Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chaiyavat Chaiyasut | -- | 2758 | 2023-02-06 08:38:59 | | | |
| 2 | Sirius Huang | Meta information modification | 2758 | 2023-02-07 04:47:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kesika, P.; Sivamaruthi, B.S.; Chaiyasut, C. Factors Affecting Hair Health. Encyclopedia. Available online: https://encyclopedia.pub/entry/40861 (accessed on 07 February 2026).
Kesika P, Sivamaruthi BS, Chaiyasut C. Factors Affecting Hair Health. Encyclopedia. Available at: https://encyclopedia.pub/entry/40861. Accessed February 07, 2026.
Kesika, Periyanaina, Bhagavathi Sundaram Sivamaruthi, Chaiyavat Chaiyasut. "Factors Affecting Hair Health" Encyclopedia, https://encyclopedia.pub/entry/40861 (accessed February 07, 2026).
Kesika, P., Sivamaruthi, B.S., & Chaiyasut, C. (2023, February 06). Factors Affecting Hair Health. In Encyclopedia. https://encyclopedia.pub/entry/40861
Kesika, Periyanaina, et al. "Factors Affecting Hair Health." Encyclopedia. Web. 06 February, 2023.
Copy Citation
Hair health is associated with personal distress and psychological well-being. Even though hair loss (alopecia) does not affect humans’ biological health, it affects an individual’s social well-being. So, treatment for hair problems and improving hair health are obligatory.
hair loss
alopecia
phytochemicals
hair health
hair growth stimulation
1. Introduction
The history of alopecia areata (AA) starts approximately 1500 before the common era (BCE), but scientific studies on hair loss have accelerated in recent decades in terms of publications [1]. Even though hair is not important for humans in terms of biological protection, hair loss has significant social, psychological, and emotional impacts on everyone. Therefore, treatment for hair loss and improving hair health is obligatory.
Humans have ~100,000 scalp hair shafts with varying degrees of hair growth, and the average life cycle of a hair shaft is ~3.5 years, with a growth rate of 0.05 inches per month. The hair has three regions, such as medulla (innermost layer, developed from transparent cells and varies among hair types), cortex (middle layer, which provides strength to the hair shaft, composed of keratin protein), and cuticle (outer layer). The hair follicle can be divided into the lower, middle (isthmus), and upper (infundibulum) segments. The lower segment includes the bulb and suprabulb regions. The middle segment of the hair follicle includes the region covering the arrector pili muscle insertion to the opening of the sebaceous gland duct. The upper segment of the follicle includes the region between the opening of the sebaceous gland duct and the follicular orifice [2].
Anagen (growth phase, lasts for 2 to 7 years), catagen (exponentiation, lasts for 2 to 4 weeks), and telogen (resting phase, lasts for 3 months) are three major phases of the hair growth cycle. The amount of scalp hairs may change based on the anagen (85 to 90.6%), telogen (10 to 15%), and catagen (1 to 2%) phases. Pigmented hair shafts are produced in the anagen phase, and the follicle achieves its maximum length and volume. During the catagen phase, the epithelium of the lower follicle breaks and grows up with the papilla until it lays below the bulge zone, establishing the club hair. Telogen is a quiescence phase of the hair cycle, characterized by the reduction of proliferation and biological activity of hair follicles [2][3][4].
Hair color, density, hair fiber curvature, and diameter are affected by the aging of hair, which overall contribute to the appearance and manageability of hair [5][6]. Numerous factors affect hair health and the hair growth cycle. Heavy metals (thallium, mercury, arsenic), toxins (Botulinum, Podostroma cornu-damae), drugs, medications, genetics, stress, smoking, menopause, lifestyle, and diet are some of the major factors associated with hair health [7][8][9][10][11][12][13][14].
Various drugs and treatment strategies were used to treat hair loss. Minoxidil was the first drug approved by the FDA to treat hair loss [15][16]. 5α reductase inhibitors (e.g., Finasteride, Dutasteride) are also used to treat male androgenetic alopecia [17][18]. The combination of finasteride and minoxidil treatment significantly improved hair health, and the combination therapy is more effective than a single treatment procedure. Furthermore, hair transplantation [19] and cell therapy [20] are effective treatments for hair loss.
Many phytochemicals include epigallocatechin gallate (EGCG), caffeine, capsaicin, procyanidin, onion juice, pumpkin seed oil, rosemary oil, saw palmetto [21][22], red ginseng extract [22], curcumin, garlic gel, and other natural products such as amino acids, marine proteins, melatonin, vitamins, and zinc [21], were reported to have hair growth-stimulating property [21][22]. Recently, researchers have been interested in herbal-based nanomedicine for hair health [23]. Furthermore, several mechanisms have been proposed and proved for the hair growth-promoting properties of phytochemicals [24][25][26][27][28][29][30].
