
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vinay Raghavendra | -- | 3529 | 2023-02-09 18:43:35 | | | |
| 2 | Jessie Wu | Meta information modification | 3529 | 2023-02-10 02:22:35 | | | | |
| 3 | Jessie Wu | Meta information modification | 3529 | 2023-02-10 02:23:20 | | | | |
| 4 | Jessie Wu | Meta information modification | 3529 | 2023-02-10 02:26:52 | | | | |
| 5 | Jessie Wu | Meta information modification | 3529 | 2023-02-10 02:27:11 | | |
Video Upload Options
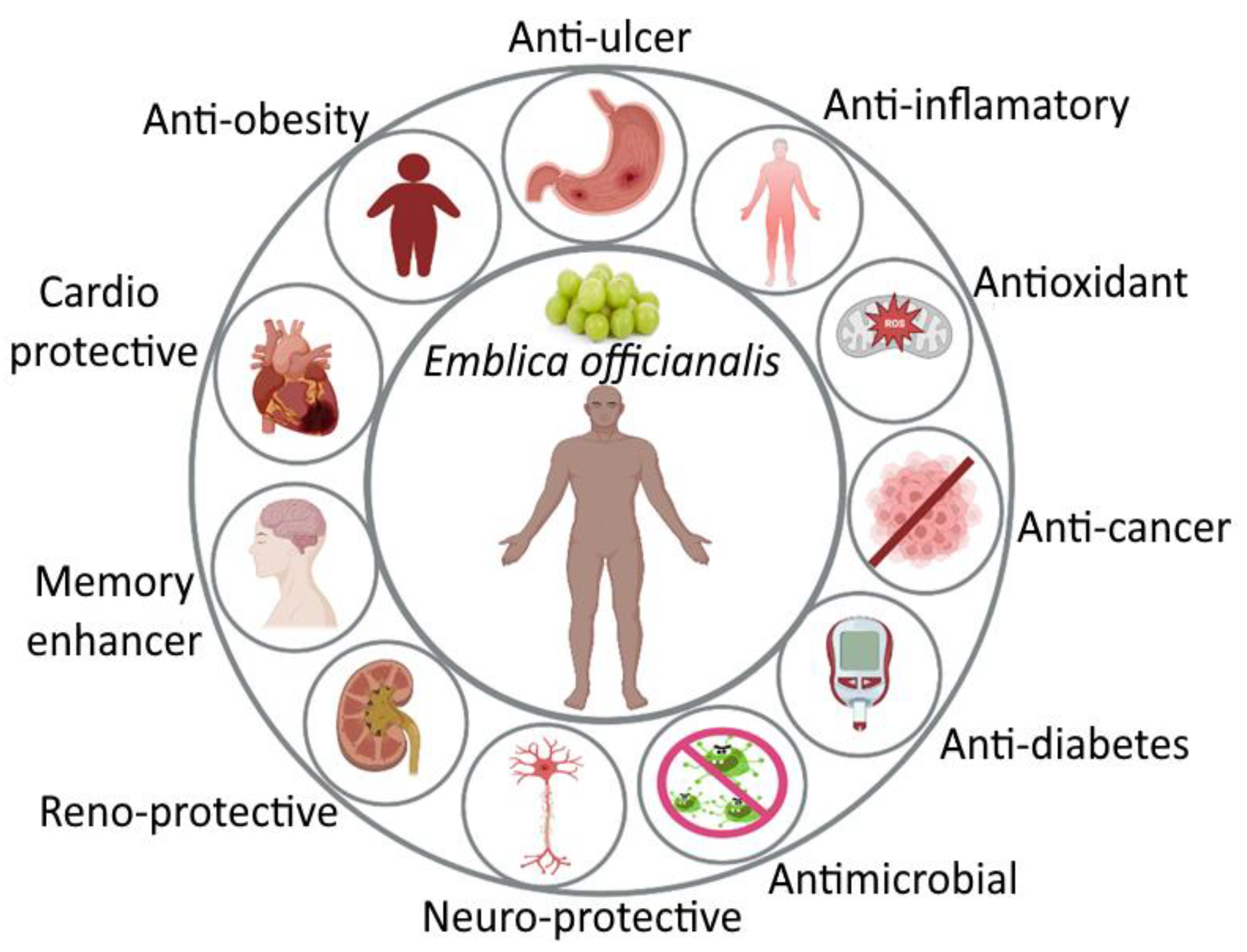
The ayurvedic herb Emblica officinalis (E. officinalis) is a gift to mankind to acquire a healthy lifestyle. It has great therapeutic and nutritional importance. Emblica officinalis, also known as Indian gooseberry or Amla, is a member of the Euphorbiaceae family. Amla is beneficial for treating illnesses in all its forms. The most crucial component is a fruit, which is also the most common. It is used frequently in Indian medicine as a restorative, diuretic, liver tonic, refrigerant, stomachic, laxative, antipyretic, hair tonic, ulcer preventive, and for the common cold and fever. Hyperlipidemia is also known as high cholesterol or an increase in one or more lipid-containing blood proteins. Various phytocompounds, including polyphenols, vitamins, amino acids, fixed oils, and flavonoids, are present in the various parts of E. officinalis. E. officinalis has been linked to a variety of pharmacological effects in earlier studies, including hepatoprotective, immunomodulatory, antimicrobial, radioprotective, and hyperlipidemic effects.
