
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kannappan Arunachalam | -- | 3511 | 2023-02-15 04:51:11 | | | |
| 2 | Lindsay Dong | Meta information modification | 3511 | 2023-02-16 07:12:09 | | |
Video Upload Options
Biofilms enable pathogenic bacteria to survive in unfavorable environments. As biofilm-forming pathogens can cause rapid food spoilage and recurrent infections in humans, especially their presence in the food industry is problematic. Using chemical disinfectants in the food industry to prevent biofilm formation raises serious health concerns. Further, the ability of biofilm-forming bacterial pathogens to tolerate disinfection procedures questions the traditional treatment methods. Thus, there is a dire need for alternative treatment options targeting bacterial pathogens, especially biofilms. As clean-label products without carcinogenic and hazardous potential, natural compounds with growth and biofilm-inhibiting and biofilm-eradicating potentials have gained popularity as natural preservatives in the food industry. However, the use of these natural preservatives in the food industry is restricted by their poor availability, stability during food processing and storage. Also there is a lack of standardization, and unattractive organoleptic qualities. Nanotechnology is one way to get around these limitations and as well as the use of underutilized bioactives. The use of nanotechnology has several advantages including traversing the biofilm matrix, targeted drug delivery, controlled release, and enhanced bioavailability, bioactivity, and stability. The nanoparticles used in fabricating or encapsulating natural products are considered as an appealing antibiofilm strategy since the nanoparticles enhance the activity of the natural products against biofilms of foodborne bacterial pathogens.
1. Introduction
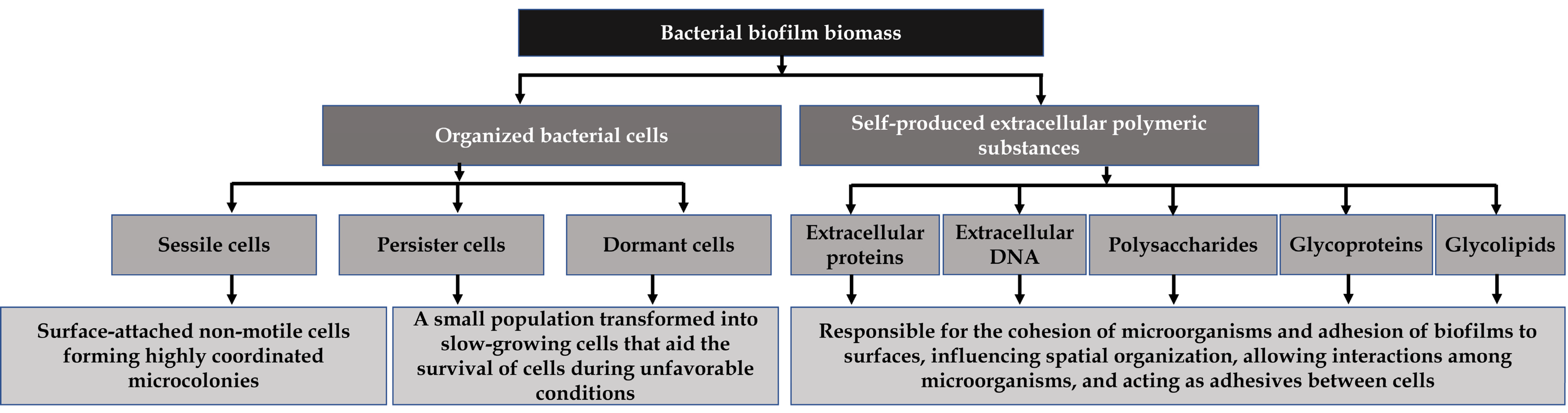
2. Significance of Pathogenic Bacteria and Their Biofilms in the Food Industry
3. Natural Products as a Promising Alternate
3.1. Natural Bioactives as Preservatives
Several natural sources like plants [13], animals [21], microorganisms [22], bacteriocins [23], and even bacteriophages [24] are being exploited for the identification of bioactive leads against the growth and biofilm formation of foodborne pathogens, which consequently ended up with an overabundance of compounds [22][25]. Phytocompounds are one of the bioactive secondary metabolites that might be used as natural food preservatives. Herbal and plant medicines continue to garner interest as potential therapeutics.