2. Types of Alopecia
Alopecia can be classified based on the cause and appearance and rarely by gender. Androgenetic alopecia (AGA), telogen effluvium (TE), alopecia areata (AA), and scarring alopecia (SA) are the common types of hair loss [31].
AGA is an androgen-dependent hair loss. About 58% of men may be affected by AGA. Hair loss starts with bitemporal hairline decline and thinning at the vertex and frontal-parietal regions. About 33% of Caucasian women aged 70 or older may be affected by AGA. Postmenopausal and genetically susceptible women are mainly more sensitive to AGA [31].
TE is a nonscarring form of hair loss, a scalp disorder exemplified by excessive hair shedding [32]. The changes in the hair cycle could cause TE, which could be acute or chronic hair loss. Older women are more susceptible to acute TE attributed to drugs, childbirth, and thyroid-related diseases [33], even though age’s influence on TE is unclear. The incidence of TE in children was about 2.7% in Southeast Nigeria [34]. Telogen gravidarum, nutrition, zinc, iron deficiency, genetics, chronic renal and hepatic failure, syphilis, early AGA, infection, stress, and malignancy are some factors that cause TE [33]. There are five types of TE based on the hair follicle cycle: immediate and delayed anagen release, immediate and delayed telogen release, and short anagen syndrome [35]. High fever, surgical trauma, starvation, and hemorrhage are the triggers for acute TE. The hair loss may occur after two to three months of the triggering event. Not all cases have the triggering event in their life. So, the cause of TE is still not yet understood completely [33][35]. The diagnosis of acute TE can be conducted by history and examination. Acute TE is a self-limiting biological event, so if the hair loss stops spontaneously, no further treatments are needed. Nevertheless, if hair loss continues (Chronic TE), medical evaluations are needed to find the cause and proper treatment [31].
AA is a nonscarring and defined hair loss due to the premature conversion of the anagen to telogen phase in hairs. Family history and autoimmune conditions are the major cause of AA; other than that, no specific cause of AA has been defined yet [36]. A higher incidence of AA has been observed in the female population. The etiology of AA is elucidated completely. The activation of immune cell androgen and estrogen receptors during pregnancy, X chromosome-mediated innate immune response and immune tolerance, and maternal micro-chimerism of fetal immune cell lines are associated with the etiology of AA. The average age of disease inception varied based on ethnicity and gender. Female patients with autoimmune diseases are reported for the incidence and progress of AA compared to their male equivalents [37].
SA is irreversible hair loss and is commonly found in European Caucasian women. SA is divided into two subgroups such as primary SA and secondary SA. In primary SA, hair follicles have microscopic inflammation and damage the follicular epithelium without affecting the interfollicular reticular dermis. Primary SA is associated with chronic cutaneous lupus erythematous (an autoimmune condition), pseudopelade of Brocq, lichen planopilaris (inflammation in hair follicles), folliculitis decalvans (chronic inflammation of hair follicles and scalp), and dissecting folliculitis. The underlying disease that causes primary SA could be predicted by knowing the type of inflammatory infiltrates around hair follicles. In secondary SA, hair follicle damage is related to a non-follicle-directed cause [38]. Exogenous factors such as trauma, endogenous infiltrative, and inflammation cause secondary SA [39]. Central centrifugal cicatricial alopecia (CCCA) caused by hair treatments using chemicals and irons is also considered secondary SA [31], and its incidence is associated with diabetes mellitus [40].
3. Factors Affecting Hair Health
Several factors affect hair health, including genetics, chemical exposure, lifestyle, smoking, drugs, stress, infection, and menopause. Evidence for some of the influencing factors of hair health has been detailed in this section (Figure 1).
