Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | shreekar pant | -- | 1594 | 2023-02-11 15:02:24 | | | |
| 2 | Sirius Huang | Meta information modification | 1594 | 2023-02-13 02:01:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wani, Z.A.; Ahmad, Z.; Asgher, M.; Bhat, J.A.; Sharma, M.; Kumar, A.; Sharma, V.; Kumar, A.; Pant, S.; Lukatkin, A.S.; et al. Major Impacts of Potentially Toxic Elements. Encyclopedia. Available online: https://encyclopedia.pub/entry/41119 (accessed on 08 February 2026).
Wani ZA, Ahmad Z, Asgher M, Bhat JA, Sharma M, Kumar A, et al. Major Impacts of Potentially Toxic Elements. Encyclopedia. Available at: https://encyclopedia.pub/entry/41119. Accessed February 08, 2026.
Wani, Zishan Ahmad, Zeeshan Ahmad, Mohd Asgher, Jahangeer A. Bhat, Manju Sharma, Ashish Kumar, Virbala Sharma, Amit Kumar, Shreekar Pant, Alexander S. Lukatkin, et al. "Major Impacts of Potentially Toxic Elements" Encyclopedia, https://encyclopedia.pub/entry/41119 (accessed February 08, 2026).
Wani, Z.A., Ahmad, Z., Asgher, M., Bhat, J.A., Sharma, M., Kumar, A., Sharma, V., Kumar, A., Pant, S., Lukatkin, A.S., & Anjum, N.A. (2023, February 11). Major Impacts of Potentially Toxic Elements. In Encyclopedia. https://encyclopedia.pub/entry/41119
Wani, Zishan Ahmad, et al. "Major Impacts of Potentially Toxic Elements." Encyclopedia. Web. 11 February, 2023.
Copy Citation
Environmental contamination with a myriad of potentially toxic elements (PTEs) is triggered by various natural and anthropogenic activities. However, the industrial revolution has increased the intensity of these hazardous elements and their concentration in the environment, which, in turn, could provoke potential ecological risks. Additionally, most PTEs pose a considerable nuisance to human beings and affect soil, aquatic organisms, and even nematodes and microbes.
potentially toxic elements
pollution
hazardous effects
plant biomass
1. Introduction
Anything that gets accumulated in the natural environment beyond its permissible limit causes deleterious effects on the biotic as well as abiotic components of the ecosystem [1]. The metal contaminant known as heavy metals is one of these examples. Metalloids, transition metals, actinides, and lanthanides are all part of the subset of elements known as heavy metals that have metallic properties. The term heavy metal is generally used for metallic elements with specific weights over 5 g/cm3 and are noxious even at low concentrations [2]. To date, 23 metals have been classified as heavy metals, with the most common being lead (Pb), cadmium (Cd), cobalt (Co), chromium (Cr), and mercury (Hg), which have potentially toxic effects at high and low concentrations [3]. These toxic elements are classified as class-B and come under non-essential elements [4][5]. These metals persist in the surroundings and accumulate at different levels of the food chain, from primary producer level to consumer levels, through consumption due to their non-biodegradable nature (unlike that of organic pollutants); thus, these are deleterious for both components (biotic as well as abiotic) of an ecosystem [6][7]. Hence, it is important for environmentalists to take strategic remediation measures to reduce heavy metals’ load in the aquatic and terrestrial environments [8]. Pourret and Hursthouse [9] prefer the term ‘potentially toxic elements (PTEs)’ for such elements instead of ‘heavy metals’ due to their toxic nature. The accumulation of toxic metals in the ecosystem is continually being fueled by the agricultural use of fertilizers and pesticides, as well as industrial inputs and metal-contaminated sewage [10]. This makes it difficult to use soils effectively and safely. Furthermore, environmental heavy metal pollution posed a grave threat to human life as well as the other biotic elements of the ecosystem. Hence, it is important for environmentalists to take strategic remediation measures to reduce heavy metals’ load in aquatic and terrestrial environments [11].
