| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jia Zeng | -- | 2915 | 2023-02-15 02:44:01 | | | |
| 2 | Peter Tang | Meta information modification | 2915 | 2023-02-15 03:07:19 | | |
Video Upload Options
Agrimonolide (AM), which is a derivative of isocoumarins, is found mainly in the herb Agrimonia pilosa Ledeb. This compound is highly lipophilic and readily crosses the blood–brain barrier. Interest has grown in the use of AM as a multitarget natural treatment for various diseases, such as cancer, inflammation, hepatic injury, myocardial damage, and diabetes mellitus. The potential mechanisms of these pharmacological effects have been clarified at cellular and molecular levels. AM shows no cytotoxicity over a range of concentrations in different types of cells, providing evidence for its good safety profile in vitro. These findings indicate that AM is a promising medicinal agent.
1. Introduction
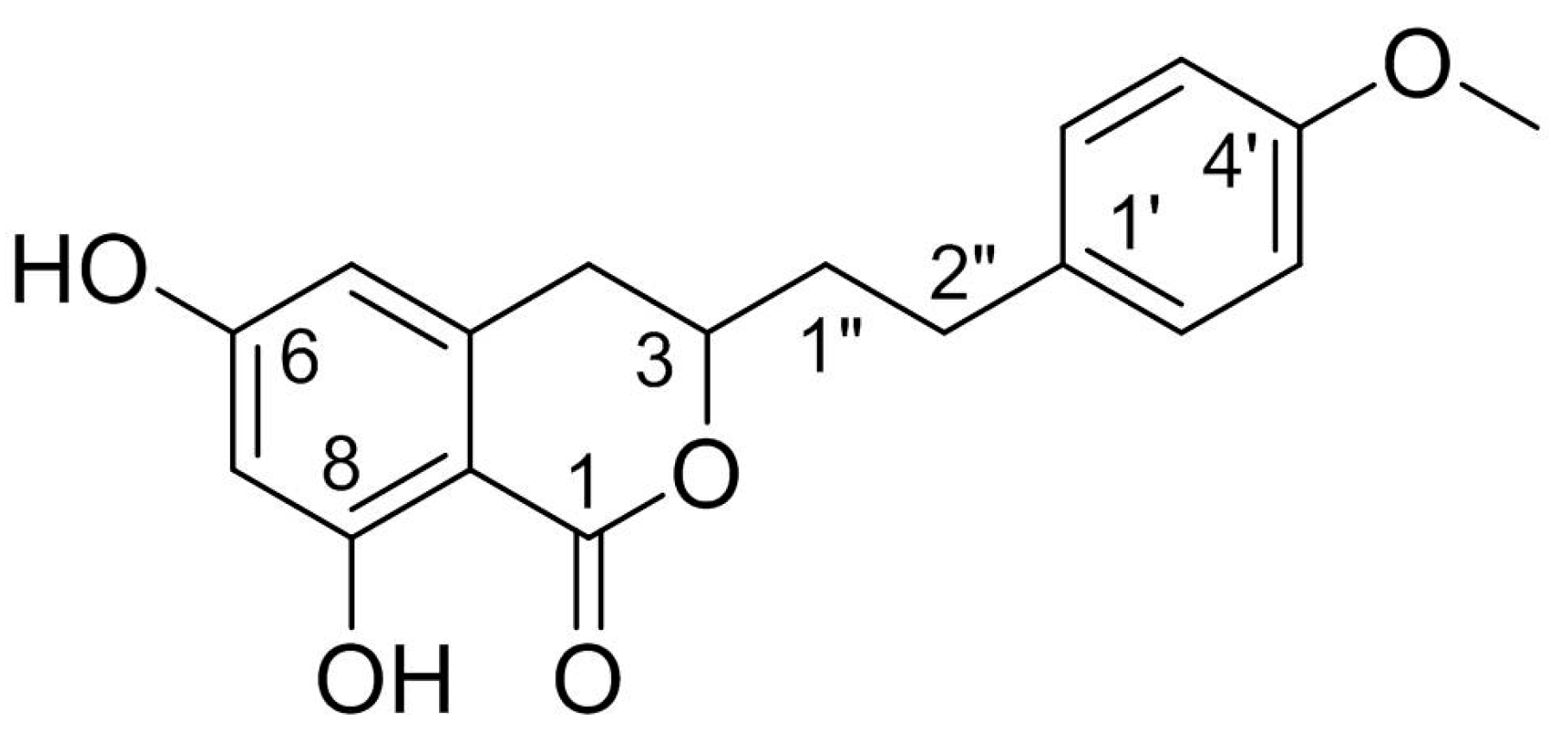
2. Sources
2.1. Derivation from Plants
|
Parts |
Methods of Extraction and Isolation |
Yield |
Content |
Ref. |
|---|---|---|---|---|
|
Fresh stems |
8.6 kg of S. formosana is extracted with hot ethanol, and the water suspension of the ethanol extract is subjected to a liquid-liquid partition to obtain chloroform, n-butanol, and water subfractions, respectively. The chloroform subfraction is then fractionated by silica gel column chromatography. |
5.6 mg |
0.65 mg/kg |
[2] |
|
Fresh roots |
10 kg of A. pilosa is extracted with methanol, and the extract is shaken with diethyl ether. The soluble part is boiled several times with petroleum ether, and the residue is heated and extracted repeatedly with benzene. Finally, the precipitated crystals are recrystallized from benzene and then from methanol. |
3000–4000 mg |
300–400 mg/kg |
[1] |
|
Dried plant |
50 kg of A. pilosa is extracted with 60% ethanol, and the 30% ethanol elution part of macroporous resin is separated by silica gel column chromatography, recrystallization, ODS column chromatography, Sephadex LH-20 gel column chromatography and preparative high-performance liquid chromatography. |
202 mg |
4.04 mg/kg |
[22] |
|
Dried aerial parts |
13 kg of A. pilosa is extracted with methanol and the extract is suspended in water. The suspension is partitioned between hexane, ethyl acetate, and n-butanol. The ethyl acetate fraction is then fractionated by repeated silica gel column chromatography. |