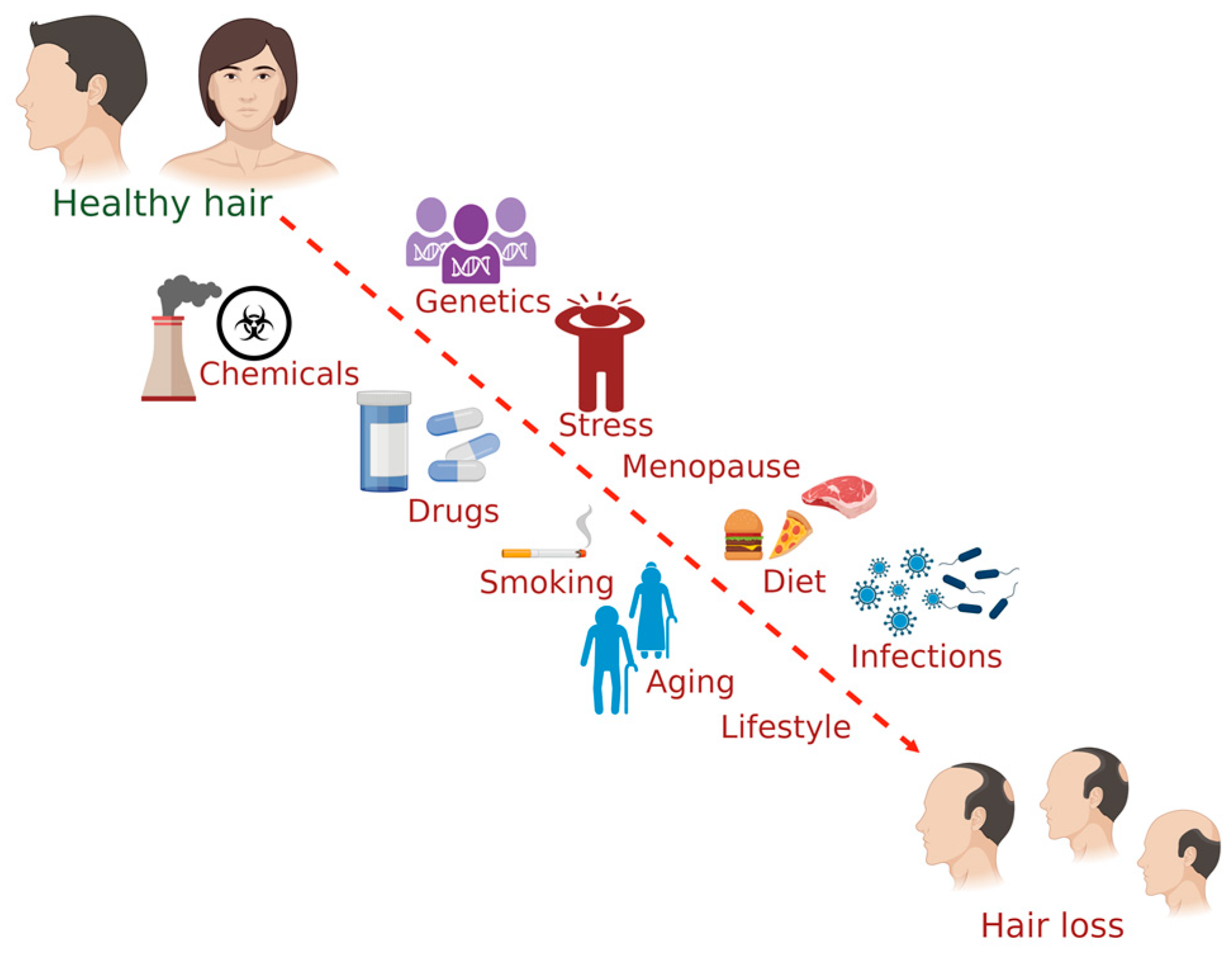
Figure 1. The common factors influencing hair health in humans. (The figure was created using BioRender.com; accessed on 17 November 2022).
3.1. Diet
Consumption of nutritional supplements such as micronutrients (vitamin A, vitamin E, and selenium) in excess leads to hair loss [41]. Toxic dose exposure to selenium shows symptoms of hair loss, fatigue, foul odor (garlic breath), changes in nails (discoloration, brittleness), nausea, and vomiting. MacFarquhar et al. [42] analyzed the outbreak of acute selenium poisoning due to the consumption of a liquid dietary supplement, which contained 200 times higher than the labeled concentration of selenium. About 201 patients with selenium poisoning showed adverse effects; among them, 140 patients showed hair loss [42]. Lecythis ollaria Loefl. nuts (L. ollaria; paradise nuts) contain a high concentration of selenium (7 to 12 g of selenium per kg dry mass of L. ollaria nuts) in the form of seleno-cystathionine [43]. Two healthy women who consumed a handful of L. ollaria nuts showed symptoms of selenium poisoning. Hair loss started for 38-year-old women twelve days after the consumption, and 46-year-old women showed hair loss two weeks after the consumption [44]. A recent study reported that high-fat diet-fed mice showed hair thinning by reducing the hair follicle stem cells compared to the standard diet-fed mice and stated that obesity accelerated the thinning of hair and leads to hair loss [45].
Carbohydrates also affect hair health. The consumption of simple sugar-containing processed foods is one of the indirect factors associated with hair loss. Simple sugar stimulates sebum secretion, and even though sebum is good, excess secretion facilitates microbial growth in the scalp, which further causes irritation and inflammation [46][47]. High sugar intake impacts hair loss by activating the polyol pathways [48][49][50]. A recent study revealed that consuming sugar-sweetened beverages is linked with a high risk of male pattern hair loss in young men [51].