1. Introduction
2. Pharmacological Activity of Amla
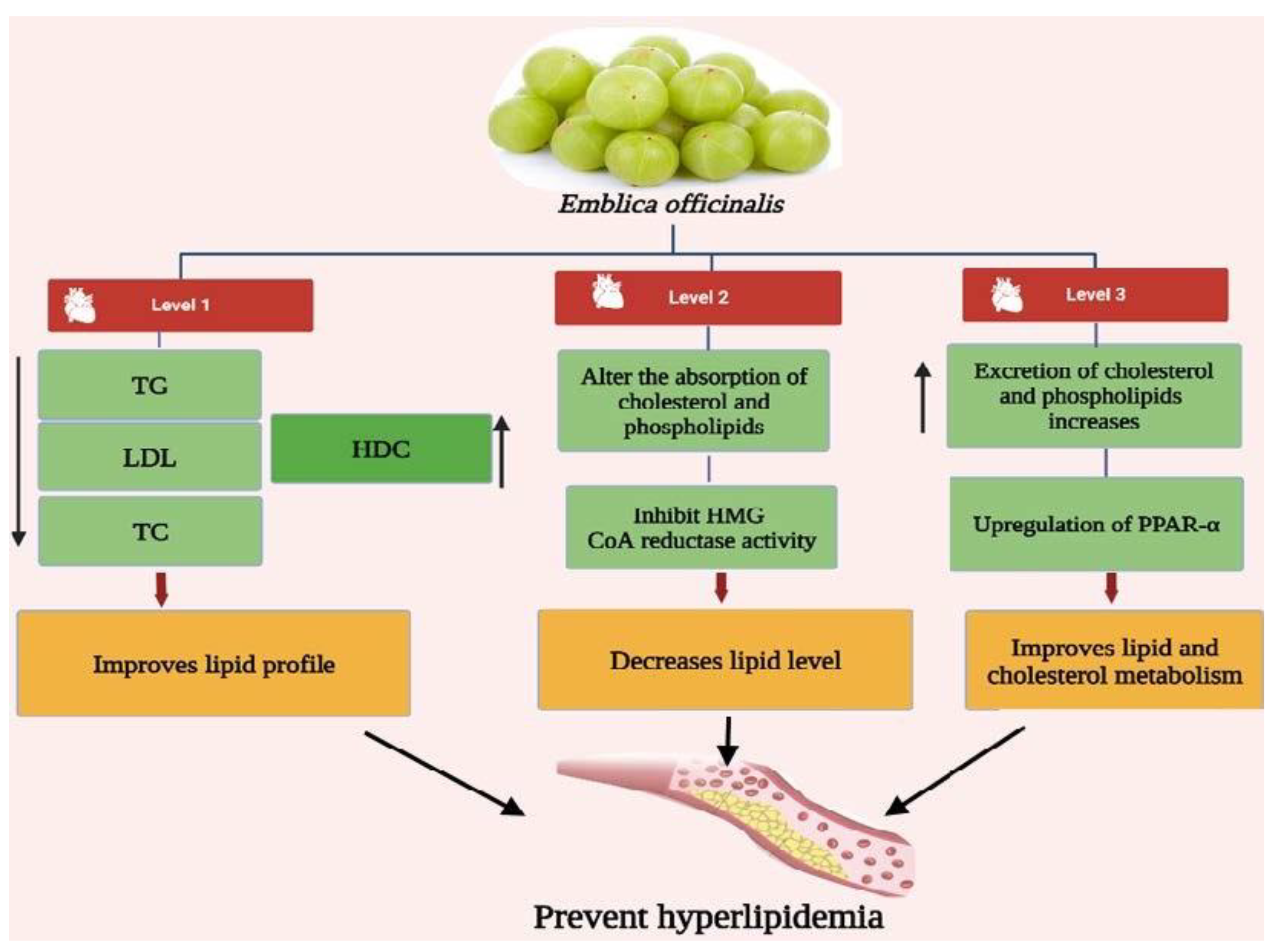
3. Antihyperlipidemic Activity of Emblica officinalis
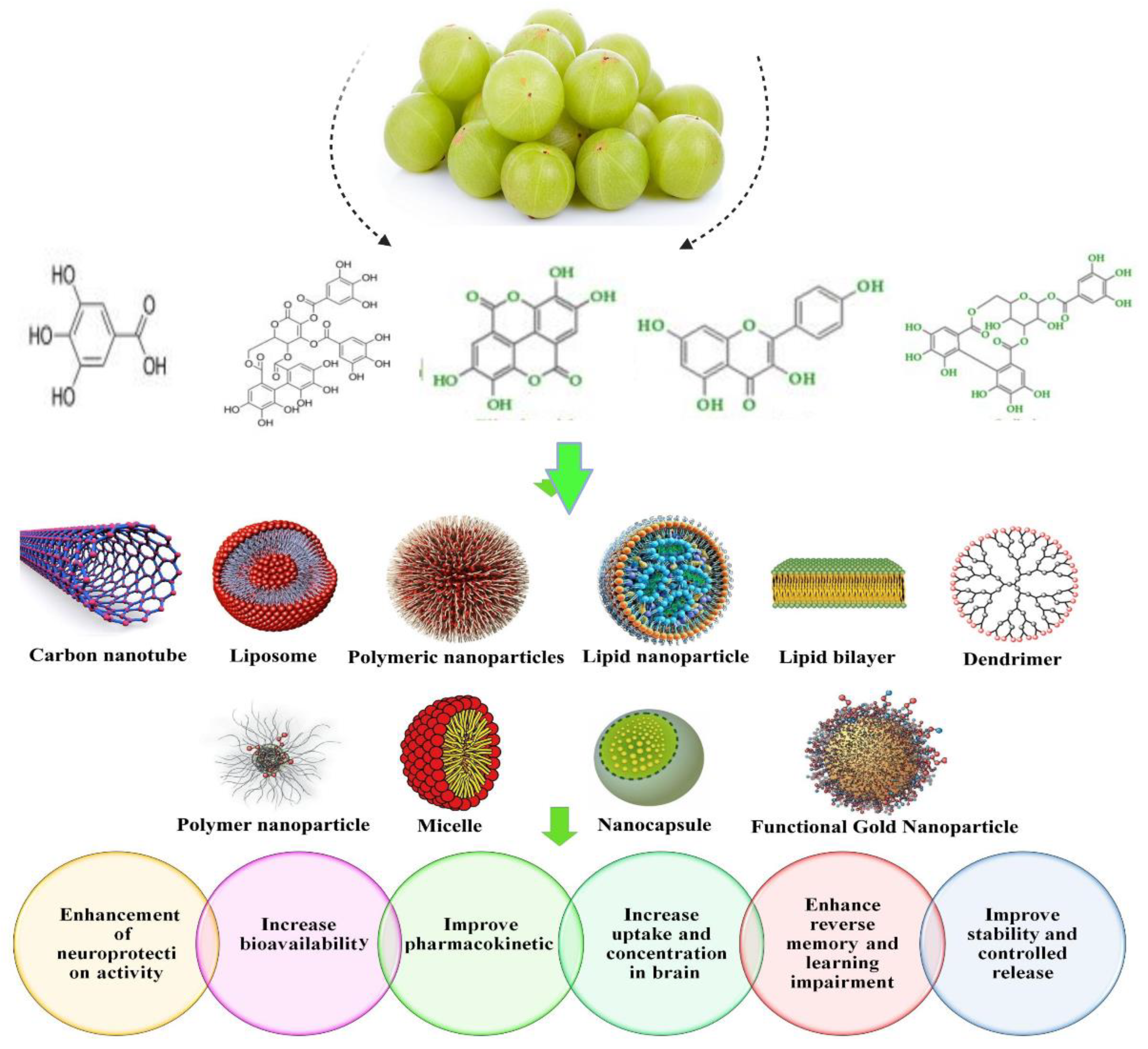
