Agrimonolide (AM), which is a derivative of isocoumarins, is found mainly in the herb Agrimonia pilosa Ledeb. This compound is highly lipophilic and readily crosses the blood–brain barrier. Interest has grown in the use of AM as a multitarget natural treatment for various diseases, such as cancer, inflammation, hepatic injury, myocardial damage, and diabetes mellitus. The potential mechanisms of these pharmacological effects have been clarified at cellular and molecular levels. AM shows no cytotoxicity over a range of concentrations in different types of cells, providing evidence for its good safety profile in vitro. These findings indicate that AM is a promising medicinal agent.
- agrimonolide
- Agrimonia pilosa Ledeb.
- pharmacological effect
- safety
1. Introduction
2. Sources
2.1. Derivation from Plants
As mentioned above, AM has been found in the plants of A. pilosa and S. formosana, which belong to the Rosaceae family. A. pilosa is a perennial herb with an erect stem that is 30–120 cm in height, and it grows along roadsides or in grassy areas at diverse altitudes. This plant is distributed in China, central Europe, the former Soviet Union, Mongolia, North Korea, Japan, and northern Vietnam [17]. A. pilosa is used in traditional Chinese medicine for mainly treating hemoptysis, metrorrhagia, hematemesis, and bloody dysentery [18]. S. formosana is a shrub endemic to Taiwan that grows in alpine woodlands at an altitude of 2100–2950 m [19]. The tender leaves, fruits, and roots of this plant have traditionally been used as diuretics, antidotes, and analgesics to treat inflammation, cough, headache, and toothache [20][21][20,21]. Conventional approaches are usually used to extract and separate AM from the above-mentioned plants. Table 1 summarizes the extraction and separation methods of AM [1][2][4][8][11][22][23][1,2,4,8,11,22,23]. The extraction and isolation process of AM from S. formosana can be summarized as follows [2]. Briefly, the fresh stems of S. formosana are extracted with hot ethanol, and the water suspension of the ethanol extract is subjected to a liquid-liquid partition to obtain chloroform, n-butanol, and water subfractions. AM is then separated from the chloroform subfraction using a combination of silica gel column chromatography. Finally, 5.6 mg of AM is obtained from 8.6 kg of S. formosana, equivalent to the content of 0.65 mg/kg. This result indicates that the content of AM in S. formosana is low.|
Parts |
Methods of Extraction and Isolation |
Yield |
Content |
Ref. |
|---|---|---|---|---|
|
Fresh stems |
8.6 kg of S. formosana is extracted with hot ethanol, and the water suspension of the ethanol extract is subjected to a liquid-liquid partition to obtain chloroform, n-butanol, and water subfractions, respectively. The chloroform subfraction is then fractionated by silica gel column chromatography. |
5.6 mg |
0.65 mg/kg |
[2] |
|
Fresh roots |
10 kg of A. pilosa is extracted with methanol, and the extract is shaken with diethyl ether. The soluble part is boiled several times with petroleum ether, and the residue is heated and extracted repeatedly with benzene. Finally, the precipitated crystals are recrystallized from benzene and then from methanol. |
3000–4000 mg |
300–400 mg/kg |
[1] |
|
Dried plant |
50 kg of A. pilosa is extracted with 60% ethanol, and the 30% ethanol elution part of macroporous resin is separated by silica gel column chromatography, recrystallization, ODS column chromatography, Sephadex LH-20 gel column chromatography and preparative high-performance liquid chromatography. |
202 mg |