3.2. Chemical Exposure
Exposure to heavy metals such as mercury and thallium is a highly associated risk factor for alopecia. Studies have evidenced the toxic effects of those chemicals on hair [52][53]. Case studies report the toxic exposure of arsenic, botulinum toxin, boric acid, Podostroma cornu-damae, synthetic opioid MT-45, and vitamin A. A case study reported heavy metal (arsenic and mercury) poisoning in a 51-year-old man showing anorexia, dizziness, fever, hair loss, and rash after the topical use of Chinese homeopathic medicine for anal fistulas [52]. Sharquie et al. [54] evaluated five patients (gender: male; age range: 10-32 years old) with thallium poisoning due to consuming cakes laced with thallium. All the patients showed symptoms of thallium poisoning, including anagen hair loss that started in the 2nd and 3rd week after thallium exposure [54]. The reported minimum lethal dose of thallium was 10 to 15 mg per kg [55]. A study reported that 1 week after thallium poisoning, three out of five patients showed profound hair loss [53].
3.3. Drugs
A retrospective hospital-based study analyzed many cases of poisoning in Sri Lanka caused by the intake of plants and mushrooms that contain toxic substances [56]. Gloriosa superba L. (G. superba) contains high levels of colchicine, an alkaloid drug. Colchicine is used in the treatment of amyloidosis, familial Mediterranean fever [57], Behçet’s disease [58], and gout [59]. It has a narrow therapeutic–toxicity window and variability in the level of tolerance among individuals [60]. The toxicity risk is dose-dependent, and intake of <0.5 mg per kg is reported with no mortality rate, intake of 0.5–0.8 mg per kg is reported with a 10% mortality rate, and intake of colchicine >0.8 mg per kg leads to 100% mortality rate [60]. The symptoms of colchicine toxicity consist of 3 phases. Gastrointestinal symptoms, diarrhea, leukocytosis, and reduced blood volume occur during the 1st phase (during the first 24 h after ingestion of a toxic colchicine dose). Bone marrow hypoplasia, leukopenia, and possible multiple organ failure occur during the second phase (from 2nd to 7th day). Renewal of the bone marrow activity, rebound of leukocytosis, recovery from multiple organ failure, and the onset of alopecia in the 3rd phase (from the 2nd week onwards is the recovery period). Hair growth starts after 3–12 weeks [61][62].
A male (age: 26 years old) started losing hair on the 9th day after consuming G. superba (2 tubers) [63]. A case study reported a child (gender: female; age: 3 years old) with acute colchicine intoxication showed symptoms of multi-system organ failure followed by alopecia, which started on the 2nd week after consuming 20 to 25 pills (a toxic dose of about 0.9 mg per kg) [64]. A case study reported that a female (age: 17 years old) attempted suicide by consuming 40 pills of colchicine (40 mg) which caused severe pancreatitis and bicytopenia, followed by anagen effluvium, which started one week after the colchicine poisoning [65]. Alaygut et al. [66] evaluated 17 pediatric cases with colchicine intoxication until their recovery as an observational case series study during January 2010–2012 at the Pediatric Intensive Care Unit of Cumhuriyet University Faculty of Medicine, Turkey. Among the 17 patients, only 1 patient (gender: female; age: 16 years old) showed total alopecia who ingested colchicine (0.88 mg per kg) on attempting suicide [66].
3.4. Diseases or Disorders
Abnormal skin conditions such as atopic dermatitis, dandruff/seborrheic dermatitis, psoriasis, and tinea capitis affect hair health. The hair samples of atopic dermatitis patients (n = 8; age range = 24 to 25 years old) showed thick scales and torn cuticular edges, which were examined using atomic force microscopy. The roughness of the hair of atopic dermatitis patients was higher than that of healthy adults (n = 15; age range = 20 to 54 years old) [67]. Plozzer et al. [68] examined the hair obtained from psoriasis patients (n = 39; age range = 24 to 74 years old) and healthy individuals using scanning electron microscopy. The altered hair conditions, such as abraded cuticular surface and cuticular breakage observed only in the hair samples obtained from the psoriasis patients [68]. Similarly, Kumar et al. [69] examined the hair obtained from psoriasis patients (n = 30; age range = 12 to 67 years old) and healthy individuals (n = 15; age range = 18 to 62 years old) using scanning electron microscopy. Hair thinning and loss in the psoriatic plaques are due to hair dystrophy (alteration in hair cuticles such as ragged appearance and roughness) and micro pits [69]. The lesioned hairs obtained from the psoriasis patients (n = 15; age range = 8 to 67 years old) showed increased macro pits, the roughness of the cuticle, and scale thickness compared to that of the healthy adult individuals (n = 15; age range = 10 to 58 years old), which was examined using atomic force microscopy [70]. Kim et al. [71] analyzed the hair shafts from psoriasis patients (n = 14; age range = 24 to 75 years) and seborrheic dermatitis patients (n = 28; age range = 19 to 78 years) in comparison with the hair samples obtained from healthy adult individuals (n = 50; age range = 21 to 60 years). The hair samples of all psoriasis patients included in the study showed macro pits, while only 4% of seborrheic dermatitis patients showed macro pits. Scale thickness of the hair samples was common among psoriasis and seborrheic dermatitis patients [71]. Seborrheic dermatitis is associated with premature hair loss [72]. Alterations in the hair, including corkscrew hairs, comma hairs, or zigzag hairs, were observed in patients with tinea capitis (fungal infection) [73].