4. Nanoparticulate Carrier System for the Treatment of Hyperlipidemia
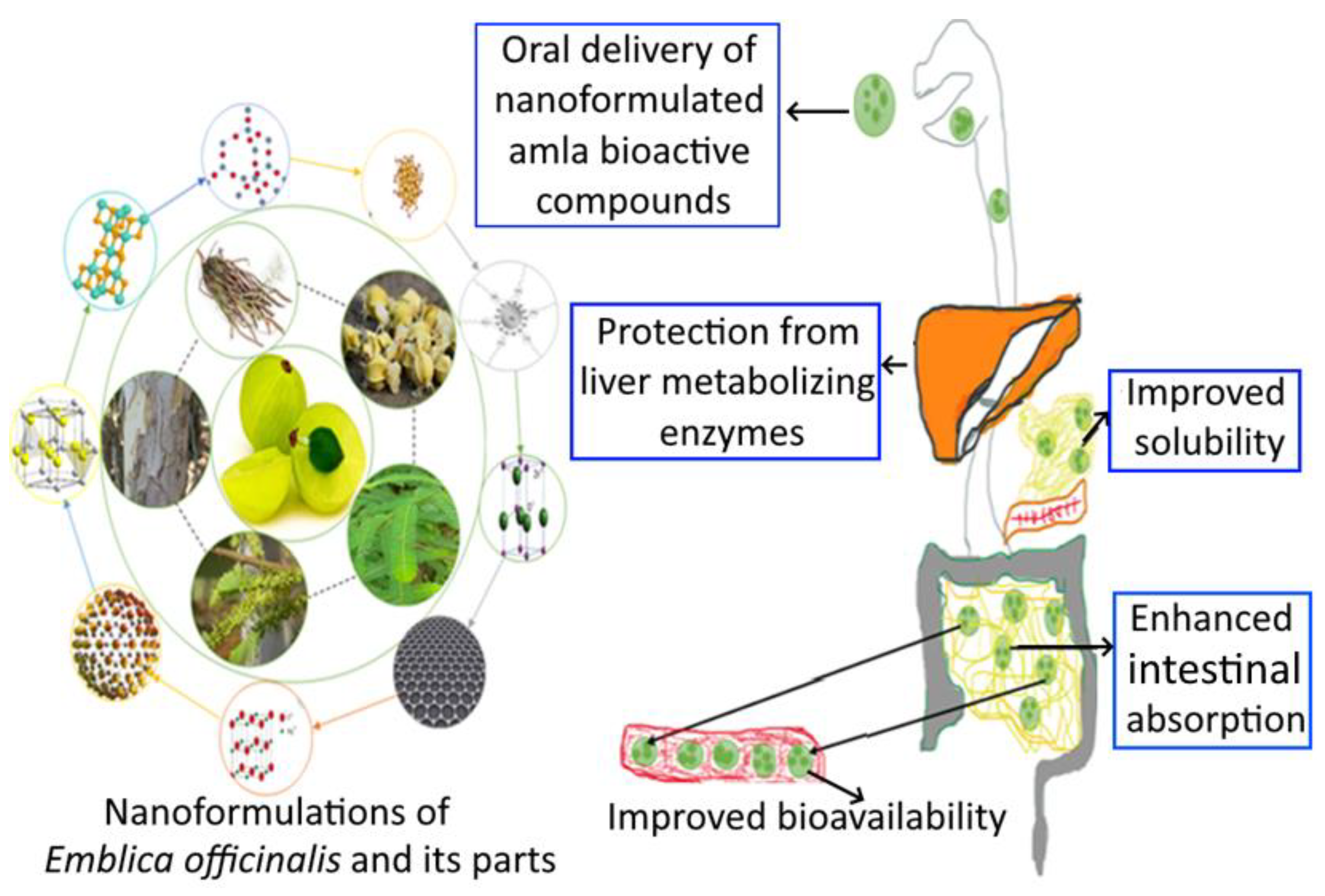
5. Nanoformulation of Emblica officinalis and Its Applications
6. Health Care Application for Emblicanin-A and Emblicanin-B Nanoformulation
7. Adversity and Toxicity of Nanoformulations
References
- Ezeh, K.J.; Ezeudemba, O. Hyperlipidemia: A Review of the Novel Methods for the Management of Lipids. Cureus 2021, 13.