2. Major Impacts of PTEs
During the last few decades, the risk of living organisms to PTE toxicity has increased due to the increase in anthropogenic activities, industrialization, and modern agricultural practices [12][13]. Due to the persistent and stable nature of PTEs, they cannot be degraded or destroyed [14]. PTEs are bio-accumulative and may gradually invade biological systems through air, water, and the series of the food chain over a definite period [15]. PTE contagion is a major ecological concern that influences the wellbeing of plants, animals, and humans, and affects the quality of the environment.
2.1. Impacts on Human
Some heavy metals have immense bio-significance to human, such asiron (Fe), zinc (Zn) and Mn. Their daily therapeutic and nutritional allowances had been suggested for good human health. However, some of these, such as arsenic (As), Pb, Cd and Hg in methylated form, have deleterious influence even at a very small amount [16]. Sources and effects of some of the PTEs are given in Table 1. PTEs such as Cd, Pb and Hg demonstrate an extensive toxicity level against biological entities, including human beings [17]. Inhalation and ingestion are the pathways through which these metals invade the human body. Hence, these lead to the diminution of some significant nutrients in the body, which consequently diminishes immunological defenses, cause growth retardation, and may increase gastrointestinal and other cancer rates [18][19][20]. Exposure to Cd and Pb might cause impaired fertility in humans. Cd is considered a metal estrogen. It can link and stimulate the alpha and beta estrogen receptors and concurrently cause the stimulation of progesterone receptors; thus, it may be designated a probable contributory mediator of estrogen-linked ailments, such as endometrial and breast cancer, endometriosis, and spontaneous abortion [21]. Pb can directly lead to an elevated risk of impulsive abortion through its possible teratogenic action [22]. Further, PTEs are probably the agents affecting the neurological system, renal functioning, and ossification process. These also attach to phosphate, deoxyribose sugar, and heterocyclic base residues of DNA, resulting in mutations by inducing alterations in DNA structures [19]. Long-term exposure to PTEs results in severe neurological diseases such as Alzheimer’s, Parkinson’s, muscular dystrophy and sclerosis.
Table 1. Sources and effects of the major PTEs on human health.
| PTE | Source(s) | Effects | Reference(s) |
|---|---|---|---|
| Arsenic (As) | Pesticides, fungicides, metal smelters | Carcinogen causes skin, lung, liver and bladder cancer. In addition, causes darkening of the skin | [23][24][25] |
| Barium (Ba) | Rodenticides, pharmaceuticals, cosmetics, diesel engines | Vomiting, abdominal cramps, diarrhea, difficulty in breathing, hypo or hypertension, numbness, paralysis or death | [26] |
| Cadmium (Cd) | Welding, electroplating, pesticides, fertilizers, batteries, nuclear plants | Stomachache, vomiting, diarrhea, kidney problems, liver damage, fragile bones, cause itai-itai | [19][24] |
| Chromium (Cr) | Mining, electroplating, textile, tanneries | Carcinogen, nose ulcers, asthma, cough, skin problems, kidney and liver damage | [23][24] |
| Lead (Pb) | Paint, batteries, pesticides, automobile emissions, mining, burning of coal | Weakness, hypertension, anemia, brain and kidney damage, miscarriage, impotence | [23][24] |
| Mercury (Hg) | Pesticides, batteries, paper industries | Minimata disease, nervous disorders, kidney damage, tremors, impaired vision and hearing, loss of memory | [23][24] |
| Selenium (Se) | Mining, agricultural wastes, petrochemicals, | Nausea, vomiting, diarrhea, sclerosis, respiratory tract irritation, bronchitis, stomach pains, bronchial spasms and cough | [27][28] |
| Silver (Au) | Industries, mining, silver plating, | Blue–grey discoloration of the skin called Argyria, breathing problems, lung and throat irritation, stomach pain, skin problems such as rashes, swelling and inflammation | [29] |
2.2. Impacts on Plants
There are four proposed mechanisms through which PTEs exert toxicities in plants [30][31] (Figure 1). In addition, PTEs affect many physiological and developmental processes in plant biosystems (Figure 2).
