Units in Host-Guest Polymers for Efficient and Stable Near-Infrared Emission from Light-Emitting Electrochemical Cells. Adv. Opt. Mater. 2019, 7, 1900280.
- Vasilopoulou, M.; Fakharuddin, A.; Arquer, F.P.G.; Georgiadou, D.G.; Kim, H.; Yusoff, A.R.M.; Gao, F.; Nazeeruddin, M.K.; Bolink, H.J.; Sargent, E.H. Advances in solution-processed near-infrared light-emitting diodes. Nat. Photon. 2021, 15, 656–669.
- Sun, Y.; Sun, W.; Liu, W.; Li, X.; Yin, J.; Zhou, L. Efficient Nondoped Pure Red/Near-Infrared TADF OLEDs by Designing and Adjusting Double Quantum Wells Structure. ACS Appl. Electron. Mater. 2022, 4, 3615–3622.
- Tu, L.; Xie, Y.; Li, Z.; Tang, B. Aggregation-induced emission: Red and near-infrared organic light-emitting diodes. SmartMat 2021, 2, 326–346.
- Xiao, Y.; Wang, H.; Xie, Z.; Shen, M.; Huang, R.; Miao, Y.; Liu, G.; Yu, T.; Huang, W. NIR TADF emitters and OLEDs: Challenges, progress, and perspectives. Chem. Sci. 2022, 13, 8906–8923.
- Kelley, M.L.; Letton, J.; Simin, G.; Ahmed, F.; Love-Baker, C.A.; Greytak, A.B.; Chandrashekhar, M.V.S. Photovoltaic and Photoconductive Action Due to PbS Quantum Dots on Graphene/SiC Schottky Diodes from NIR to UV. ACS Appl. Electron. Mater. 2020, 2, 134–139.
- Boopathi, K.M.; Hanmandlu, C.; Singh, A.; Chen, Y.-F.; Lai, C.S.; Chu, C.W. UV- and NIR-Protective Semitransparent Smart Windows Based on Metal Halide Solar Cells. ACS Appl. Energy Mater. 2018, 1, 632–637.
- Chen, C.; Zheng, S.; Song, H. Photon management to reduce energy loss in perovskite solar cells. Chem. Soc. Rev. 2021, 50, 7250–7329.
- Leccardi, M.J.I.A.; Chenais, N.A.L.; Ferlauto, L.; Kawecki, M.; Zollinger, E.G.; Ghezzi, D. Photovoltaic organic interface for neuronal stimulation in the near-infrared. Commun. Mater. 2020, 1, 21.
- Zhou, X.; Wang, R.; Xiang, G.; Jiang, S.; Li, L.; Luo, X.; Pang, Y.; Tian, Y. Multi-parametric thermal sensing based on NIR emission of Ho(III) doped CaWO4 phosphors. Opt. Mater. 2017, 66, 12–16.
- Shang, K.; He, W.; Sun, J.; Hu, D.; Liu, J. Synthesis, crystal structure and Near-infrared luminescence of rare earth metal (YIII, ErIII, HoIII) complexes containing semi-rigid tricarboxylic acid ligand. J. Mol. Struct. 2021, 1246, 131097.
- Wu, J.; Pan, X.; Wen, L.; Luo, L.; Zhou, Q. Design a rare-earth free broadband NIR phosphor and improve the photoluminescence intensity by alkali charge compensation. Mater. Today Commun. 2022, 30, 102997.
- Rao, V.R.; Jayasankar, C.K. Spectroscopic investigations on multi-channel visible and NIR emission of Sm3+-doped alkali-alkaline earth fluoro phosphate glasses. Opt. Mater. 2019, 91, 7–16.
- Wang, S.-F.; Su, B.-K.; Wang, X.-Q.; Wei, Y.-C.; Kuo, K.-H.; Wang, C.-H.; Liu, S.-H.; Liao, L.-S.; Hung, W.-Y.; Fu, L.-W.; et al. Polyatomic molecules with emission quantum yields >20% enable efficient organic light-emitting diodes in the NIR(II) window. Nat. Photon. 2022, 16, 843–850.