3.5. Smoking
An observational study stated that smoking is associated with premature graying of hair in both men and women and baldness in men [74].
Su and Chen [12] studied the association between smoking and androgenetic alopecia by conducting a community-based cross-sectional survey (10th April and 12th June 2005) among men (n = 740; ≥40 years old) living in Tainan County, Taiwan. Based on the survey, the family history of androgenetic alopecia among 1st and 2nd degree relatives is associated with an increased risk of moderate or severe androgenetic alopecia. Besides the family history and age, the risk factors for androgenetic alopecia include cigarette smoking [12]. A cross-sectional study conducted by Chaudhry et al. [75] with men (n = 398; ≥36.50 ± 12.11 years old) concluded that smoking is the risk factor for baldness. A recent study showed that premature graying and hair loss prevalence is higher in smokers than in nonsmokers [76].
3.6. Genetics
Chumlea et al. [9] investigated the association between the expression of androgenic alopecia in male individuals (from the general community) and their family history of androgenic alopecia. They stated that family history and age are the high-risk factors for the onset of male pattern hair loss. Other risk factors might include hair loss in the father and hair loss in the mother or maternal grandfather [9]. Lukasik et al. [10] studied the association between the family history of androgenic alopecia in females and the onset of female pattern hair loss in women from the Polish population. Family histories of female individuals (n = 111 unrelated patients) with androgenic alopecia and female individuals (n = 129) with no hair thinning were analyzed. It stated that a family history of maternal hair loss is a high-risk factor for the onset of female pattern hair loss in women. Other high-risk factors might include a family history of androgenic alopecia in grandparents [10].
3.7. Stress
Stress is a risk factor for hair loss and inhibition of hair growth. Chronic or acute stress is a primary inducer of hair growth disorder, namely, telogen effluvium; it acts as an aggravating factor for hair growth disorders such as androgenetic alopecia and alopecia areata [77]. Adrenal glands release stress hormones induced by stress signals. Cortisol is a stress hormone released in humans during stress conditions, and corticosterone is a stress hormone in rodents. A recent study stated that corticosterone inhibits the activity of hair follicle stem cells by suppressing the expression of the growth arrest-specific 6 (gas6) gene, thereby extending the hair follicles’ resting phase. Corticosterone-induced inhibition of hair follicle’s stem cell activity recovered by restoring the expression of the gas6 gene [11].
3.8. Menopause
References
- Broadley, D.; McElwee, K.J. A “hair-raising” history of alopecia areata. Exp. Dermatol. 2020, 29, 208–222.
- Park, A.M.; Khan, S.; Rawnsley, J. Hair biology: Growth and pigmentation. Facial Plast. Surg. Clin. N. Am. 2018, 26, 415–424.
- Mulinari-Brenner, F.; Neto, F.J.; Rosas, F.M.B.; Torres, L.F.B. Morphometry of normal scalp hair follicles. An. Bras. Dermatol. 2006, 81, 46–52.
- Wosicka, H.; Cal, K. Targeting to the hair follicles: Current status and potential. J. Dermatol. Sci. 2010, 57, 83–89.
- Trüeb, R.M. Aging of hair. J. Cosmet. Dermatol. 2005, 4, 60–72.
- Trüeb, R.M.; Henry, J.P.; Davis, M.G.; Schwartz, J.R. Scalp condition impacts hair growth and retention via oxidative stress. Int. J. Trichol. 2018, 10, 262–270.
- Yu, V.; Juhász, M.; Chiang, A.; Atanaskova Mesinkovska, N. Alopecia and associated toxic agents: A systematic review. Skin Appendage Disord. 2018, 4, 245–260.
- Mercke, Y.; Sheng, H.; Khan, T.; Lippmann, S. Hair loss in psychopharmacology. Ann. Clin. Psychiatry 2000, 12, 35–42.
- Chumlea, W.C.; Rhodes, T.; Girman, C.J.; Johnson-Levonas, A.; Lilly, F.R.; Wu, R.; Guo, S.S. Family history and risk of hair loss. Dermatology 2004, 209, 33–39.