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia: Review. Arch. Physiol. Biochem. 2019, 125, 128–135.
- Mirunalini, S.; Krishnaveni, M. Therapeutic potential of Phyllanthus emblica (amla): The ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 93–105.
- Muzaffar, K.; Sofi, S.A.; Makroo, H.A.; Majid, D.; Dar, B.N. Insight about the biochemical composition, postharvest processing, therapeutic potential of Indian gooseberry (amla), and its utilization in development of functional foods—A comprehensive review. J. Food Biochem. 2022, e14132.
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016, 111, 180–200.
- Brahm, A.J.; Hegele, R.A. Combined hyperlipidemia: Familial but not (usually) monogenic. Curr. Opin. Lipidol. 2016, 27, 131–140.
- Abo-Zalam, H.B.; El-Denshary, E.S.; Abdelsalam, R.M.; Khalil, I.A.; Khattab, M.M.; Hamzawy, M.A. Therapeutic advancement of simvastatin-loaded solid lipid nanoparticles (SV-SLNs) in treatment of hyperlipidemia and attenuating hepatotoxicity, myopathy and apoptosis: Comprehensive study. Biomed. Pharmacother. 2021, 139, 111494.
- Braich, A.K.; Kaur, G.; Singh, A.; Dar, B.N. Amla essential oil-based nano-coatings of Amla fruit: Analysis of morphological, physiochemical, enzymatic parameters, and shelf-life extension. J. Food Process. Preserv. 2022, 46, e16498.
- Goyal, R.; Patel, S. Emblica officinalis Geart.: A Comprehensive Review on Phytochemistry, Pharmacology and Ethnomedicinal Uses. Res. J. Med. Plant 2012, 6, 6–16.
- Fitriansyah, S.N.; Aulifa, D.L.; Febriani, Y.; Sapitri, E. Correlation of total phenolic, flavonoid and carotenoid content of Phyllanthus emblica extract from Bandung with DPPH scavenging activities. Pharmacog. J. 2018, 10, 447–452.
- Priya, G.; Parminder, N.; Jaspreet, S. Antimicrobial and antioxidant activity on Emblica officinalis seed extract. Int. J. Res. Ayur. Pharma. 2012, 3, 591–596.
- Usha, T.; Middha, S.K.; Goyal, A.K.; Lokesh, P.; Yardi, V.; Mojamdar, L.; Keni, D.S.; Babu, D. Toxicological Evaluation of Emblica officinalis Fruit Extract and its Anti-inflammatory and Free Radical Scavenging Properties. Pharmacogn. Mag. 2015, 11, S427–S433.
- Satish, S.; Mohana, D.C.; Raghavendra, M.P.; Raveesha, K.A. Antifungal activity of some plant extracts against important seed borne pathogens of Aspergillus sp. J. Agric. Technol. 2007, 3, 109–119.
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570.
- Patil, S.G.; Deshmukh, A.A.; Padol, A.R.; Kale, D.B. In vitro antibacterial activity of Emblica officinalis fruit extract by tube Dilution Method. Int. J. Toxicol. Appl. Pharmacol. 2012, 2, 49–51.
- Kamal, R.; Yadav, S.; Mathur, M.; Katariya, P. Antiradical efficiency of 20 selected medicinal plants. Nat. Prod. Res. 2012, 26, 1054–1062.
- Liu, G.; Xiong, S.; Xiang, S.; Guo, C.W.; Ge, F.; Yang, C.R.; Zhang, Y.; Wang, Y.; Kitazato, K. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Arch. Virol. 2011, 156, 1359–1369.
- Usharani, P.; Merugu, P.L.; Nutalapati, C. Evaluation of the effects of a standardized aqueous extract of Phyllanthus emblica fruits on endothelial dysfunction, oxidative stress, systemic inflammation and lipid profile in subjects with metabolic syndrome: A randomised, double blind, placebo controlled clinical study. BMC Complement. Altern. Med. 2019, 19, 97.