- Xiong, W.; Meng, F.; You, C.; Wang, P.; Yu, J.; Wu, X.; Pei, Y.; Zhu, W.; Wang, Y.; Su, S. Molecular Isomeric Engineering of Naphtyl-quinoline-Containing Dinuclear Platinum Complexes to Tune Emission from Deep Red to Near Infrared. J. Mater. Chem. C 2019, 7, 630–638.
- Zhu, Z.-L.; Tan, J.-H.; Chen, W.-C.; Yuan, Y.; Fu, L.-W.; Cao, C.; You, C.-J.; Ni, S.-F.; Chi, Y.; Lee, C.-S. High Performance NIR OLEDs with Low Efficiency Roll-Off by Leveraging Os(II) Phosphors and Exciplex Co-Host. Adv. Funct. Mater. 2021, 31, 2102.
- Penconi, M.; Kajjam, A.B.; Jung, M.-C.; Cazzaniga, M.; Baldoli, C.; Ceresoli, D.; Thompson, M.E.; Bossi, A. Advancing Near-Infrared Phosphorescence with Heteroleptic Iridium Complexes Bearing a Single Emitting Ligand: Properties and Organic Light-Emitting Diode Applications. Chem. Mater. 2022, 34, 574–583.
- Hu, Y.; Yuan, Y.; Shi, Y.; Lin, J.; Jiang, Z.; Liao, L. Efficient near-infrared organic light-emitting diodes based on a bipolar host. J. Mater. Chem. C 2018, 6, 1407–1412.
- Liu, Y.; Yang, J.; Mao, Z.; Chen, X.; Yang, Z.; Ge, X.; Peng, X.; Zhao, J.; Su, S.-J.; Chi, Z. Asymmetric Thermally Activated Delayed Fluorescence Emitter for Highly Efficient Red/Near-Infrared Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2022, 14, 33606–33613.
- Yu, Y.; Xing, H.; Liu, D.; Zhao, M.; Sung, H.H.-Y.; Williams, I.D.; Lam, J.W.Y.; Xie, G.; Zhao, Z.; Tang, B.Z. Solution-processed AIEgen NIR OLEDs with EQE Approaching 15%. Angew. Chem. Int. Ed. 2022, 61, 202204279.
- Yang, R.Q.; Tian, R.Y.; Yan, J.G.; Zhang, Y.; Yang, J.; Hou, Q.; Yang, W.; Zhang, C.; Cao, Y. Deep-Red Electroluminescent Polymers: Synthesis and Characterization of New Low-Band-Gap Conjugated Copolymers for Light-Emitting Diodes and Photovoltaic Devices. Macromolecules 2005, 38, 244–253.
- Li, P.; Fenwick, O.; Yilmaz, S.; Breusov, D.; Caruana, D.J.; Allard, S.; Scherf, U.; Cacialli, F. Dual functions of a novel low-gap polymer for near infrared photovoltaics and light-emitting diodes. Chem. Commun. 2011, 47, 8820–8822.
- Crossley, D.L.; Urbano, L.; Neumann, R.; Bourke, S.; Jones, J.; Dailey, L.A.; Green, M.; Humphries, M.J.; King, S.M.; Turner, M.L.; et al. Post-polymerization C−H Borylation of Donor−Acceptor Materials Gives Highly Efficient Solid State Near-Infrared Emitters for Near-IROLEDs and Effective Biological Imaging. ACS Appl. Mater. Interfaces 2017, 9, 28243–28249.
- Du, X.; Qi, J.; Zhang, Z.; Ma, D.; Wang, Z.Y. Efficient Non-doped Near Infrared Organic Light-Emitting Devices Based on Fluorophores with Aggregation-Induced Emission Enhancement. Chem. Mater. 2012, 24, 2178–2185.