- Łukasik, A.; Kozicka, K.; Kłosowicz, A.; Jaworek, A.; Wojas-Pelc, A. The role of family history and its influence on the onset time in female pattern hair loss. Postepy Dermatol. Alergol. 2021, 38, 815–818.
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432.
- Su, L.H.; Chen, T.H. Association of androgenetic alopecia with smoking and its prevalence among Asian men: A community-based survey. Arch. Dermatol. 2007, 143, 1401–1406.
- Chaikittisilpa, S.; Rattanasirisin, N.; Panchaprateep, R.; Orprayoon, N.; Phutrakul, P.; Suwan, A.; Jaisamrarn, U. Prevalence of female pattern hair loss in postmenopausal women: A cross-sectional study. Menopause 2022, 29, 415–420.
- Guo, E.L.; Katta, R. Diet and hair loss: Effects of nutrient deficiency and supplement use. Dermatol. Pract. Concept. 2017, 7, 1–10.
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194.
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–566.
- Arca, E.; Açikgöz, G.; Taştan, H.B.; Köse, O.; Kurumlu, Z. An open, randomized, comparative study of oral finasteride and 5% topical minoxidil in male androgenetic alopecia. Dermatology 2004, 209, 117–125.
- Olsen, E.A.; Whiting, D.; Bergfeld, W.; Miller, J.; Hordinsky, M.; Wanser, R.; Zhang, P.; Kohut, B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2007, 57, 767–774.
- Leavitt, M.; Perez-Meza, D.; Rao, N.A.; Barusco, M.; Kaufman, K.D.; Ziering, C. Effects of finasteride (1 mg) on hair transplant. Dermatol. Surg. 2005, 31, 1268–1276.
- Sung, J.H. Effective and economical cell therapy for hair regeneration. Biomed. Pharmacother. 2023, 157, 13988.
- Hosking, A.M.; Juhasz, M.; Atanaskova Mesinkovska, N. Complementary and alternative treatments for alopecia: A comprehensive review. Skin Appendage Disord. 2019, 5, 72–89.
- Daniels, G.; Akram, S.; Westgate, G.E.; Tamburic, S. Can plant-derived phytochemicals provide symptom relief for hair loss? A critical review. Int. J. Cosmet. Sci. 2019, 41, 332–345.
- Padule, K.; Shinde, S.; Chitlange, S.; Giram, P.; Nagore, D. The advancement of herbal-based nanomedicine for hair. Cosmetics 2022, 9, 118.
- Saansoomchai, P.; Limmongkon, A.; Surangkul, D.; Chewonarin, T.; Srikummool, M. Enhanced VEGF expression in hair follicle dermal papilla cells by Centella asiatica Linn. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 25–37.
- Pandit, S.; Chauhan, N.S.; Dixit, V.K. Effect of Cuscuta reflexa Roxb on androgen-induced alopecia. J. Cosmet. Dermatol. 2008, 7, 199–204.
- Mustarichie, R.; Wicaksono, I.A. Hair growth stimulants activity from Sterculia urceolata JE Smith ethanol extract. Res. J. Pharm. Technol. 2019, 12, 4111–4116.
- Yuen, G.K.; Ho, B.S.; Lin, L.S.; Dong, T.T.; Tsim, K.W. Tectoridin stimulates the activity of human dermal papilla cells and promotes hair shaft elongation in mouse vibrissae hair follicle culture. Molecules 2022, 27, 400.
- Ahmed, N.S.; Ghatak, S.; El Masry, M.S.; Gnyawali, S.C.; Roy, S.; Amer, M.; Everts, H.; Sen, C.K.; Khanna, S. Epidermal E-cadherin dependent β-catenin pathway is phytochemical inducible and accelerates anagen hair cycling. Mol. Ther. 2017, 5, 2502–2512.
- Choi, B.Y. Hair-growth potential of ginseng and its major metabolites: A review on its molecular mechanisms. Int. J. Mol. Sci. 2018, 19, 2703.
- Lin, W.H.; Xiang, L.J.; Shi, H.X.; Zhang, J.; Jiang, L.P.; Cai, P.T.; Lin, Z.L.; Lin, B.B.; Huang, Y.; Zhang, H.L.; et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Res. Int. 2015, 2015, 730139.
- Coleman, E. Types and Treatment of Hair Loss in Men and Women. Plast. Surg. Nurs. 2020, 40, 222–235.
- Asghar, F.; Shamim, N.; Farooque, U.; Sheikh, H.; Aqeel, R. Telogen effluvium: A review of the literature. Cureus 2020, 12, e8320.
- Harrison, S. Sinclair, R. Telogen effluvium. Clin. Exp. Dermatol. 2002, 27, 389–395.
- Nnoruka, E.N.; Obiagboso, I.; Maduechesi, C. Hair loss in children in South-East Nigeria: Common and uncommon cases. Int. J. Dermatol. 2007, 46, 18–22.