Essential oils are highly concentrated, volatile, hydrophobic chemicals present in a wide variety of plants. The hydroxyl groups of essential oil components, such as those in thymol, carvacrol, and eugenol, react with the phospholipid bilayer of microorganisms resulting in leakage of ions, nucleic acids, and ATP and water imbalance, leading to cell death [25]. The said compounds at sub-MIC were reported to target the biofilm formation of bacterial pathogens in a concentration-dependent manner.
Furthermore, the bioactives from thyme and rosemary plants reduce the biofilm growth of L. monocytogenes and garlic extract can prevent quorum sensing (QS) signaling in multidrug-resistant bacterial pathogens [25][26]. In addition, flavones under the flavonoid class form a complex with the components of the bacterial cell wall and impede cell adherence and proliferation. In this regard, the genes Staphylococcus accessory regulator (sarA) and intercellular adhesins (ica) are both downregulated by baicalein to suppress the virulence regulation of S. aureus [27].
Glycolipid is a biosurfactant with potential anticancer and antibacterial effects and currently has a wide variety of therapeutic uses [28] including in the pharmaceutical, food, and petroleum sectors. Sophorolipid is one of the glycolipids produced by the yeast Starmerella bombicola that has antibacterial and antibiofilm properties against foodborne pathogens such as C. jejuni, E. coli, Listeria spp., and Salmonella spp. [29][30][31].
3.2. Challenges in the Use of Natural Preservatives for Preservation
4. Nanotechnology Approach
Nanotechnology is a promising technology that has the ability to convert an individual particle to one billionth of its original size. The converted particles are nano-sized (1–100 nm), have a large surface area and mass ratio, and are highly reactive, making them completely different from the exact composition of the bulk material [37]. The converted nanoparticles have many advantages, including an increased impact against the target pathogenic microorganisms with multiple functional sites. The exact antibacterial mechanism of nanoparticles has not been entirely elucidated. Many studies have suggested possible mechanisms of action. The absorption of nanoparticles into the cell membrane and the subsequent disintegration are the initial steps involved in the antibacterial mechanism of nanoparticles [38]. Following absorption and disintegration, the cell-penetrating nanoparticles target bacterial growth through intracellular content leakage, generation of reactive oxygen species, impairment of the electron transport system, inactivation of efflux pumps, and most importantly, interference with the enzymatic and metabolic activities of the cell [38].
4.1. Techniques for Targeting Biofilms through Natural Product Delivery
4.1.1. Biofabrication of Nanoparticles with Antibiofilm Activity Using Surface Functionalization
4.1.2. Nanoencapsulation of Natural Compounds with Antibiofilm Activity
Polymeric Nanoparticles
Lipid Nanoparticles
Silica Nanoparticles
5. Strategies to Enhance Biofilm Clearance
5.1. Size and Surface Modifications
5.2. Stimuli-Responsive Release
5.3. Combined Strategies
References
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306.
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59.
- Arunachalam, K.; Davoodbasha, M. Imaging Bacteria and Biofilm by Field Emission Scanning Electron Microscopy. In Analytical Methodologies for Biofilm Research; Springer Protocols Handbooks; Springer: New York, NY, USA, 2021; pp. 205–222.
- van Wolferen, M.; Orell, A.; Albers, S.-V. Archaeal biofilm formation. Nat. Rev. Microbiol. 2018, 16, 699–713.
- Cámara, M.; Green, W.; MacPhee, C.E.; Rakowska, P.D.; Raval, R.; Richardson, M.C.; Slater-Jefferies, J.; Steventon, K.; Webb, J.S. Microbiomes. Economic significance of biofilms: A multidisciplinary and cross-sectoral challenge. Npj Biofilms Microbiomes 2022, 8, 42.
- Alvarez-Ordóñez, A.; Coughlan, L.M.; Briandet, R.; Cotter, P.D. Biofilms in food processing environments: Challenges and opportunities. Annu. Rev. Food Sci. Technol. 2019, 10, 173–195.
- Galie, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898.
- Rodríguez-Campos, D.; Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Persistent Listeria monocytogenes isolates from a poultry-processing facility form more biofilm but do not have a greater resistance to disinfectants than sporadic strains. Pathogens 2019, 8, 250.
- Cadena, M.; Kelman, T.; Marco, M.L.; Pitesky, M.J.F. Understanding antimicrobial resistance (AMR) profiles of Salmonella biofilm and planktonic bacteria challenged with disinfectants commonly used during poultry processing. Foods 2019, 8, 275.