- Cao, L.; Li, J.; Zhu, Z.-Q.; Huang, L.; Li, J. Stable and Efficient Near-Infrared Organic Light-Emitting Diodes Employing a Platinum(II) Porphyrin Complex. ACS Appl. Mater. Interfaces 2021, 13, 60261–60268.
- Chen, Z.; Zhang, H.; Wen, D.; Wu, W.; Zeng, Q.; Chen, S.; Wong, W.-Y. A simple and efficient approach toward deep-red to near-infrared-emitting iridium(III) complexes for organic light-emitting diodes with external quantum efficiencies of over 10%. Chem. Sci. 2020, 11, 2342–2349.
- Zhang, H.; Chen, Z.; Zhu, L.; Wu, Y.; Xu, Y.; Chen, S.; Wong, W.-Y. High Performance NIR OLEDs with Emission Peak Beyond 760 nm and Maximum EQE of 6.39%. Adv. Optical Mater. 2022, 10, 2200111.
- Shen, L.; Wu, Q.; Lu, J.; Zhao, H.; Liu, H.; Meng, Q.; Li, X. Design of potential singlet fission chromophores based on diketofurofuran: An alternative to diketopyrrolopyrrole. J. Mater. Chem. C 2022, 10, 10404–10411.
- Zhang, X.; Wang, Z.; Hou, Y.; Yan, Y.; Zhao, J.; Dick, B. Recent development of heavy-atom-free triplet photosensitizers: Molecular structure design, photophysics and application. J. Mater. Chem. C 2021, 9, 11944–11973.
- Song, Y.; Yu, R.; Meng, X.; He, L. Donor-σ-acceptor molecules with alkyl σ-linkers of different lengths: Exploration on the exciplex emission and their use for efficient organic light-emitting diodes. Dyes Pigments 2022, 208, 110876.
- Yee, N.; Dadvand, A.; Perepichka, D.F. Band gap engineering of donor–acceptor co-crystals by complementary two-point hydrogen bonding. Mater. Chem. Front. 2020, 4, 3669–3677.
- Pschirer, N.G.; Kohl, C.; Nolde, F.; Qu, J.; Mullen, K. Pentarylene- and Hexarylenebis(dicarboximide)s: Near-Infrared-Absorbing Polyaromatic Dyes. Angew. Chem. Int. Ed. 2006, 45, 1401.
- Muller, S.; Mullen, K. Expanding benzene to giant graphenes: Towards molecular devices. Philos. Trans. R. Soc. A 2007, 365, 1453–1472.
- Zhang, X.; Chen, X.; Zhao, J. Electron spin-controlled charge transfer and the resulting long-lived charge transfer state: From transition metal complexes to organic compounds. Dalton Trans. 2021, 50, 59–67.
- Carbas, B.B.; NOORI, H.A.; Kavak, E.; Kaya, Y.; Kıvrak, A. Optical, electrochemical and DFT studies of donor-acceptor typed indole derivatives. J. Mol. Struct. 2023, 1271, 134129.
- Zhu, Y.; Qu, C.; Ye, J.; Xu, Y.; Zhang, Z.; Wang, Y. Donor-Acceptor Type of Fused-Ring Thermally Activated Delayed Fluorescence Compounds Constructed through an Oxygen-Containing Six-Membered Ring. ACS Appl. Mater. Interfaces 2022, 14, 47971–47980.
- Dufresne, S.; Bourgeaux, M.; Skene, W.G. Tunable spectroscopic and electrochemical properties of conjugated push-push, push-pull and pull-pull thiopheno azomethines. J. Mater. Chem. 2007, 17, 1166–1177.
- Scharber, M.C.; Sariciftci, N.S. Low Band Gap Conjugated Semiconducting Polymers. Adv. Mater. Technol. 2021, 6, 2000857.