43.7 mg |
3.36 mg/kg |
[11] |
|
NA |
Ethyl acetate fraction of methanol extract of A. pilosa is chromatographed repeatedly with silica gel columns and purified by preparative thin layer chromatography. |
6.5 mg |
NA |
[4] |
|
Dried roots |
290 g of A. pilosa is extracted with hot water and the filtrated aqueous solution is partitioned with ethyl acetate and n-butanol, successively. The ethyl acetate soluble fraction is chromatographed by silica gel column repeatedly. |
44 mg |
151.7 mg/kg |
[8] |
|
Dried plant |
500 g of A. pilosa is extracted with 70% ethanol. The extract is then eluted with different concentrations of ethanol on the macroporous resin. The 50% ethanol eluted fractions is collected and used for subsequent high-speed counter-current chromatography separation. |
385.2 mg |
770.4 mg/kg |
[23] |
2.2. Obtaining AM by Chemical Synthesis
3. Properties
3.1. Physicochemical Properties
|
Physicochemical Properties |
Property Value |
Ref. |
|---|---|---|
|
color/form |
white powder |
[28] |
|
molecular weight |
314.3 g/moL |
[28] |
|
partition coefficient |
3.649 |
[27] |
|
distribution coefficient |
2.949 |
[27] |
|
acid dissociation constant |
8.10 ± 0.40 |
[28] |
|
density |
1.293 g/cm3 |
[28] |
|
melting point |
175.5–176.5 °C |
[29] |
|
boiling point |
581.1 °C at 760 mmHg |
[28] |
|
refractive index |
1.611 |
[28] |
|
flash point |
215.5 °C |
[28] |
|
vapour pressure |
4.2E–14 mmHg at 25 °C |
[28] |
3.2. Predicted Absorption, Distribution, Metabolism, Excretion, and Toxicity Properties
|
ADMET Properties |
Prediction Value |
Level |
|---|---|---|
|
ADMET absorption |
/ |
0 |
|
ADMET BBB |
–0.241 |
2 |
|
ADMET solubility |
–4.092 |
2 |
|
ADMET hepatotoxicity |
0.655 |
1 |
|
ADMET CYP2D6 |
0.356 |
0 |
|
ADMET PPB |
/ |
2 |
|
drug-likeness |
0.842 |
good |
4. Pharmacological Effects
|
Pharmacological Effects |
Levels |
Models |
Concentrations or Doses of AM |
Mechanisms |
Ref. |
|---|---|---|---|---|---|
|
anti-gastric cancer |
in vitro |
AGS cells |
40 µM, IC50 = 25.9 μM |
decrease the expression of Bcl-2; increase the expression of Bax; increase the level of phospho-ERK/ERK protein and the expression of phosphor-p38 protein; increase the activity of caspase-3; down-regulate the levels of the inactive pro-caspase-3, -8, and -9 proteins |
[6] |
|
anti-ovarian cancer |
in vitro |