- Pang, X.; Song, X.; Chen, M.; Tian, S.; Lu, Z.; Sun, J.; Li, X.; Lu, Y.; Yuk, H.-G. Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1657–1676.
- Uddin Mahamud, A.G.M.S.; Nahar, S.; Ashrafudoulla, M.; Park, S.H.; Ha, S.-D. Insights into antibiofilm mechanisms of phytochemicals: Prospects in the food industry. Crit. Rev. Food Sci. Nutr. 2022.
- Deng, Y.; Liu, Y.; Li, J.; Wang, X.; He, S.; Yan, X.; Shi, Y.; Zhang, W.; Ding, L. Marine natural products and their synthetic analogs as promising antibiofilm agents for antibiotics discovery and development. Eur. J. Med. Chem. 2022, 239, 114513.
- Martinengo, P.; Arunachalam, K.; Shi, C.J.F. Polyphenolic Antibacterials for Food Preservation: Review, Challenges, and Current Applications. Foods 2021, 10, 2469.
- Chitlapilly Dass, S.; Wang, R. Biofilm through the Looking Glass: A Microbial Food Safety Perspective. Pathogens 2022, 11, 346.
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585.
- Lin, Y.; Briandet, R.; Kovács, Á.T. Bacillus cereus sensu lato biofilm formation and its ecological importance. Biofilm 2022, 4, 100070.
- Liu, X.-Y.; Hu, Q.; Xu, F.; Ding, S.-Y.; Zhu, K. Characterization of Bacillus cereus in dairy products in China. Toxins 2020, 12, 454.
- Carter, M.Q.; Feng, D.; Li, H.H. Curli fimbriae confer shiga toxin-producing Escherichia coli a competitive trait in mixed biofilms. Food Microbiol. 2019, 82, 482–488.
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825.
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabulsi, A.A.; Abu Ghoush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292.
- Arteaga, V.; Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Antimicrobial activity of apitoxin from Apis mellifera in Salmonella enterica strains isolated from poultry and its effects on motility, biofilm formation and gene expression. Microb. Pathog. 2019, 137, 103771.
- Srinivasan, R.; Kannappan, A.; Shi, C.; Lin, X. Marine bacterial secondary metabolites: A treasure house for structurally unique and effective antimicrobial compounds. Mar. Drugs 2021, 19, 530.
- Camargo, A.C.; de Paula, O.A.; Todorov, S.D.; Nero, L.A. In vitro evaluation of bacteriocins activity against Listeria monocytogenes biofilm formation. Appl. Biochem. Biotechnol. 2016, 178, 1239–1251.
- Abdelhadi, I.M.A.; Sofy, A.R.; Hmed, A.A.; Refaey, E.E.; Soweha, H.E.; Abbas, M.A. Discovery of Polyvalent Myovirus (vB_STM-2) Phage as a natural antimicrobial system to lysis and biofilm removal of Salmonella Typhimurium Isolates from various food sources. Sustainability 2021, 13, 11602.
- Martínez-Graciá, C.; González-Bermúdez, C.A.; Cabellero-Valcárcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015, 6, 38–43.
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619.
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein inhibits Staphylococcus aureus biofilm formation and the quorum sensing system in vitro. PLoS ONE 2016, 11, e0153468.
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of Glycolipid Biosurfactants and Glycolipid-Modified Materials to Antimicrobial Strategy: A Review. Pharmaceutics 2021, 13, 227.
- Silveira, V.A.I.; Nishio, E.K.; Freitas, C.A.U.Q.; Amador, I.R.; Kobayashi, R.K.T.; Caretta, T.; Macedo, F.; Celligoi, M.A.P. Production and antimicrobial activity of sophorolipid against Clostridium perfringens and Campylobacter jejuni and their additive interaction with lactic acid. Biocatal. Agric. Biotechnol. 2019, 21, 101287.
- Zhang, X.; Ashby, R.; Solaiman, D.K.Y.; Uknalis, J.; Fan, X. Inactivation of Salmonella spp. and Listeria spp. by palmitic, stearic, and oleic acid sophorolipids and thiamine dilauryl sulfate. Front. Microbiol. 2016, 7, 2076.
- Elgamoudi, B.A.; Korolik, V. Campylobacter Biofilms: Potential of Natural Compounds to Disrupt Campylobacter jejuni Transmission. Int. J. Mol. Sci. 2021, 22, 12159.
