Conjugated polymers with fluorescence units have attracted a multitude of attention due to the academic and commercial value when used as the active materials in PLEDs [
76]. The turn-on voltage, color purity, and stability of the devices should be optimized to accommodate PLEDs. Some of the principal advantages of conjugated polymers are easy manufacture, solution processability, low-cost, flexibility and suitability to form large area surfaces [
77]. The synthetic organic flexibility is the most obvious feature of the conjugated polymers. Through the manipulation of the structures of the monomer and polymer, the physical, thermal, optical, and electrochemical properties could be adjusted for specific applications.
For conjugated polymer, the value of the energy level band gap is the key to their performance in PLEDs. The most common and effective approach is introducing D-A units consisting of various donors and acceptors to tune the HOMO-LUMO levels. Another approach to lower the band gap is utilizing the polar effect caused by the heavy atoms. The notable cases of decreasing the energy gap involve the replacement of oxygen and sulfur atoms with heavier ones like selenium and tellurium in a conjugated system [
71].
4. NIR Fluorescent Materials Based on Small Molecules
Due to the parity-forbidden radiative 4f-4f transitions of the rare earth ions, the corresponding LEDs usually have a nonmeasurable or very low EQE and low power output. In contrast, the luminescence of organic molecules originates from their allowed S1–S0 transitions and thus free from the luminescence efficiency limitation. By using phosphorescent heavy metal complexes that can effectively harvest both the singlet and triplet excitons. Unfortunately, the EL quantum efficiency drops rapidly at high current densities.
Initially, attempts were made to construct NIR luminescent materials using molecules with a large area of conjugated systems. In 2006, Kageyama et al. [95] investigated that OLED using tris(8-quinolinolato)aluminum (Alq3) highly doped with N,N′ -bis(neopentyl)-3,4:9,10-perylenebis(dicarboximide) as an emitting layer exhibit near-infrared EL with a peak at 805 nm originating from N,N′ -bis(neopentyl)-3,4:9,10-perylenebis(dicarboximide) aggregates. Phthalocyanines are known to be organic semiconductors and have attracted much attention because of their high chemical stability, various synthetic modifications, epitaxial growth of thin films by organic molecular beam epitaxy and unique absorption bands extending from the ultraviolet region to infrared region [96,97]. Cheng et al. [98] reported the OLED device used purple phthalocyanine single crystal as an active light-emitting layer with the emission of 936 nm.
5. NIR Phosphorescent Materials Based on Small Molecules
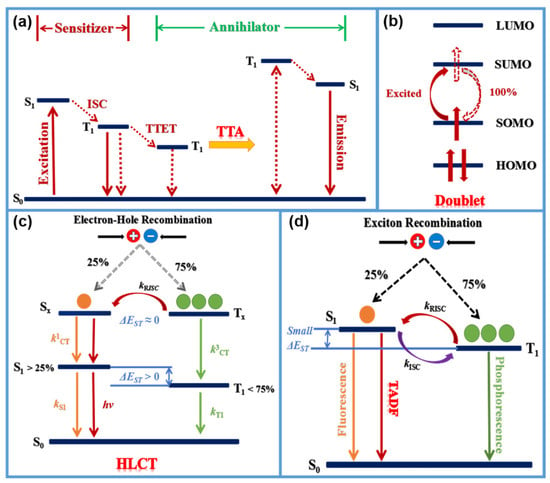
In general, holes and electrons injected from electrodes to emitters generate excitons, and the excitons are classified into singlet and triplet excitons that are formed at a ratio of 1:3. In the case of fluorescent emitting materials, only singlet excitons can be transformed into photons, and so only 25% internal quantum efficiency (QE) is theoretically possible, where the remaining 75% of non-radiation energy is lost. Therefore, breaking spin statistics to utilize the other 75% triplet energy is the key factor to improving OLED efficiency.