A2780 and SKOV-3 cells |
40 µM |
increase the cleavage of caspase-3 and -9; increase the levels of ROS, total iron and ferrous ion, and down-regulate the levels of SLC7A11 and GPX4, thus inducing ferroptosis; direct inhibit tumor cell migration and invasion; inhibit the protein levels of SCD1 |
[5] |
|
in vivo |
SKOV-3 xenograft model (BALB/c mice) |
50 mg/kg |
down-regulate the expressions of Ki-67 and SCD1; lower the expressions of SCD1 mRNA and protein |
[5] |
|
|
anti-diabetic |
in vitro |
PANC-1 cell |
1 μM; 5 μM |
promote the expression of PDX-1 |
[22] |
|
in vitro |
/ |
IC50 = 37.4 μM |
inhibit α-glucosidase |
[11] |
|
|
in vitro |
Insulin-resistance HepG2 cell |
20 µM |
elevate the activity of GK, and increase the content of glycogen; lower the activities of PEPCK and G6Pase, and constrain the gluconeogensis |
[12] |
|
|
anti-oxidative and hepatoprotective |
in vitro |
HepG2 cell; rat primary hepatocytes |
EC50 = 88.2 μM; EC50 = 37.7 μM |
scavenge the free radical |
[8] |
|
in vitro |
HepG2 cell |
200 μM |
scavenge the free radical; activate Nrf2-driven pathways; activate ERK, JNK, and MAPK phosphorylation; inhibit p38 phosphorylation; elevate the activity of antioxidative enzymes |
[7] |
|
|
anti-inflammatory |
in vitro |
RAW 264.7 cells |
80 μM |
reduce the levels of IL-1β, IL-6, and TNF-α; attenuate the expression of iNOS and COX-2; inhibit the activation of JNK and p38 MAPKs; decrease the activation of JAK-STAT and NF-κB |
[4] |
|
myocardial protective |
in vitro |
H9c2 cell |
15 μM |
regulate the gene expression involved in mitochondrial function; decrease the levels of cleaved Caspase 3 and Bax; boost the level of Bcl2; prevent the rate of apoptosis and shield H9c2 cells from hypoxia-induced apoptosis; reduce ROS production and preserve the normal shape of mitochondria; regulate the functional proteins to enhance the mitochondrial activity |
[10] |
|
in vivo |
CLP rat model |
5 mg/kg |
attenuate myocardial injury by Akt signaling; suppress cardiac injury indicators, oxidative stress, and inflammation; restrain the activation of Akt, Erk, mTOR and the apoptosis of cardiomyocytes |
[9] |
|
|
blocking α1A adrenergic receptor |
in vitro |
rat prostate cell membrane |
/ |
/ |
[3] |
5. Safety
References
- Yamato, M. On the chemical structure of agrimonolide, a new constituent of agrimonia pilosa Ledel. Yakugaku Zasshi 1958, 78, 1086–1089.