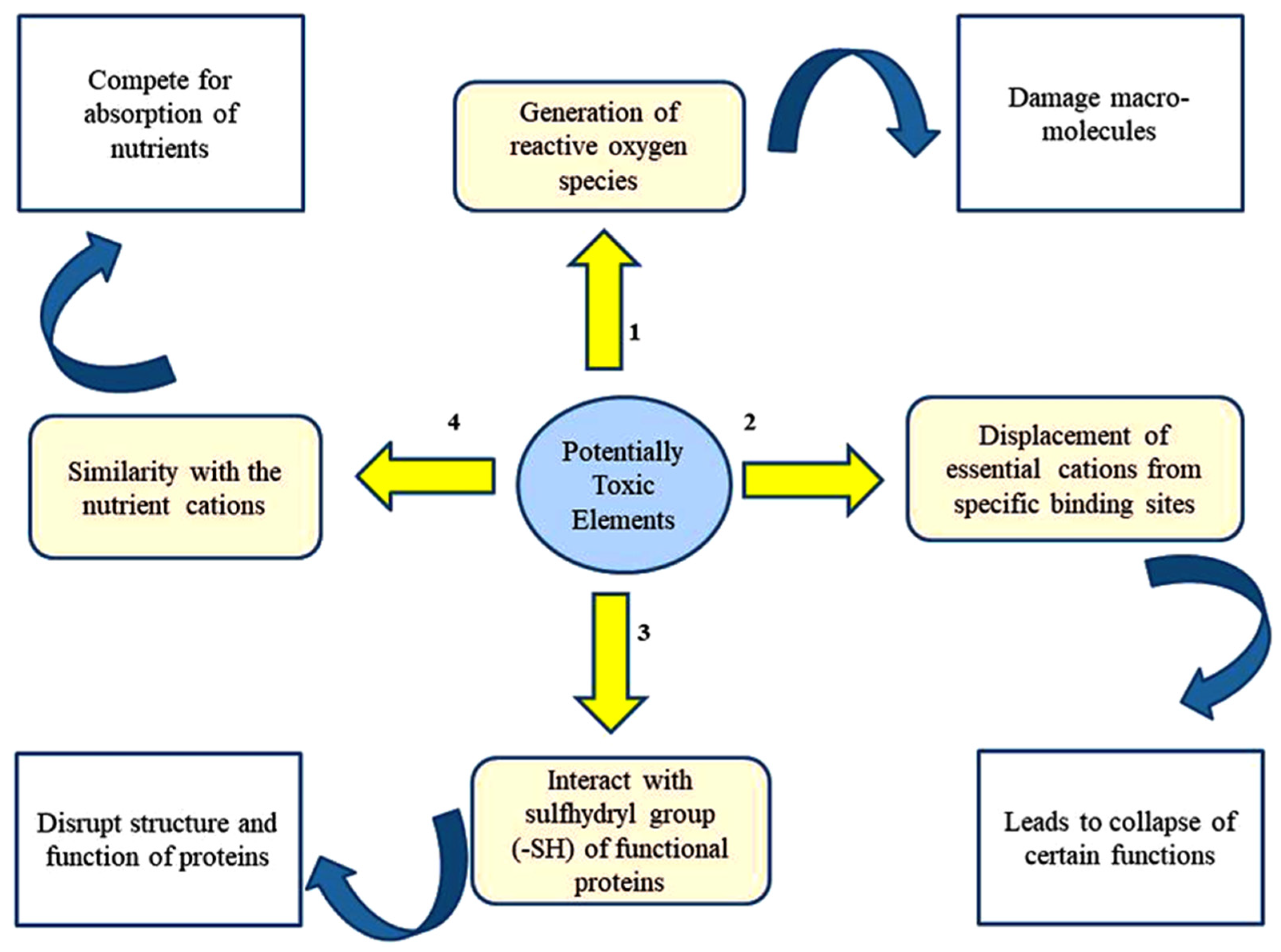
Figure 1. Major mechanisms underlying PTE-caused toxicity in plants.

