Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Environmental Sciences
Environmental contamination with a myriad of potentially toxic elements (PTEs) is triggered by various natural and anthropogenic activities. However, the industrial revolution has increased the intensity of these hazardous elements and their concentration in the environment, which, in turn, could provoke potential ecological risks. Additionally, most PTEs pose a considerable nuisance to human beings and affect soil, aquatic organisms, and even nematodes and microbes.
- potentially toxic elements
- pollution
- hazardous effects
- plant biomass
1. Introduction
Anything that gets accumulated in the natural environment beyond its permissible limit causes deleterious effects on the biotic as well as abiotic components of the ecosystem [1]. The metal contaminant known as heavy metals is one of these examples. Metalloids, transition metals, actinides, and lanthanides are all part of the subset of elements known as heavy metals that have metallic properties. The term heavy metal is generally used for metallic elements with specific weights over 5 g/cm3 and are noxious even at low concentrations [2]. To date, 23 metals have been classified as heavy metals, with the most common being lead (Pb), cadmium (Cd), cobalt (Co), chromium (Cr), and mercury (Hg), which have potentially toxic effects at high and low concentrations [3]. These toxic elements are classified as class-B and come under non-essential elements [4,5]. These metals persist in the surroundings and accumulate at different levels of the food chain, from primary producer level to consumer levels, through consumption due to their non-biodegradable nature (unlike that of organic pollutants); thus, these are deleterious for both components (biotic as well as abiotic) of an ecosystem [6,7]. Hence, it is important for environmentalists to take strategic remediation measures to reduce heavy metals’ load in the aquatic and terrestrial environments [8]. Pourret and Hursthouse [9] prefer the term ‘potentially toxic elements (PTEs)’ for such elements instead of ‘heavy metals’ due to their toxic nature. The accumulation of toxic metals in the ecosystem is continually being fueled by the agricultural use of fertilizers and pesticides, as well as industrial inputs and metal-contaminated sewage [10]. This makes it difficult to use soils effectively and safely. Furthermore, environmental heavy metal pollution posed a grave threat to human life as well as the other biotic elements of the ecosystem. Hence, it is important for environmentalists to take strategic remediation measures to reduce heavy metals’ load in aquatic and terrestrial environments [11].
2. Major Impacts of PTEs
During the last few decades, the risk of living organisms to PTE toxicity has increased due to the increase in anthropogenic activities, industrialization, and modern agricultural practices [17,18]. Due to the persistent and stable nature of PTEs, they cannot be degraded or destroyed [19]. PTEs are bio-accumulative and may gradually invade biological systems through air, water, and the series of the food chain over a definite period [20]. PTE contagion is a major ecological concern that influences the wellbeing of plants, animals, and humans, and affects the quality of the environment.
2.1. Impacts on Human
Some heavy metals have immense bio-significance to human, such asiron (Fe), zinc (Zn) and Mn. Their daily therapeutic and nutritional allowances had been suggested for good human health. However, some of these, such as arsenic (As), Pb, Cd and Hg in methylated form, have deleterious influence even at a very small amount [21]. Sources and effects of some of the PTEs are given in Table 1. PTEs such as Cd, Pb and Hg demonstrate an extensive toxicity level against biological entities, including human beings [22]. Inhalation and ingestion are the pathways through which these metals invade the human body. Hence, these lead to the diminution of some significant nutrients in the body, which consequently diminishes immunological defenses, cause growth retardation, and may increase gastrointestinal and other cancer rates [23,24,25]. Exposure to Cd and Pb might cause impaired fertility in humans. Cd is considered a metal estrogen. It can link and stimulate the alpha and beta estrogen receptors and concurrently cause the stimulation of progesterone receptors; thus, it may be designated a probable contributory mediator of estrogen-linked ailments, such as endometrial and breast cancer, endometriosis, and spontaneous abortion [26]. Pb can directly lead to an elevated risk of impulsive abortion through its possible teratogenic action [27]. Further, PTEs are probably the agents affecting the neurological system, renal functioning, and ossification process. These also attach to phosphate, deoxyribose sugar, and heterocyclic base residues of DNA, resulting in mutations by inducing alterations in DNA structures [24]. Long-term exposure to PTEs results in severe neurological diseases such as Alzheimer’s, Parkinson’s, muscular dystrophy and sclerosis.