- Malik, S.; Suchal, K.; Bhatia, J.; Khan, S.I.; Vasisth, S.; Tomar, A.; Goyal, S.; Kumar, R.; Arya, D.S.; Ojha, S.K. Therapeutic potential and molecular mechanisms of Emblica officinalis gaertn in countering nephrotoxicity in rats induced by the chemotherapeutic agent cisplatin. Front. Pharmacol. 2016, 7, 350.
- Purena, R.; Seth, R.; Bhatt, R. Protective role of Emblica officinalis hydro-ethanolic leaf extract in cisplatin induced nephrotoxicity in Rats. Toxicol. Rep. 2018, 5, 270–277.
- Dhingra, D.; Joshi, P.; Gupta, A.; Chhillar, R. Possible Involvement of Monoaminergic Neurotransmission in Antidepressant-like activity of Emblica officinalis Fruits in Mice. CNS Neurosci. Ther. 2012, 18, 419–425.
- Suja, R.S.; Nair, A.M.C.; Sujith, S.; Preethy, J.; Deepa, A.K. Evaluation of immunomodulatory potential of Emblica officinalis fruit pulp extract in mice. Indian J. Anim. Res. 2009, 43, 103–106.
- Ansari, A.; Shahriar, S.Z.; Hassan, M.; Das, S.R.; Rokeya, B.; Haque, A.; Haque, E.; Biswas, N.; Sarkar, T. Emblica officinalis improves glycemic status and oxidative stress in STZ induced type 2 diabetic model rats. Asian Pac. J. Trop. Med. 2014, 7, 21–25.
- Akhtar, M.S.; Ramzan, A.; Ali, A.; Ahmad, M. Effect of Amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients. Int. J. Food Sci. Nutr. 2011, 62, 609–616.
- Gopa, B.; Bhatt, J.; Hemavathi, K.G. A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3- hydroxy-3-methylglutaryl-coenzyme-a reductase inhibitor simvastatin. Indian J. Pharmacol. 2012, 44, 238.
- Variya, B.C.; Bakrania, A.K.; Chen, Y.; Han, J.; Patel, S.S. Suppression of abdominal fat and anti-hyperlipidemic potential of Emblica officinalis: Upregulation of PPARs and identification of active moiety. Biomed. Pharmacother. 2018, 108, 1274–1281.
- Husain, I.; Zameer, S.; Madaan, T.; Minhaj, A.; Ahmad, W.; Iqubaal, A.; Ali, A.; Najmi, A.K. Exploring the multifaceted neuroprotective actions of Emblica officinalis (Amla): A review. Metab. Brain Dis. 2019, 34, 957–965.
- Shubhi, M.; Rohitash, J.; Radhey, S.; Dharmendra, K.M.; Kshipra, M.; Rajashree, P.; De, R.; Mukhopadhyay, A.; Srivastava, A.K.; Shoma, P.N. Anti-Helicobacter pylori and antioxidant properties of Emblica officinalis pulp extract: A potential source for therapeutic use against gastric ulcer. J. Med. Plants Res. 2011, 5, 2577–2583.
- Al-Rehaily, A.J.; Al-Howiriny, T.S.; Al-Sohaibani, M.O.; Rafatullah, S. Gastroprotective effects of ‘Amla’Emblica officinalis on in vivo test models in rats. Phytomedicine 2002, 9, 515–522.
- Santoshkumar, J.; Manjunath, S.; Sakhare, P.M. A study of anti-hyperlipedemia, hypolipedemic and anti-atherogenic activity of fruit of Emblica officinalis (amla) in high fat fed Albino rats. Int. J. Med. Res. Health Sci. 2013, 2, 70–77.
- Kapoor, M.P.; Suzuki, K.; Derek, T.; Ozeki, M.; Okubo, T. Clinical evaluation of Emblica officinalis Gatertn (Amla) in healthy human subjects: Health benefits and safety results from a randomized, double-blind, crossover placebo-controlled study. Contemp. Clin. Trials Commun. 2020, 17, 100499.
- Gul, M.; Liu, Z.-W.; Haq, I.U.; Rabail, R.; Faheem, F.; Walayat, N.; Nawaz, A.; Shabbir, M.A.; Munekata, P.E.S.; Lorenzo, J.M.; et al. Functional and Nutraceutical Significance of Amla (Phyllanthus emblica L.): A Review. Antioxidants 2022, 11, 816.