Figure 2. Major toxic effects of PTEs on plants.
The main entry points for PTE ions into plant systems are the leaves and roots. High PTE concentrations have detrimental effects, including the inhibition of cytoplasmic enzymes and oxidative stress-related cell structure damage [32]. Additionally, PTEs have detrimental effects on the development and activity of soil microorganisms, which ultimately may affect plant growth. Further, due to PTEs’ interference with the activities of soil microbes, enzyme activities important for plant metabolisms may also be hindered. PTEs are also attributed with increasing enzyme activity, such as glucose-6-phosphate dehydrogenase, and peroxides in the leaves of plants growing in contaminated soils [33]. Further, PTEs are also responsible for stomatal closure, increased ethylene production and cause iron deficiency by inhibiting uptake from the medium or due to immobilization in root tissues [34]. PTEs such as Cd, As, Cr, and Cu inhibit PSII and photosynthetic genes (psbA and psbB), and increased accumulation of PTEs in roots, stems and leaves causes reduced growth in plants [35][36][37]. Aluminum damages root tips by stimulating the synthesis and accumulation of callose by inhibiting the assembly and release of border cells [38].Further, Al can accrue in plant cells and have the potential to bind at multiple sites, such as cell wall, cell membrane and nucleic acids, and impede various biological, physiological and cellular processes [38]. Pb is one of the most prevalent toxic elements in the soil, is widely dispersed and harms the various enzymatic activities in plants [39]. PTE toxicity causes a reduction in the decomposition of litter, a reduction in the mineralization of carbon and nitrogen, and an inhibition of microbial growth [14]. Plants growing in PTE-contaminated soil experience both reduced growth and yield, as the PTEs hinder growth and developmental processes [40]. PTE toxicity has a variety of effects on plants at the cellular and molecular levels. PTEs, for example, affect germination, chlorophyll biosynthesis, photosynthesis, exchange of gases, respiration, blocking functional groups of crucial molecules for metabolism, and processes of the central dogma of life [41]. These toxic effects cause overall plant growth to slow down, which may eventually cause the plant to die [39].
2.3. Impact on Aquatic Life
Due to PTEs’ toxic nature, accumulation/biomagnification, and level of environmental persistence, the accumulation of PTEs in fresh and marine water systems is one of the principal hazards for aquatic organisms [42]. The main sources of these toxic metals in aquatic ecosystems are biological activities such as weathering, soil leaching, urban storms, as well as anthropogenic activities such as land filling, coal mining, agricultural activities, and effluent discharges [43]. PTEs can take on many different forms in aquatic systems, including being adsorbed on particulate matter, precipitating as insoluble salts, or existing in an ionic state. However, all forms are harmful to aquatic life, especially the benthic fauna, and may cause several oxidative stresses in those living things [44]. Whole plant or animal bodies are exposed to PTE toxicity in aquatic ecosystems. PTEs in the water are especially harmful to young fish and may even lead to the extinction of the entire fish population in contaminated reservoirs [45].
2.4. Impacts on Soil Health
Different levels of heavy metal concentration are measured as one of potent soil contaminants and their elevated concentrations may decrease soil productivity to a great extent by harming soil flora and fauna, which, in turn, causes alterations in the size of the population and community composition of soil microbes, and by altering the physical and chemical status of soil, including increasing soil acidity, redox potential, and enhanced decomposition of organic matter. Through other means as well, contaminants have the potential to cause shifts in microbial populations, according to the isolation-based technique [44]. Generally, an increase in PTE concentration in soil negatively affects soil microbial estates and causes an overall decrease in the growth of microbes by affecting their growth, morphology and metabolism [46][47]. PTEs are also toxic for nematodes and the most toxic PTE for nematodes is selenium (Se) [48].