Table 1. Sources and effects of the major PTEs on human health.
| PTE | Source(s) | Effects | Reference(s) |
|---|---|---|---|
| Arsenic (As) | Pesticides, fungicides, metal smelters | Carcinogen causes skin, lung, liver and bladder cancer. In addition, causes darkening of the skin | [28,29,30] |
| Barium (Ba) | Rodenticides, pharmaceuticals, cosmetics, diesel engines | Vomiting, abdominal cramps, diarrhea, difficulty in breathing, hypo or hypertension, numbness, paralysis or death | [31] |
| Cadmium (Cd) | Welding, electroplating, pesticides, fertilizers, batteries, nuclear plants | Stomachache, vomiting, diarrhea, kidney problems, liver damage, fragile bones, cause itai-itai | [24,29] |
| Chromium (Cr) | Mining, electroplating, textile, tanneries | Carcinogen, nose ulcers, asthma, cough, skin problems, kidney and liver damage | [28,29] |
| Lead (Pb) | Paint, batteries, pesticides, automobile emissions, mining, burning of coal | Weakness, hypertension, anemia, brain and kidney damage, miscarriage, impotence | [28,29] |
| Mercury (Hg) | Pesticides, batteries, paper industries | Minimata disease, nervous disorders, kidney damage, tremors, impaired vision and hearing, loss of memory | [28,29] |
| Selenium (Se) | Mining, agricultural wastes, petrochemicals, | Nausea, vomiting, diarrhea, sclerosis, respiratory tract irritation, bronchitis, stomach pains, bronchial spasms and cough | [32,33] |
| Silver (Au) | Industries, mining, silver plating, | Blue–grey discoloration of the skin called Argyria, breathing problems, lung and throat irritation, stomach pain, skin problems such as rashes, swelling and inflammation | [34] |
2.2. Impacts on Plants
There are four proposed mechanisms through which PTEs exert toxicities in plants [35,36] (Figure 1). In addition, PTEs affect many physiological and developmental processes in plant biosystems (Figure 2).
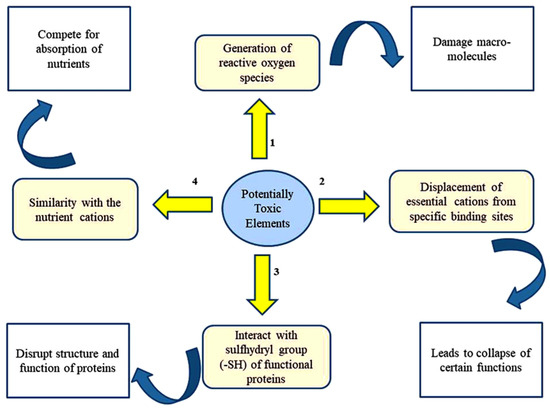
Figure 1. Major mechanisms underlying PTE-caused toxicity in plants.
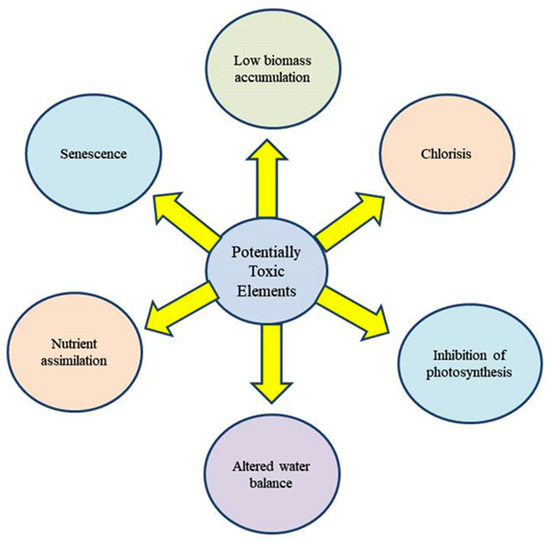
Figure 2. Major toxic effects of PTEs on plants.