4.04 mg/kg |
[22] |
|
Dried aerial parts |
13 kg of A. pilosa is extracted with methanol and the extract is suspended in water. The suspension is partitioned between hexane, ethyl acetate, and n-butanol. The ethyl acetate fraction is then fractionated by repeated silica gel column chromatography. |
43.7 mg |
3.36 mg/kg |
[11] |
|
NA |
Ethyl acetate fraction of methanol extract of A. pilosa is chromatographed repeatedly with silica gel columns and purified by preparative thin layer chromatography. |
6.5 mg |
NA |
[4] |
|
Dried roots |
290 g of A. pilosa is extracted with hot water and the filtrated aqueous solution is partitioned with ethyl acetate and n-butanol, successively. The ethyl acetate soluble fraction is chromatographed by silica gel column repeatedly. |
44 mg |
151.7 mg/kg |
[8] |
|
Dried plant |
500 g of A. pilosa is extracted with 70% ethanol. The extract is then eluted with different concentrations of ethanol on the macroporous resin. The 50% ethanol eluted fractions is collected and used for subsequent high-speed counter-current chromatography separation. |
385.2 mg |
770.4 mg/kg |
[23] |
2.2. Obtaining AM by Chemical Synthesis
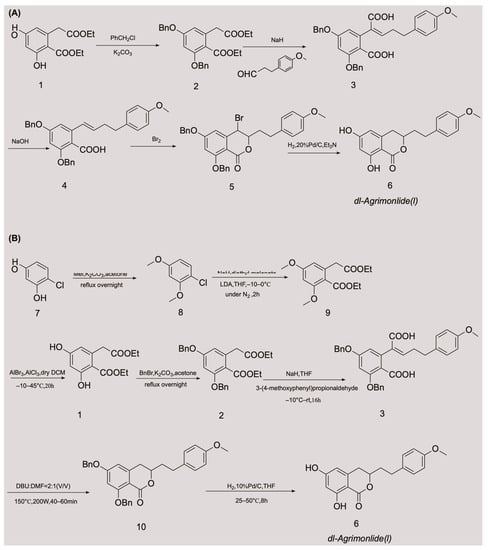
The low accumulation of AM in plants, its cumbersome extraction and separation processes, and the overexploitation of natural resources are generally considered the main driving factors for its high production cost. These factors are also the main causes of supply shortages of AM. These obstacles have made chemical synthesis an appealing alternative method for obtaining AM. Although AM has a variety of pharmacological activities, there have been relatively few advances in its chemical synthesis. A few attempts have been made to synthesize AM, with variable success. In 1976, Yamato et al. synthesized the racemate of AM in five steps for the first time, and confirmed its structure using nuclear magnetic resonance imaging [24]. They started with compound 1 and obtained compound 2 by protecting two phenolic hydroxyl groups with a benzyl group. An overall yield of 2.6% AM was obtained through a series of reactions, including Stobbe condensation, ester hydrolysis, benzyl decarboxylation, bromine addition, and reduction [24]. The chemical synthesis route is shown in Figure 2A. Unfortunately, there were no subsequent reports of the chemical synthesis process of AM for many years. In 2018, a Chinese invention patent was published that contained a novel chemical synthesis method for AM [25]. The authors improved on the synthesis route of Yamato et al., and constructed the 3,4-dihydroisocoumarin core structure using microwave-assisted intramolecular esterification for the first time. The synthetic process began with 4-chlororesorcinol as the material, and the goal product was obtained in seven steps. The chemical synthesis route is shown in Figure 2B. This synthetic route increased the overall yield of AM to 20.7% [25][26][25,26], which is nearly eight times higher than that of Yamato et al. This exciting result suggests that microwave-assisted synthesis is a promising approach for the chemical construction of AM.3. Properties
3.1. Physicochemical Properties
Physicochemical properties, such as solubility in water and organic solvents, the acid dissociation constant, the oil/water partition coefficient, and chemical stability, are key factors that affect the pharmacokinetics, biopharmaceutics, and quality of drugs. The physicochemical properties of AM are summarized in detail in Table 2 [27][28][29][27,28,29].|
Physicochemical Properties |
Property Value |
Ref. |
|||||||