- Hintz, T.; Matthews, K.K.; Di, R. The Use of Plant Antimicrobial Compounds for Food Preservation. BioMed Res. Int. 2015, 2015, 246264.
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1147, 132–136.
- Driessen, A.J.M.; van den Hooven, H.W.; Kuiper, W.; Van de Camp, M.; Sahl, H.-G.; Konings, R.N.H.; Konings, W.N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry 1995, 34, 1606–1614.
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247.
- Alvarez, M.; Moreira, M.d.R.; Roura, S.; Ayala-Zavala, J.; González-Aguilar, G.A. Using Natural Antimicrobials to Enhance the Safety and Quality of Fresh and Processed Fruits and Vegetables: TYPES of Antimicrobials. In Handbook of Natural Antimicrobials for Food Safety and Quality; Woodhead Publishing: Cambridge, UK, 2015; pp. 287–313.
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769.
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65.
- Barros, C.H.N.; Devlin, H.; Hiebner, D.W.; Vitale, S.; Quinn, L.; Casey, E. Enhancing curcumin’s solubility and antibiofilm activity via silica surface modification. Nanoscale Adv. 2020, 2, 1694–1708.
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Effic. Technol. 2017, 3, 516–527.
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600.
- Zahoor, M.; Nazir, N.; Iftikhar, M.; Naz, S.; Zekker, I.; Burlakovs, J.; Uddin, F.; Kamran, A.W.; Kallistova, A.; Pimenov, N.; et al. A review on silver nanoparticles: Classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 2021, 13, 2216.
- Lotha, R.; Shamprasad, B.R.; Sundaramoorthy, N.S.; Nagarajan, S.; Sivasubramanian, A. Biogenic phytochemicals (cassinopin and isoquercetin) capped copper nanoparticles (ISQ/) inhibits MRSA biofilms. Microb. Pathog. 2019, 132, 178–187.
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403.
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics 2020, 12, 298.
- Elbialy, N.S.; Aboushoushah, S.F.; Sofi, B.F.; Noorwali, A. Multifunctional curcumin-loaded mesoporous silica nanoparticles for cancer chemoprevention and therapy. Microporous Mesoporous Mater. 2020, 291, 109540.
- Di, J.; Kim, J.; Hu, Q.; Jiang, X.; Gu, Z. Spatiotemporal drug delivery using laser-generated-focused ultrasound system. J. Control. Release 2015, 220, 592–599.
- Sirivisoot, S.; Harrison, B.S. Magnetically stimulated ciprofloxacin release from polymeric microspheres entrapping iron oxide nanoparticles. Int. J. Nanomed. 2015, 10, 4447–4458.
- Wang, Z.; Chen, Z.; Gao, N.; Ren, J.; Qu, X. Transmutation of personal glucose meters into portable and highly sensitive microbial pathogen detection platform. Small 2015, 11, 4970–4975.
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7.
- Pircalabioru, G.G.; Chifiriuc, M.-C. Nanoparticulate drug-delivery systems for fighting microbial biofilms: From bench to bedside. Future Microbiol. 2020, 15, 679–698.
- Michalak, G.; Głuszek, K.; Piktel, E.; Deptuła, P.; Puszkarz, I.; Niemirowicz, K.; Bucki, R. Polymeric nanoparticles—A novel solution for delivery of antimicrobial agents. Med. Stud. 2016, 32, 56–62.
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20.
- Siddhardha, B.; Pandey, U.; Kaviyarasu, K.; Pala, R.; Syed, A.; Bahkali, A.H.; Elgorban, A.M. Chrysin-loaded chitosan nanoparticles potentiates antibiofilm activity against Staphylococcus aureus. Pathogens 2020, 9, 115.
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm effect of cinnamaldehyde-chitosan nanoparticles against the biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403.
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515.
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and thyme essential oils encapsulated in chitosan nanoparticles as effective antimicrobial agents against foodborne pathogens. Molecules 2021, 26, 4055.
- Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Antibacterial and antibiofilm activities of chitosan nanoparticles loaded with Ocimum basilicum L. essential oil. Int. J. Biol. Macromol. 2022, 202, 122–129.
- Khan, F.; Yu, H.; Kim, Y.-M. Bactericidal activity of usnic acid-chitosan nanoparticles against persister cells of biofilm-forming pathogenic bacteria. Mar. Drugs 2020, 18, 270.