- Wu, T.S.; Hwang, C.C.; Kuo, P.C.; Kuo, T.H.; Damu, A.G.; Su, C.R. New Neolignans from Spiraea formosana. Chem. Pharm. Bull. 2004, 52, 1227–1230.
- Han, S.; Zhang, P.; Wei, F.; Wang, S. Screening active components acting on α1A adrenergic receptors from agrimony using a Sprague-Dawley rat prostate cell membrane chromatography online coupled HPLC/MS method. Anal. Methods 2012, 4, 3351–3357.
- Chen, L.; Teng, H.; Fang, T.; Xiao, J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-kappaB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016, 23, 846–855.
- Liu, Y.; Liu, X.; Wang, H.; Ding, P.; Wang, C. Agrimonolide inhibits cancer progression and induces ferroptosis and apoptosis by targeting SCD1 in ovarian cancer cells. Phytomedicine 2022, 101, 154102.
- Teng, H.; Huang, Q.; Chen, L. Inhibition of cell proliferation and triggering of apoptosis by agrimonolide through MAP kinase (ERK and p38) pathways in human gastric cancer AGS cells. Food Funct. 2016, 7, 4605–4613.
- Chen, L.; Teng, H.; Zhang, K.Y.; Skalicka-Woźniak, K.; Georgiev, M.I.; Xiao, J. Agrimonolide and Desmethylagrimonolide Induced HO-1 Expression in HepG2 Cells through Nrf2-Transduction and p38 Inactivation. Front. Pharmacol. 2017, 7, 513.
- Park, E.J.; Oh, H.; Kang, T.H.; Sohn, D.H.; Kim, Y.C. An isocoumarin with hepatoprotective activity in Hep G2 and primary hepatocytes from Agrimonia Pilosa. Arch. Pharm. Res. 2004, 27, 944–946.
- Zhao, Z.; Hu, X. Protective effect of agrimonolide against myocardial injury in cecal ligation and puncture (CLP) induced sepsis in wistar rats via inhibition of akt. Acta Pol. Pharm. 2021, 78, 845–851.
- Wang, C.; Qi, C.; Liu, M.; Wang, L.; Cheng, G.; Li, L.; Xing, Y.; Zhao, X.; Liu, J. Protective effects of agrimonolide on hypoxia-induced H9c2 cell injury by maintaining mitochondrial homeostasis. J. Cell. Biochem. 2022, 123, 306–321.
- Park, M.J.; Kang, Y.H. Isolation of Isocoumarins and Flavonoids as alpha-Glucosidase Inhibitors from Agrimonia pilosa L. Molecules 2020, 25, 2572.
- Teng, H.; Chen, L.; Song, H. The potential beneficial effects of phenolic compounds isolated from A. pilosa Ledeb on insulin-resistant hepatic HepG2 cells. Food Funct. 2016, 7, 4400–4409.
- Wen, S.; Zhang, X.; Wu, Y.; Yu, S.; Zhang, W.; Liu, D.; Yang, K.; Sun, J. Agrimonia pilosa Ledeb.: A review of its traditional uses, botany, phytochemistry, pharmacology, and toxicology. Heliyon 2022, 8, e09972.
- Jin, T.; Chi, L.; Ma, C. Agrimonia pilosa: A Phytochemical and Pharmacological Review. Evid. Based Complement. Alternat. Med. 2022, 2022, 3742208.
- Le, U.; Joshi, R.K.; Lay, H.; Ming, C. Agrimonia pilosa Ledeb: Phytochemistry, Ethnopharmacology, Pharmacology of an important traditional herbal medicine. J. Pharmacogn. Phytochem. 2018, 7, 3202–3211.