- Kim, H.Y.; Okubo, T.; Juneja, L.R.; Yokozawa, T. The protective role of amla (Emblica officinalis Gaertn.) against fructose-induced metabolic syndrome in a rat model. Br. J. Nutr. 2010, 103, 502–512.
- Nisar, M.F.; He, J.; Ahmed, A.; Yang, Y.; Li, M.; Wan, C. Chemical components and biological activities of the genus Phyllanthus: A review of the recent literature. Molecule 2018, 23, 2567.
- Kaur, J.; Kaur, D.; Singh, H.; Khan, M.U. Emblica officinalis: A meritocratic drug for treating various disorders. Indo Am. J. Pharm. Res. 2013, 3, 2231–6876.
- Nambiar, S.S.; Paramesha, M.; Shetty, N.P. Comparative analysis of phytochemical profile, antioxidant activities and foam prevention abilities of whole fruit, pulp and seeds of Emblica officinalis. J. Food Sci. Technol. 2015, 52, 7254–7262.
- Zahid, S.; Anjum, K.M.; Mughal, M.S.; Yaqub, A.; Yameen, M. Evaluation of antioxidant and antihyperlipidemic activity of Indian gooseberry (Emblica officinalis) fruit in high fat-fed rabbits. JAPS J. Animal Plant Sci. 2018, 28, 1007–1013.
- Iyer, U.; Shah, A.; Venugopal, S. Amla (Emblica officinalis) and Guava (Psidium guajava) supplementation: Impact of Low Carbon footprint local seasonal fruits on Lipemic Status of Morning Walkers. Eco. Environ. Cons. 2021, 27, S233–S238.
- Sandeep, B.S.; Panda, N.; Sethy, K.; Nath, S. Effect of dietary supplementation of amla (Emblica officinalis) powder and equivalent synthetic vitamin C on growth performance in black rock broiler chicken. Pharma Innov. J. 2022, 11, 2002–2007.
- Dabulici, C.M.; Sârbu, I.; Vamanu, E. The Bioactive Potential of Functional Products and Bioavailability of Phenolic Compounds. Foods 2020, 9, 953.
- Taleuzzaman, M.; Mahapatra, D.K.; Gupta, D.K. Emblicanin-A and Emblicanin-B: Pharmacological and Nano-Pharmacotherapeutic Perspective for Healthcare Applications. In Applied Pharmaceutical Practice and Nutraceuticals; Apple Academic Press: Waretown, NJ, USA, 2021; pp. 13–27.
- Li, T.; Liang, W.; Xiao, X.; Qian, Y. Nanotechnology, an alternative with promising prospects and advantages for the treatment of cardiovascular diseases. Int. J. Nanomed. 2018, 13, 7349–7362.
- Han, H.S.; Koo, S.Y.; Choi, K.Y. Emerging nanoformulation strategies for phytocompounds and applications from drug delivery to phototherapy to imaging. Bioact. Mater. 2022, 14, 182–205.
- Moradi, S.Z.; Momtaz, S.; Bayrami, Z.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of Herbal Extracts in Treatment of Neurodegenerative Disorders. Front. Bioeng. Biotechnol. 2020, 8, 238.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Kapoor-Narula, U.; Lenka, N. Phytochemicals and their nanoformulation in sustained drug delivery and therapy. In Innovations in Fermentation and Phytopharmaceutical Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–220.
- Enrico, C. Nanotechnology-Based Drug Delivery of Natural Compounds and Phytochemicals for the Treatment of Cancer and Other Diseases. Stud. Nat. Prod. Chem. 2019, 62, 91–123.
- Omran, Z.; Bader, A.; Porta, A.; Vandamme, T.; Anton, N.; Alehaideb, Z.; El-Said, H.; Faidah, H.; Essa, A.; Vassallo, A.; et al. Evaluation of Antimicrobial Activity of Triphala Constituents and Nanoformulation. Evid.-Based Complement. Altern. Med. 2020, 2020, 6976973.
- Rosarin, S.; Fathima, S.; Vadivel, A.; Samuthira, N.; Sankaran, M. Antiproliferative effect of silver nanoparticles synthesized using amla on Hep2 cell line. Asian Pac. J. Trop Med. 2013, 6, 1–10.