- Pompilio, A.; Riviello, A.; Crocetta, V.; Giuseppe, F.D.; Pomponio, S.; Sulpizio, M.; Ilio, C.D.; Angelucci, S.; Barone, L.; Giulio, A.D.; et al. Evaluation of antibacterial and antibiofilm mechanisms by usnic acid against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2016, 11, 1315–1338.
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: Current applications and future prospects. Molecules 2014, 19, 14066–14079.
- del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193.
- Ahmed, K.; Muiruri, P.W.; Jones, G.H.; Scott, M.J.; Jones, M.N. The effect of grafted poly(ethylene glycol) on the electrophoretic properties of phospholipid liposomes and their adsorption to bacterial biofilms. Colloids Surf. A Physicochem. Eng. Asp. 2001, 194, 287–296.
- Wang, D.-Y.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 2020, 7, 872.
- Ribeiro, L.N.d.M.; de Paula, E.; Rossi, D.A.; Martins, F.A.; de Melo, R.T.; Monteiro, G.P.; Breitkreitz, M.C.; Goulart, L.R.; Fonseca, B.B. Nanocarriers from natural lipids with in vitro activity against Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2021, 10, 571040.
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22.
- Wang, Y. Liposome as a delivery system for the treatment of biofilm-mediated infections. J. Appl. Microbiol. 2021, 131, 2626–2639.
- Cui, H.; Li, W.; Li, C.; Vittayapadung, S.; Lin, L. Liposome containing cinnamon oil with antibacterial activity against methicillin-resistant Staphylococcus aureus biofilm. Biofouling 2016, 32, 215–225.
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Spósito, L.; De Toledo, L.G.; Bonifácio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213.
- Rukavina, Z.; Vanić, Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, 18.
- Wu, Y.; Long, Y.; Li, Q.-L.; Han, S.; Ma, J.; Yang, Y.-W.; Gao, H. Layer-by-layer (LBL) self-assembled biohybrid nanomaterials for efficient antibacterial applications. ACS Appl. Mater. Interfaces 2015, 7, 17255–17263.
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534.
- Balaure, P.C.; Boarca, B.; Popescu, R.C.; Savu, D.; Trusca, R.; Vasile, B.Ș.; Grumezescu, A.M.; Holban, A.M.; Bolocan, A.; Andronescu, E. Bioactive mesoporous silica nanostructures with anti-microbial and anti-biofilm properties. Int. J. Pharm. 2017, 531, 35–46.
- Thorn, C.R.; Carvalho-Wodarz, C.d.S.; Horstmann, J.C.; Lehr, C.-M.; Prestidge, C.A.; Thomas, N. Tobramycin liquid crystal nanoparticles eradicate cystic fibrosis-related Pseudomonas aeruginosa biofilms. Small 2021, 17, 2100531.
- Nosrati, M.; Ranjbar, R. Investigation of the antibacterial and biofilm inhibitory activities of Prangos acaulis (DC.) Bornm in nanoparticulated formulation. Nanotechnology 2022, 33, 385103.
- Dalcin, A.J.F.; Santos, C.G.; Gündel, S.S.; Roggia, I.; Raffin, R.P.; Ourique, A.F.; Santos, R.C.V.; Gomes, P. Anti biofilm effect of dihydromyricetin-loaded nanocapsules on urinary catheter infected by Pseudomonas aeruginosa. Colloids Surf. B Biointerfaces 2017, 156, 282–291.
- Lima, R.A.; de Souza, S.L.X.; Lima, L.A.; Batista, A.L.X.; de Araújo, J.T.C.; Sousa, F.F.O.; Rolim, J.; Bandeira, T. Antimicrobial effect of anacardic acid-loaded zein nanoparticles loaded on Streptococcus mutans biofilms. Braz. J. Microbiol. 2020, 51, 1623–1630.
- Pu, C.; Tang, W. The antibacterial and antibiofilm efficacies of a liposomal peptide originating from rice bran protein against Listeria monocytogenes. Food Funct. 2017, 8, 4159–4169.
- Liu, Y.; Shi, L.; Su, L.; van der Mei, H.C.; Jutte, P.C.; Ren, Y.; Busscher, H.J. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem. Soc. Rev. 2019, 48, 428–446.
- Peulen, T.O.; Wilkinson, K.J. Diffusion of nanoparticles in a biofilm. Environ. Sci. Technol. 2011, 45, 3367–3373.