|---|---|---|---|---|---|---|---|---|---|
Concentrations or | Doses of AM |
Mechanisms |
Ref. |
||||||
|
color/form |
|||||||||
|
anti-gastric cancer |
white powder |
in vitro [28] |
|||||||
AGS cells |
40 µM, IC50 = 25.9 μM |
decrease the expression of Bcl-2; increase the expression of Bax; increase the level of phospho-ERK/ERK protein and the expression of phosphor-p38 protein; increase the activity of caspase-3; down-regulate the levels of the inactive pro-caspase-3, -8, and -9 proteins |
[6] |
molecular weight |
314.3 g/moL |
[ | |||
|
anti-ovarian cancer |
in vitro | 28] |
|||||||
A2780 and SKOV-3 cells |
40 µM |
increase the cleavage of caspase-3 and -9; increase the levels of ROS, total iron and ferrous ion, and down-regulate the levels of SLC7A11 and GPX4, thus inducing ferroptosis; direct inhibit tumor cell migration and invasion; inhibit the protein levels of SCD1 |
[5] |
partition coefficient |
3.649 |
[27] |
|||
|
in vivo |
SKOV-3 xenograft model (BALB/c mice) |
50 mg/kg |
down-regulate the expressions of Ki-67 and SCD1; lower the expressions of SCD1 mRNA and protein |
[ |
distribution coefficient |
2.949 |
[27] |
||
] |
1 |
||||||||
|
anti-diabetic |
in vitro |
PANC-1 cell |
1 μM; 5 μM |
promote the expression of PDX-1 |
[22] |
acid dissociation constant |
ADMET CYP2D6 8.10 ± 0.40 |
||
|
in vitro | 0.356 | [28] |
|||||||
0 |
density |
||||||||
|
ADMET PPB |
1.293 g/cm3 |
/ [28] |
|||||||
2 |
melting point |
||||||||
|
drug-likeness |
175.5–176.5 °C |
0.842 [29] |
|||||||
|
boiling point |
581.1 °C at 760 mmHg |
[28] |
|||||||
|
refractive index |
1.611 |
[28] |
|||||||
|
flash point |
215.5 °C |
[28] |
|||||||
|
vapour pressure |
4.2E–14 mmHg at 25 °C |
[28] |
3.2. Predicted Absorption, Distribution, Metabolism, Excretion, and Toxicity Properties
Pharmacokinetic behaviors of drugs in vivo include absorption, distribution, metabolism, excretion, and toxicity (ADMET). The ADMET properties account for 50% of drug research and development (R&D) failures. Computer-aided design is an effective and alternative method of biological experimental evaluation, and helps to improve the R&D success rate. Computational approaches have increasingly been used to predict ADMET properties of compounds, especially in evaluating the ADMET properties of herbal medicines [32]. The predicted ADMET profiles of AM regarding its absorption, solubility, permeability across the blood-brain barrier (BBB), interactions with cytochrome P450 2D6, hepatotoxicity, and plasma protein binding (PPB) are shown in Table 3 [27]. ADMET absorption levels: 0, 1, 2, and 3 represent good, moderate, low, or very low absorption, respectively. ADMET BBB levels: 0, 1, 2, 3, 4, and 5 represent very high, high, medium, low, undefined, and molecules with one or more unknown AlogP98 types, respectively. ADMET solubility levels: 0, 1, 2, 3, 4, 5, and 6 represent extremely low, very low but possible, low, good, optimal, too soluble, and molecules with one or more unknown AlogP98 types, respectively. ADMET hepatotoxicity: 0 and 1 represent nontoxic and toxic effects, respectively. ADMET CYP2D6: 0 and 1 represent non-inhibitor and inhibitor, respectively. ADMET PPB levels: 0, 1, and 2 represent binding <90%, binding ≥90% and binding ≥95%, respectively. AM appears to show a good absorption capacity in vivo with a predicted absorption level of 0. However, AM is predicted to have a low aqueous solubility, with a solubility level of 2, which contradicts the prediction result for in vivo absorption and needs to be further confirmed by in vivo testing. Regarding the prediction of BBB