- Headington, J. Telogen effluvium. new concepts and review. Arch. Dermatol. 1993, 129, 356–363.
- Messenger, A.G.; McKillop, J.; Farrant, P.; McDonagh, A.J.; Sladden, M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br. J. Dermatol. 2012, 166, 916–926.
- Lundin, M.; Chawa, S.; Sachdev, A.; Bhanusali, D.; Seiffert-Sinha, K.; Sinha, A.A. Gender differences in alopecia areata. J. Drugs Dermatol. 2014, 13, 409–413.
- Filbrandt, R.; Rufaut, N.; Jones, L.; Sinclair, R. Primary cicatricial alopecia: Diagnosis and treatment. CMAJ 2013, 185, 1579–1585.
- Villablanca, S.; Fischer, C.; García-García, S.C.; Mascaró-Galy, J.M.; Ferrando, J. Primary scarring alopecia: Clinical-pathological review of 72 cases and review of the literature. Skin Appendage Disord. 2017, 3, 132–143.
- Kyei, A.; Bergfeld, W.F.; Piliang, M.; Summers, P. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia. Arch. Dermatol. 2011, 147, 909–914.
- Finner, A.M. Nutrition and hair. Deficiencies and supplements. Dermatol. Clin. 2013, 31, 167–172.
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261.
- Hammel, C.; Kyriakopoulos, A.; Behne, D.; Gawlik, D.; Brätter, P. Protein-bound selenium in the seeds of coco de mono (Lecythis ollaria). J. Trace. Elem. Med. Biol. 1996, 10, 96–102.
- Müller, D.; Desel, H. Acute selenium poisoning by paradise nuts (Lecythis ollaria). Hum. Exp. Toxicol. 2010, 29, 431–434.
- Morinaga, H.; Mohri, Y.; Grachtchouk, M.; Asakawa, K.; Matsumura, H.; Oshima, M.; Takayama, N.; Kato, T.; Nishimori, Y.; Sorimachi, Y.; et al. Obesity accelerates hair thinning by stem cell-centric converging mechanisms. Nature 2021, 595, 266–271.
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343S–348S.
- Goluch-Koniuszy, Z.S. Nutrition of women with hair loss problem during the period of menopause. Menopause Rev. 2016, 15, 56–61.
- Lee, W.S.; Ro, B.I.; Hong, S.P.; Bak, H.; Sim, W.Y.; Kim, D.W.; Park, J.K.; Ihm, C.W.; Eun, H.C.; Kwon, O.S.; et al. A new classification of pattern hair loss that is universal for men and women: Basic and specific (BASP) classification. J. Am. Acad. Dermatol. 2007, 57, 37–46.
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017–1026.
- Figlak, K.; Paus, R.; Williams, G.; Philpott, M. 597 Outer root sheath is able to synthesise glycogen from lactate-investigating glycogen metabolism in human hair follicles. J. Investig. Dermatol. 2019, 139, S317.
- Shi, X.; Tuan, H.; Na, X.; Yang, H.; Yang, Y.; Zhang, Y.; Xi, M.; Tan, Y.; Yang, C.; Zhang, J.; et al. The association between sugar-sweetened beverages and male pattern hair loss in young men. Nutrients 2023, 15, 214.
- Wu, M.L.; Deng, J.F.; Lin, K.P.; Tsai, W.J. Lead, mercury, and arsenic poisoning due to topical use of traditional Chinese medicines. Am. J. Med. 2013, 126, 451–454.
- Namba, Y.; Suzuki, R.; Sasaki, J.; Takayasu, M.; Watanabe, K.; Kenji, D.; Hayashi, M.; Kitamura, Y.; Kawamo, M.; Masaki, H.; et al. Thallium group poisoning incident in Japan 2011. Crit. Care 2013, 17 (Suppl. 2), P269.
- Sharquie, K.E.; Ibrahim, G.A.; Noaimi, A.A.; Hamudy, H.K. Outbreak of thallium poisoning among Iraqi patients. JSSDDS 2011, 15, 29–32.
- Moeschlin, S. Thallium poisoning. Clin. Toxicol. 1980, 17, 133–146.
- Fernando, R.; Fernando, D.N. Poisoning with plants and mushrooms in Sri Lanka: A retrospective hospital-based study. Vet. Hum. Toxicol. 1990, 32, 579–581.
- Nakamura, N.; Fujita, T.; Murakami, R.; Kumasaka, R.; Shimada, M.; Shimaya, Y.; Osawa, H.; Yamabe, H.; Okumura, K.; Yachie, A. A case of familial Mediterranean fever-associated systemic amyloidosis. CEN Case Rep. 2012, 1, 4–6.