- MubarakAli, D.; Arunkumar, J.; Pooja, P.; Subramanian, G.; Thajuddin, N.; Alharbi, N.S. Synthesis and characterization of biocompatibility of tenorite nanoparticles and potential property against biofilm formation. Saudi Pharm. J. 2015, 23, 421–428.
- Forier, K.; Messiaen, A.-S.; Raemdonck, K.; Nelis, H.; De Smedt, S.; Demeester, J.; Coenye, T.; Braeckmans, K. Probing the size limit for nanomedicine penetration into Burkholderia multivorans and Pseudomonas aeruginosa biofilms. J. Control. Release 2014, 195, 21–28.
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12.
- Jiang, F.; Deng, Y.; Yeh, C.-K.; Sun, Y. Quaternized chitosans bind onto preexisting biofilms and eradicate pre-attached microorganisms. J. Mater. Chem. B 2014, 2, 8518–8527.
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S. pH-activated nanoparticles for controlled topical delivery of farnesol to disrupt oral biofilm virulence. ACS Nano 2015, 9, 2390–2404.
- Wang, Y.; Shukla, A. Bacteria-responsive biopolymer-coated nanoparticles for biofilm penetration and eradication. Biomater. Sci. 2022, 10, 2831–2843.
- Liu, Y.; Ren, Y.; Li, Y.; Su, L.; Zhang, Y.; Huang, F.; Liu, J.; Liu, J.; van Kooten, T.G.; An, Y.; et al. Nanocarriers with conjugated antimicrobials to eradicate pathogenic biofilms evaluated in murine in vivo and human ex vivo infection models. Acta Biomater. 2018, 79, 331–343.
- Ding, Y.; Hao, Y.; Yuan, Z.; Tao, B.; Chen, M.; Lin, C.; Liu, P.; Cai, K. A dual-functional implant with an enzyme-responsive effect for bacterial infection therapy and tissue regeneration. Biomater. Sci. 2020, 8, 1840–1854.
- Sims, K.R.; Liu, Y.; Hwang, G.; Jung, H.I.; Koo, H.; Benoit, D.S.W. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale 2018, 11, 219–236.
- Ren, Y.; Liu, H.; Liu, X.; Zheng, Y.; Li, Z.; Li, C.; Yeung, K.W.K.; Zhu, S.; Liang, Y.; Cui, Z.; et al. Photoresponsive Materials for Antibacterial Applications. Cell Rep. Phys. Sci. 2020, 1, 100245.
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888.
- Olakanmi, O.; Britigan, B.E.; Schlesinger, L.S. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 2000, 68, 5619–5627.
- Halwani, M.; Yebio, B.; Suntres, Z.E.; Alipour, M.; Azghani, A.O.; Omri, A. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 62, 1291–1297.
- Domenico, P.; Baldassarri, L.; Schoch, P.E.; Kaehler, K.; Sasatsu, M.; Cunha, B.A. Activities of bismuth thiols against staphylococci and staphylococcal biofilms. Antimicrob. Agents Chemother. 2001, 45, 1417–1421.
- Domenico, P.; Reich, J.; Madonia, W.; Cunha, B.A. Resistance to bismuth among Gram-negative bacteria is dependent upon iron and its uptake. J. Antimicrob. Chemother. 1996, 38, 1031–1040.
- Huang, C.T.; Stewart, P.S. Reduction of polysaccharide production in Pseudomonas aeruginosa biofilms by bismuth dimercaprol (BisBAL) treatment. J. Antimicrob. Chemother. 1999, 44, 601–605.
- Folsom, J.P.; Baker, B.; Stewart, P.S. In vitro efficacy of bismuth thiols against biofilms formed by bacteria isolated from human chronic wounds. J. Appl. Microbiol. 2011, 111, 989–996.
- de Annunzio, S.R.; de Freitas, L.M.; Blanco, A.L.; da Costa, M.M.; Carmona-Vargas, C.C.; de Oliveira, K.T.; Fontana, C.R. Susceptibility of Enterococcus faecalis and Propionibacterium acnes to antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 2018, 178, 545–550.
- Silva, A.F.; de Oliveira Silva, J.V.; Campanerut-Sá, P.A.Z.; Mikcha, J.M.G. Chapter 13—Photoinactivation of biofilms. In Recent Trends in Biofilm Science and Technology; Simoes, M., Borges, A., Chaves Simoes, L., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 295–306.