- Brutting, W.; Frischeisen, J.; Scholz, B.J.; Schmidt, T.D. More light from organic light-emitting diodes Europhys. News 2011, 42, 20–24.
- Kertesz, M. Pancake Bonding: An Unusual Pi-Stacking Interaction. Chem. Eur. J. 2019, 25, 400–416.
- Bulovic, V.; Kozlov, V.G.; Khalfin, V.B.; Forrest, S.R. Transform-Limited, Narrow-Linewidth Lasing Action in Organic Semiconductor Microcavities. Science 1998, 279, 553–555.
- Xu, S.; Yang, D.; Sun, L.; Lv, W.; Wu, X.; Wei, Y.; Fang, X.; Song, X.; Wang, Y.; Tang, Y.; et al. Toward an Ultrahigh-Performance Near-Infrared Photoresponsive Field-Effect Transistor Using a Lead Phthalocyanine/MoS2 Organic-Inorganic Planar Heterojunction. ACS Appl. Electron. Mater. 2022, 4, 2777–2786.
- Cranston, R.R.; Lessard, B.H. Metal phthalocyanines: Thin-film formation, microstructure, and physical properties. RSC Adv. 2021, 11, 21716–21737.
- Bai, Q.; Zhang, C.; Song, J.; Liu, J.; Feng, Y.; Duan, L.; Cheng, C. Metal-free phthalocyanine single crystal: Solvothermal synthesis and near-infrared electroluminescence. Chin. Chem. Lett. 2016, 27, 764–768.
- Pun, A.B.; Sanders, S.N.; Sfeir, M.Y.; Campos, L.M.; Congreve, D.N. Annihilator dimers enhance triplet fusion upconversion. Chem. Sci. 2019, 10, 3969–3975.
- Yang, L.; Chua, X.W.; Yang, Z.; Ding, X.; Yu, Y.; Suwardi, A.; Zhao, M.; Ke, K.L.; Ehrler, B.; Di, D. Photon-upconverters for blue organic light emitting diodes: A low-cost, sky-blue example. Nanoscale Adv. 2022, 4, 1318–1323.
- Tang, X.; Liu, H.; Xu, L.; Xu, X.; He, X.; Liu, F.; Chen, J.; Peng, Q. Achieving High Efficiency at High Luminance in Fluorescent Organic Light-Emitting Diodes through Triplet-Triplet Fusion Based on Phenanthroimidazole-Benzothiadiazole Derivatives. Chem. Eur. J. 2021, 27, 13828–13839.
- Xue, J.; Li, C.; Xin, L.; Duan, L.; Qiao, J. High-efficiency and low efficiency roll-off near-infrared fluorescent OLEDs through triplet fusion. Chem. Sci. 2016, 7, 2888–2895.
- Gu, Q.; Abdurahman, A.; Friend, R.H.; Li, F. Polymer Light Emitting Diodes with Doublet Emission. J. Phys. Chem. Lett. 2020, 11, 5638–5642.
- Jayabharathi, J.; Thilagavathy, S.; Thanikachalam, V.; Anudeebhana, J. A triphenylacrylonitrile phenanthroimidazole cored butterfly shaped AIE chromophore for blue and HLCT sensitized fluorescent OLEDs. J. Mater. Chem. C 2022, 10, 4342–4354.
- Liu, Y.; Liu, H.; Bai, Q.; Du, C.; Shang, A.; Jiang, S.; Tang, X.; Lu, P. Pyreneimidazole-Based Derivatives with Hybridized Local and Charge-Transfer State for Highly Efficient Blue and White Organic Light-Emitting Diodes with Low Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2020, 12, 16715–16725.
- Usta, H.; Cosut, B.; Alkan, F. Understanding and Tailoring Excited State Properties in SolutionProcessable Oligo(p-phenyleneethynylene)s: Highly Fluorescent Hybridized Local and Charge Transfer Character via Experiment and Theory. J. Phys. Chem. B 2021, 125, 11717–11731.