- Li, J.; Yang, J. Research Advances on the Main Chemical Constituents and Pharmacological Actions of Agrimoniae Herba. Chin. Wild Plant Resour. 2020, 39, 54–60.
- Yu, T.; Lu, L.; Gu, C.; Guan, C.; Li, C. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1985; Volume 37, p. 457.
- Kuang, L.; Li, C.; Cao, S.; Li, H.; Wang, Z.; Wu, S.; Fan, Y.; Zhang, F.; Li, Q.; Liu, T.; et al. Pharmacopoeia of the People’s Republic of China; China Pharmaceutical Science and Technology Press: Beijing, China, 2020; p. 106.
- Ohashi, H.; Hsieh, C.F. Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, China, 1993; Volume 3, p. 149.
- Zhao, G.; Dei, S.; Chen, R. ZhongyaoDacidian, 2nd ed.; Shanghai Science and Technology Press: Shanghai, China, 1977; pp. 1117–1118+1978.
- Xie, Z.; Fang, C.; Zhu, Z. QuanGuoZhongCaoYao Huibian, 2nd ed.; People’s Hygenic Publishing House: Beijing, China, 1996; pp. 514–515+767.
- Huang, Y. Chemical Composition of Agrimonia pilosa Ledeb. and Its Hypoglycemic Activity; Xiamen University: Xiamen, China, 2020.
- Wang, Y.; Liu, M.; Zheng, L.; Yin, L.; Xu, L.; Qi, Y.; Ma, X.; Liu, K.; Peng, J. Preparative purification of five bioactive components from Agrimonia pilosa Ledeb by high-speed counter-current chromatography. J. Sep. Sci. 2012, 35, 1977–1984.
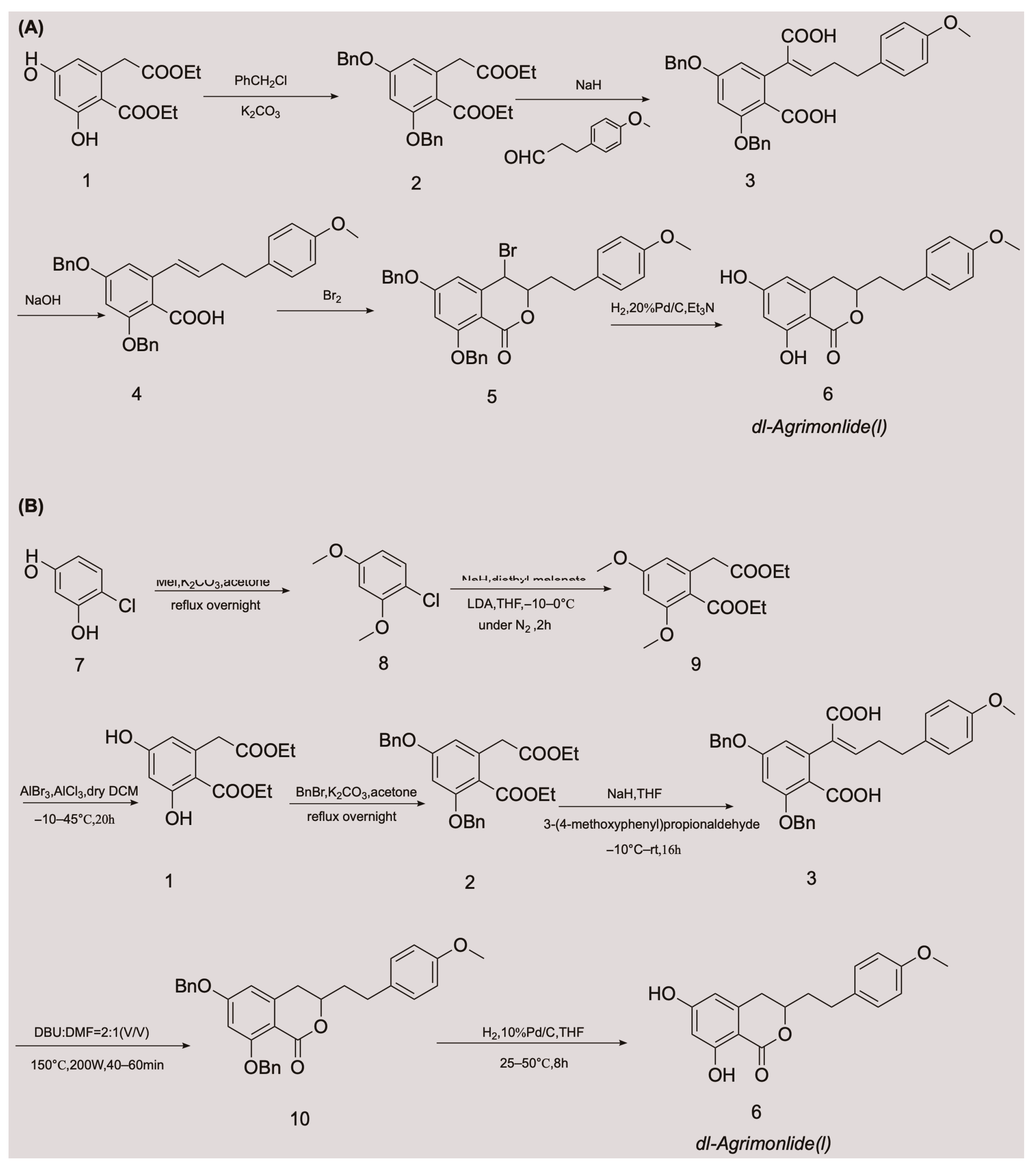
- Yamato, M.; Hashigaki, K. Synthesis of dl-Agrimonolide (Constituent of Rhizome of Agrimonia pilosa LEDEB). Chem. Pharm. Bull. 1976, 24, 200–203.