- Abitha, S.T.; Rajeshkumar, S.; Lakshmi, T.; Roy, A. Cytotoxic effects of silver nanoparticles synthesized using amla fruit seed. Drug Invent. Today 2019, 1, 11.
- Soundarajan, S.; Sankari, M.; Rajeshkumar, S. Antibacterial activity and cytotoxicity of amla seed mediated graphene oxide, silver nanoparticle and go-ag nanoparticle-an in vitro study. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 55–67.
- Ranjani, S.; Hemalatha, S. Triphala decorated multipotent green nanoparticles and its applications. Mater. Lett. 2021, 308, 131184.
- Naik, S.; Jarnain, T.; David, N. Phytofabrication of silver and zinc oxide nanoparticles using the fruit extract of Phyllanthus emblica and its potential anti-diabetic and anti-cancer activity. Part Sci. Technol. 2022, 1–13.
- Harakeh, S.; Mohammed, A.; Al Soad, J.; Saad, A.; Saber, H.; Al Turki, A.; Najiah; Azhar, E. Antidiabetic effects of novel ellagic acid nanoformulation: Insulin-secreting and anti-apoptosis effects. Saudi J. Biol. Sci. 2020, 27, 3474–3480.
- Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263.
- Patil, P.; Suresh, K. Chitosan and glyceryl monooleate nanostructures containing gallic acid isolated from amla fruit: Targeted delivery system. Heliyon 2021, 7, e06526.
- Alfei, S.; Barbara, M.; Guendalina, Z.; Federica, T.; Cinzia, D. Dendrimer nanodevices and gallic acid as novel strategies to fight chemoresistance in neuroblastoma cells. Nanomaterials 2020, 10, 1243.
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New perspectives on the efficacy of gallic acid in cosmetics & nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187.
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448.
- Alfei, S.; Catena, S.; Turrini, F. Biodegradable and biocompatible spherical dendrimer nanoparticles with a gallic acid shell and a double-acting strong antioxidant activity as potential device to fight diseases from “oxidative stress”. Drug Deliv. Transl. Res. 2019, 10, 259–270.
- Mady, F.; Ibrahim, S.R.-M. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076.
- Rosman, R.; Saifullah, B.; Maniam, S.; Dorniani, D.; Hussein, M.Z.; Fakurazi, S. Improved Anticancer Effect of Magnetite Nanocomposite Formulation of Gallic Acid (Fe3 O4 -PEG-GA) Against Lung, Breast and Colon Cancer Cells. Nanomaterials 2018, 8, 83.
- Klooster, S.T.; Villeneuve, P.; Bourlieu-Lacanal, C.; Durand, E.; Schroën, K.; Berton-Carabin, C. Alkyl chain length modulates antioxidant activity of gallic acid esters in spray-dried emulsions. Food Chem. 2022, 387, 132880.
- Pathak, K.; Das, R.J.; Saikia, R.; Sahariah, J.J.; Pathak, H.; Sarma, H.; Das, A. Design and fabrication of gallic acid loaded chitosan nanoformulation. Drug Deliv. Lett. 2022, 12, 135–148.
- Harakeh, S.; Qari, M.; Rajeh, N.; Ali, S.; El-Shitany, N.; Hassan, S.; Abd-Allah, E.A.; Tashkandi, H.; Malik, M.F.A.; Aljabri, F.K.; et al. Ellagic acid nanoparticles attenuate oxidative stress and testicular damage in high fat Diet/Streptozotocin-Induced diabetic rats. J. King Saud Univ. Sci. 2022, 34, 101720.
- Dayar, E.; Pechanova, O. Targeted Strategy in Lipid-Lowering Therapy. Biomedicines 2022, 10, 1090.
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217.
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303–5321.
- Martakov, I.S.; Shevchenko, O.G.; Torlopov, M.A.; Gerasimov, E.Y.; Sitnikov, P.A. Formation of gallic acid layer on γ-AlOOH nanoparticles surface and their antioxidant and membrane-protective activity. J. Inorg. Biochem. 2019, 199, 110782.
- Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.B.; Sridhar, K.; Sharma, M. Advances in Nanofabrication Technology for Nutraceuticals: New Insights and Future Trends. Bioengineering 2022, 9, 478.