As discussed previously, there are several approaches to design high efficiency materials with NIR emitting through utilizing triplet energy. One of these is to harness the triplet excitons of organic fluorescent materials involves triplet fusion (TF) [
133,
134,
135]. The theoretical maximum singlet exciton production yield through TF is 50%, which would contribute a maximum radiative exciton ratio of up to 62.5%. To enable highly efficient NIR-OLEDs through TF, Qiao et al. [
136] used the more feasible approach of efficient TF via the host rather than direct TF from the emitter, since the triplet excitons of the NIR emitter may decay dominantly via non-radiative transition according with the energy gap law. They realized high performance NIR-OLEDs via the high-efficiency TF of a bipolar host doped with a special naphthoselenadiazole emitter 4,9-bis(4-(2,2-diphenylvinyl)phenyl)naphtho[2,3-c][1,2,5]selenadiazole. Unlike typical NIR organic D-A chromophores, 4,9-bis(4-(2,2-diphenylvinyl)phenyl)naphtho[2,3-c][1,2,5]selenadiazole features a non-D-A structure and a very large HOMO/LUMO overlap, displaying strong deep-red to NIR emitting and unique ambipolar character. The corresponding photoluminescence quantum efficiency of NSeD reached 52% in solution and retained 17% in the blend film. The optimized NIR-OLEDs demonstrated a strong emission at 700 nm with a high
EQEmax of 2.1% and the
EQE remained at around 2% over a wide range of current densities from 18 to 200 mA cm
−2. However, this method would lose some of the energy of triplet excitons, so the quantum efficiency in theory is not 100%.
For standard closed-shell organic semiconductors, holes and electrons occupy the HOMO and LUMO respectively, and recombine to form singlet or triplet excitons. The radical emitter has a SOMO in the ground state, giving an overall spin 1/2 dipole. In the high energy ground state, where both electrons and holes occupy the SOMO level, recombination returns the system to the ground state and does not emit light. However, in 2015, Li et al. [
49] achieved selective hole injection into HOMO and electron injection into SOMO to form a fluorescent two-photon excited state with near unit internal quantum efficiency and proposed an open-shell organic molecule 9-(4-(bis(2,4,6-trichlorophenyl)methyl)-3,5-dichlorophenyl)-9H-carbazole as an NIR-emitter of OLEDs.
The hybridized local and charge-transfer excited state (HLCT) possesses two combined and compatible characteristics with a large transition moment from a local excited (LE) state and a weakly bound exciton from a charge transfer (CT) state [
137,
138,
139]. The former contributes to a high-efficiency radiation of fluorescence, while the latter is responsible for the generation of a high fraction of singlet excitons. The twisting D-A molecule may be an ideal carrier to realize this strategy that may possess two combined and compatible characteristics with large transition moment from LE state and weakly bound exciton from CT state.
6. Conclusions
Shifting the spectral range of OLEDs/PLEDs from the visible to the NIR region of the electromagnetic spectrum is of great interest. To date, much efforts have been made to develop NIR phosphorescent OLEDs/PLEDs using transition metal complexes. However, high costs, limited resources of phosphorescent materials, and efficiency roll-offs at high current densities remain challenges for their applications in long-term mass production. To reduce cost and improve environmental sustainability, the development of highly efficient OLEDs/PLEDs that does not rely on heavy metal-containing compounds remains an important need. As an alternative material system, the “heavy metal-free” NIR fluorophores have been widely investigated for their cost advantage and versatility in tuning molecules. However, the EQE of traditional organic near-infrared fluorescent OLEDs is generally about 0.1% or even lower due to low exciton utilization rate and low fluorescence quantum yield in solid state, which has become an almost insurmountable obstacle for their further development. Therefore, several strategies have been proposed to realize high quantum efficiency in pure organic dyes by utilizing triplet energy. Nevertheless, this research field is still in its infancy, and while many examples harvesting triplet excitons are reported, only a few studies have focused on their NIR emission, particularly in terms of OLEDs/PLEDs applications.