- Xie, W.; Wu, X.; Rao, X. Synthesis of a racemic natural product Agrimonolide (I) extracted from Agrimonia pilosa. Patent CN201811078365.0, 13 November 2018.
- Cui, Y.; Zhang, G.; Li, Y.; Li, W.; Tanabe, G.; Osamu, M.; Xie, W. Microwave-Assisted Synthesis of d/l-Agrimonolide. Asian J. Org. Chem. 2022, 11, e202100334.
- The Encyclopedia of Traditional Chinese Medicine. In Agrimonolide. Available online: http://www.tcmip.cn/ETCM/index.php/Home/Index/cf_details.html?id=5545 (accessed on 3 September 2022).
- Chemical Trading Guide. In (1S,4R)-N-phenyl]-2-bicycloheptanecarboxamide;2,2,2-trifluoroacetic Acid. 2022. Available online: https://www.guidechem.com/encyclopedia/agrimonolide-dic74411.html (accessed on 1 September 2022).
- Arakawa, H.; Torimoto, N.; Masui, Y. Die absolute konfiguration des (−)-β-tetralols und des agrimonolides. Tetrahedron Lett. 1968, 9, 4115–4117.
- BioCrick. In Agrimonolide. Available online: https://www.biocrick.com/Agrimonolide-BCN4925.html (accessed on 11 September 2022).
- Shukla, A.K.; Bishnoi, R.S.; Dev, S.K.; Kumar, M.; Fenin, V. Biopharmaceutical Classification System: Tool based prediction for drug dosage formulation. Adv. Pharm. J. 2017, 2, 204–209.
- Qiu, J.; Zhou, Z.; He, Z.; Zhang, X.; Zhou, S.; Zhu, S. Estimation of the binding modes with important human cytochrome P450 enzymes, drug interaction potential, pharmacokinetics, and hepatotoxicity of ginger components using molecular docking, computational, and pharmacokinetic modeling studies. Drug Des. Devel. Ther. 2015, 9, 841–866.