- Alpsoy, E.; Leccese, P.; Emmi, G.; Ohno, S. Treatment of Behçet’s disease: An algorithmic multidisciplinary approach. Front. Med. 2021, 8, 624795.
- Richette, P.; Bardin, T. Colchicine for the treatment of gout. Expert Opin. Pharmacother. 2010, 11, 2933–2938.
- Finkelstein, Y.; Aks, S.E.; Hutson, J.R.; Juurlink, D.N.; Nguyen, P.; Dubnov-Raz, G.; Pollak, U.; Koren, G.; Bentur, Y. Colchicine poisoning: The dark side of an ancient drug. Clin. Toxicol. 2010, 48, 407–414.
- Folpini, A.; Furfori, P. Colchicine toxicity-clinical features and treatment. Massive overdose case report. J. Toxicol. Clin. Toxicol. 1995, 33, 71–77.
- Levsky, M.E.; Miller, M.A.; Masneri, D.A.; Borys, D. Colchicine exposures: The Texas experience. South. Med. J. 2008, 101, 480–483.
- Premaratna, R.; Weerasinghe, M.S.; Premawardana, N.P.; de Silva, H.J. Gloriosa superba poisoning mimicking an acute infection- a case report. BMC Pharmacol. Toxicol. 2015, 16, 27.
- Biçer, S.; Soysal, D.D.; Ctak, A.; Uçsel, R.; Karaböcüoğlu, M.; Uzel, N. Acute colchicine intoxication in a child: A case report. Pediatr. Emerg. Care 2007, 23, 314–317.
- Combalia, A.; Baliu-Piqué, C.; Fortea, A.; Ferrando, J. Anagen effluvium following acute colchicine poisoning. Int. J. Trichol. 2016, 8, 171–172.
- Alaygut, D.; Kilic, S.C.; Kaya, A.; Oflaz, M.B.; Bolat, F.; Cevit, Ö.; Icagasioglu, F.D. Assessment of 17 pediatric cases with colchicine poisoning in a 2-year period. Pediatr. Emerg. Care 2016, 32, 168–172.
- Kim, K.; Shin, M.K.; Kim, J.H.; Kim, M.H.; Haw, C.R.; Park, H.K. Effects of atopic dermatitis on the morphology and water content of scalp hair. Micros. Res. Tech. 2012, 75, 620–625.
- Plozzer, C.; Coletti, C.; Kokelj, F.; Trevisan, G. Scanning electron microscopy study of hair shaft disorders in psoriasis. Acta Derm. Venereol. Suppl. (Stockh) 2000, 211, 9–11.
- Kumar, B.; Soni, A.; Saraswat, A.; Kaur, I.; Dogra, S. Hair in psoriasis: A prospective, blinded scanning electron microscopic study. Clin. Exp. Dermatol. 2008, 33, 491–494.
- Shin, M.K.; Kim, K.S.; Ahn, J.J.; Kim, N.I.; Park, H.K.; Haw, C.R. Investigation of the hair of patients with scalp psoriasis using atomic force microscopy. Clin. Exp. Dermatol. 2012, 37, 156–163.
- Kim, K.S.; Shin, M.K.; Ahn, J.J.; Haw, C.R.; Park, H.K. A comparative study of hair shafts in scalp psoriasis and seborrheic dermatitis using atomic force microscopy. Skin Res. Technol. 2013, 19, e60–e64.
- Pitney, L.; Weedon, D.; Pitney, M. Is seborrhoeic dermatitis associated with a diffuse, low-grade folliculitis and progressive cicatricial alopecia? Australas J. Dermatol. 2016, 57, e105–e107.
- Dhaille, F.; Dillies, A.S.; Dessirier, F.; Reygagne, P.; Diouf, M.; Baltazard, T.; Lombart, F.; Hébert, V.; Chopinaud, M.; Verneuil, L.; et al. A single typical trichoscopic feature is predictive of tinea capitis: A prospective multicentre study. Br. J. Dermatol. 2019, 181, 1046–1051.
- Mosley, J.G.; Gibbs, A.C. Premature grey hair and hair loss among smokers: A new opportunity for health education? BMJ 1996, 313, 1616.
- Chaudhry, M.; Ashraf, T.; Zeeshan, N.; Hanif, A.; Khan, M.; Ghazanfar, I. Association of smoking with baldness and graying of hair among male adults. Biomedica 2018, 34, 53–56.
- Babadjouni, A.; Pouldar Foulad, D.; Hedayati, B.; Evron, E.; Mesinkovska, N. The effects of smoking on hair health: A systematic review. Skin Appendage Disord. 2021, 7, 251–264.
- Thom, E. Stress and the hair growth cycle: Cortisol-induced hair growth disruption. J. Drugs Dermatol. 2016, 15, 1001–1004.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
8.1K
Revisions:
2 times
(View History)
Update Date:
07 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