penetration, AM exhibits a moderate BBB penetration capability, with a level of 2. This indicates that AM may enter the brain tissue through the BBB and could be used to treat brain diseases. Furthermore, the ADMET predictor shows that AM exhibits potential hepatotoxicity, with a level of 1. Preliminary explorations and in-depth investigations are required to determine the specific mechanism of hepatotoxicity and whether it is dose dependent. In addition, AM is predicted to be a non-inhibitor of the cytochrome P450 2D enzyme and may be metabolized and excreted successfully. Therefore, drug-drug interactions are less likely when AM and the cytochrome P450 2D6 substrates are used simultaneously. Moreover, the PPB level is predicted to be 2, indicating that the binding rate of AM with plasma protein is ≥95%. The high degree of PPB limits the partitioning of AM from the blood into the tissues, where it could be metabolized. This limited partitioning may result in a delayed onset of action and longer half-life period, thereby reducing the elimination of AM. AM has been predicted to have a good drug-likeness, with a drug-likeness weight of 0.842. Generally, AM demonstrates promising ADMET profiles. However, to fully confirm the ADMET properties of AM, real-world tests are required to validate these properties, and more animal and human studies are required.4. Pharmacological Effects
AM possesses a wide range of pharmacological activities, such as antitumor activity, antioxidation and hepatoprotection, antidiabetic activity, anti-inflammatory activity, myocardial protection, and α1A adrenergic receptor antagonist activity. The mechanisms of action of these effects are shown in Table 4.|
Pharmacological Effects | Prediction Value |
Level |
|||
|---|---|---|---|---|---|
Levels |
Models | ||||
|
ADMET absorption |
/ |
0 |
|||
|
ADMET BBB |
–0.241 |
2 |
|||
|
ADMET solubility |
–4.092 |
2 |
|||
|
ADMET hepatotoxicity |
0.655 |
||||
/ |
IC50 = 37.4 μM |
inhibit α-glucosidase |
[11] |
||
|
in vitro |
Insulin-resistance HepG2 cell |
20 µM |
elevate the activity of GK, and increase the content of glycogen; lower the activities of PEPCK and G6Pase, and constrain the gluconeogensis |
[12] |
|
|
anti-oxidative and hepatoprotective |
in vitro |
HepG2 cell; rat primary hepatocytes |
EC50 = 88.2 μM; EC50 = 37.7 μM |
scavenge the free radical |
[8] |
|
in vitro |
HepG2 cell |
200 μM |
scavenge the free radical; activate Nrf2-driven pathways; activate ERK, JNK, and MAPK phosphorylation; inhibit p38 phosphorylation; elevate the activity of antioxidative enzymes |
[7] |
|
|
anti-inflammatory |
in vitro |
RAW 264.7 cells |
80 μM |
reduce the levels of IL-1β, IL-6, and TNF-α; attenuate the expression of iNOS and COX-2; inhibit the activation of JNK and p38 MAPKs; decrease the activation of JAK-STAT and NF-κB |
[4] |
|
myocardial protective |
in vitro |
H9c2 cell |
15 μM |
regulate the gene expression involved in mitochondrial function; decrease the levels of cleaved Caspase 3 and Bax; boost the level of Bcl2; prevent the rate of apoptosis and shield H9c2 cells from hypoxia-induced apoptosis; reduce ROS production and preserve the normal shape of mitochondria; regulate the functional proteins to enhance the mitochondrial activity |
[10] |
|
in vivo |
CLP rat model |
5 mg/kg |
attenuate myocardial injury by Akt signaling; suppress cardiac injury indicators, oxidative stress, and inflammation; restrain the activation of Akt, Erk, mTOR and the apoptosis of cardiomyocytes |
[9] |
|
|
blocking α1A adrenergic receptor |
in vitro |
rat prostate cell membrane |
/ |
/ |
[3] |