References
- Zhu, X.; Yao, J.; Wang, F.; Yuan, Z.; Liu, J.; Jordan, G.; Knudsen, T.S.; Avdalovic, J. Combined effects of antimony and sodium diethyldithiocarbamate on soil microbial activity and speciation change of heavy metals. Implications for contaminated lands hazardous material pollution in nonferrous metal mining areas. J. Hazard. Mater. 2018, 349, 160–167.
- Mahiya, S.; Lofrano, G.; Sharma, S.K. Heavy metals in water, their adverse health effects and biosorptive removal: A review. Int. J. Chem. 2014, 3, 132–149.
- Al-Rubaie, A.S.A.; Al-Kubaisa, A.R.A. Removal of Lead from water using aquatic plants (Ceratophyllumdemersum and Eichhorina crassipes). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 45–51.
- Song, H.; Kumar, A.; Zhang, Y. Microbial-induced carbonate precipitation prevents Cd2+ migration through the soil profile. Sci. Total Environ. 2022, 844, 157167.
- Song, H.; Kumar, A.; Zhang, Y. A novel approach for the removal of Pb2+ and Cd2+ from wastewater by sulfur-ferromagnetic nanoparticles (SFMNs). Chemosphere 2022, 287, 132156.
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front. Plant Sci. 2018, 9, 1476.
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359.
- Kumar, A.; Jigyasu, D.K.; Subrahmanyam, G.; Mondal, R.; Shabnam, A.A.; Cabral-Pinto, M.M.S.; Malyan, S.K.; Chaturvedi, A.K.; Gupta, D.K.; Fagodiya, R.K.; et al. Nickel in terrestrial biota: Comprehensive review on contamination, toxicity, tolerance and its remediation approaches. Chemosphere 2021, 275, 129996.
- Pourret, O.; Hursthouse, A. It’s time to replace the term ‘Heavy Metals’ with ‘Potentially Toxic Elements’ when reporting environmental research. Int. J. Environ. Res. Public Health 2019, 16, 4446.
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.; Alstrup, A.K.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006.
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Yu, Z.-G. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2020, 268, 128855.
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics and lonomics. Front. Plant Sci. 2016, 6, 1143.
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut. 2020, 231, 47.
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological responses of heavy metal pollution with special emphasis on soil health and plant performances. Front. Environ. Sci. 2017, 5, 64.
- Chormare, R.; Kumar, M.A. Environmental health and risk assessment metrics with special mention to biotransfer, bioaccumulation and biomagnification of environmental pollutants. Chemosphere 2022, 302, 134836.
- Munir, N.; Jahangeer, M.; Bouyahya, A.; El Omari, N.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Shah, S.M.A.; et al. Heavy metal contamination of natural foods is a serious health issue: A review. Sustainability 2021, 14, 161.
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264.
- Zafarzadeh, A.; Rahimzadeh, H.; Mahvi, A.H. Health risk assessment of heavy metals in vegetables in an endemic esophageal cancer region in Iran. Health Scope 2018, 7, e12340.
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and health effects of heavy metal toxicity in humans. In Poisoning in the Modern World-New Tricks for an Old Dog? IntechOpen: London, UK, 2019; Volume 10, pp. 70–90.
- Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573.
- Rzymski, P.; Rzymski, P.; Tomczyk, K.; Niedzielski, P.; Jakubowski, K.; Poniedziałek, B.; Opala, T. Metal status in human endometrium: Relation to cigarette smoking and histological lesions. Environ. Res. 2014, 132, 328–333.
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. 2021, 29, 62067–62092.
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72.
- Saxena, G.; Purchase, D.; Mulla, S.I.; Saratale, G.D.; Bharagava, R.N. Phytoremediation of Heavy Metal-Contaminated Sites: Eco-environmental Concerns, Field Studies, Sustainability Issues, and Future Prospects. In Reviews of Environmental Contamination and Toxicology; Springer: Cham, Swizerland, 2019; Volume 249, pp. 71–131.
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A Review on a Great Health Issue Worldwide. Appl. Sci. 2022, 12, 6184.