The main entry points for PTE ions into plant systems are the leaves and roots. High PTE concentrations have detrimental effects, including the inhibition of cytoplasmic enzymes and oxidative stress-related cell structure damage [37]. Additionally, PTEs have detrimental effects on the development and activity of soil microorganisms, which ultimately may affect plant growth. Further, due to PTEs’ interference with the activities of soil microbes, enzyme activities important for plant metabolisms may also be hindered. PTEs are also attributed with increasing enzyme activity, such as glucose-6-phosphate dehydrogenase, and peroxides in the leaves of plants growing in contaminated soils [38]. Further, PTEs are also responsible for stomatal closure, increased ethylene production and cause iron deficiency by inhibiting uptake from the medium or due to immobilization in root tissues [39]. PTEs such as Cd, As, Cr, and Cu inhibit PSII and photosynthetic genes (psbA and psbB), and increased accumulation of PTEs in roots, stems and leaves causes reduced growth in plants [40,41,42]. Aluminum damages root tips by stimulating the synthesis and accumulation of callose by inhibiting the assembly and release of border cells [43].Further, Al can accrue in plant cells and have the potential to bind at multiple sites, such as cell wall, cell membrane and nucleic acids, and impede various biological, physiological and cellular processes [43]. Pb is one of the most prevalent toxic elements in the soil, is widely dispersed and harms the various enzymatic activities in plants [44]. PTE toxicity causes a reduction in the decomposition of litter, a reduction in the mineralization of carbon and nitrogen, and an inhibition of microbial growth [19]. Plants growing in PTE-contaminated soil experience both reduced growth and yield, as the PTEs hinder growth and developmental processes [45]. PTE toxicity has a variety of effects on plants at the cellular and molecular levels. PTEs, for example, affect germination, chlorophyll biosynthesis, photosynthesis, exchange of gases, respiration, blocking functional groups of crucial molecules for metabolism, and processes of the central dogma of life [46]. These toxic effects cause overall plant growth to slow down, which may eventually cause the plant to die [44].
2.3. Impact on Aquatic Life
Due to PTEs’ toxic nature, accumulation/biomagnification, and level of environmental persistence, the accumulation of PTEs in fresh and marine water systems is one of the principal hazards for aquatic organisms [47]. The main sources of these toxic metals in aquatic ecosystems are biological activities such as weathering, soil leaching, urban storms, as well as anthropogenic activities such as land filling, coal mining, agricultural activities, and effluent discharges [48]. PTEs can take on many different forms in aquatic systems, including being adsorbed on particulate matter, precipitating as insoluble salts, or existing in an ionic state. However, all forms are harmful to aquatic life, especially the benthic fauna, and may cause several oxidative stresses in those living things [49]. Whole plant or animal bodies are exposed to PTE toxicity in aquatic ecosystems. PTEs in the water are especially harmful to young fish and may even lead to the extinction of the entire fish population in contaminated reservoirs [50].
2.4. Impacts on Soil Health
Different levels of heavy metal concentration are measured as one of potent soil contaminants and their elevated concentrations may decrease soil productivity to a great extent by harming soil flora and fauna, which, in turn, causes alterations in the size of the population and community composition of soil microbes, and by altering the physical and chemical status of soil, including increasing soil acidity, redox potential, and enhanced decomposition of organic matter. Through other means as well, contaminants have the potential to cause shifts in microbial populations, according to the isolation-based technique [49]. Generally, an increase in PTE concentration in soil negatively affects soil microbial estates and causes an overall decrease in the growth of microbes by affecting their growth, morphology and metabolism [51,52]. PTEs are also toxic for nematodes and the most toxic PTE for nematodes is selenium (Se) [53].
This entry is adapted from the peer-reviewed paper 10.3390/plants12030429
This entry is offline, you can click here to edit this entry!