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ. Geochem. Health 2014, 36, 797–814.
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268.
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14.
- Drake, P.; Hazelwood, K.J. Exposure related health effects of silver and silver compounds: A review. Ann. Occup. Hyg. 2005, 49, 575–585.
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50.
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132.
- Shoaib, A.; Javaid, A. Oxidative Stress in Plants Exposed to Heavy Metals. In Organic Solutes, Oxidative Stress, and Antioxidant Enzymes under Abiotic Stressors; CRC Press: Boca Raton, FL, USA, 2021; pp. 133–152.
- Sciedlecka, A.; Krupa, Z. Functions of Enzymes in Heavy Metal Treated Plants. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2002.
- Rehman, A.U.; Nazir, S.; Irshad, R.; Tahir, K.; Rehman, K.U.; Islam, R.U.; Wahab, Z. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles. J. Mol. Liq. 2021, 321, 114455.
- Asgher, M.; Per, T.S.; Verma, S.; Pandith, S.A.; Masood, A.; Khan, N.A. Ethylene Supplementation Increases PSII Efficiency and Alleviates Chromium-Inhibited Photosynthesis Through Increased Nitrogen and Sulphur Assimilation in Mustard. J. Plant Growth Regul. 2018, 37, 1300–1317.
- Asgher, M.; Ahmed, S.; Sehar, Z.; Gautam, H.; Gandhi, S.G.; Khan, N.A. Hydrogen peroxide modulates activity and expression of antioxidant enzymes and protects photosynthetic activity from arsenic damage in rice (Oryza sativa L.). J. Hazard. Mater. 2021, 401, 123365.
- Emamverdian, A.; Ding, Y.; Barker, J.; Liu, G.; Hasanuzzaman, M.; Li, Y.; Ramakrishnan, M.; Mokhberdoran, F. Co-application of 24-epibrassinolide and titanium oxide nanoparticles promotes pleioblastus pygmaeus plant tolerance to Cu and Cd toxicity by increasing antioxidant activity and photosynthetic capacity and reducing heavy metal accumulation and translocation. Antioxidants 2022, 11, 451.
- Rahman, R.; Upadhyaya, H. Aluminium toxicity and its tolerance in plant: A review. J. Plant Biol. 2021, 64, 101–121.
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effects on plants and Bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708.
- Anjum, N.A.; Gill, S.S.; Gill, R.; Hasanuzzaman, M.; Duarte, A.C.; Pereira, E.; Ahmad, I.; Tuteja, R.; Tuteja, N. Metal/metalloid stress tolerance in plants: Role of ascorbate, its redox couple, and associated enzymes. Protoplasma 2014, 251, 1265–1283.
- Wani, P.A.; Khan, M.S.; Zaidi, A. Toxic effects of heavy metal on germination and physiological processes of plants. In Toxicity of Heavy Metal to Legumes and Bioremediation; Zaidi, A., Wani, P.A., Khan, M.S., Eds.; Springer-Verlag Wien: Vienna, Austria, 2012; pp. 45–66.
- Masindi, V.; Muedi, K.L. Environmental contamination by heavy metals. Heavy Metals 2018, 10, 115–132.
- Singh, U.K.; Kumar, B. Pathways of heavy metals contamination and associated human health risk in Ajay River Basin, India. Chemosphere 2017, 174, 183–199.
- Singh, J.; Kalamdhad, A.S. Effects of heavy metals on soil, plants, human health and aquatic life. Int. J. Res. Chem. Environ. 2011, 1, 15–21.
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Lett. Appl. NanoBioScience 2020, 10, 2148–2166.
- Chu, D. Effects of heavy metals on soil microbial community. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012009.
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195.
- Chauvin, C.; Trambolho, M.; Hedde, M.; Makowski, D.; Cérémonie, H.; Jimenez, A.; Villenave, C. Soil nematodes as indicators of heavy metal pollution: A meta-analysis. Open J. Soil Sci. 2020, 10, 579–601.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
13 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




