Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, Y.; Liang, J.; Deng, B.; Jiang, Y.; Zhu, J.; Chen, L.; Li, M.; Li, J. CRISPR/Cas9-Mediated Base Editing in Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/40814 (accessed on 07 February 2026).
Li Y, Liang J, Deng B, Jiang Y, Zhu J, Chen L, et al. CRISPR/Cas9-Mediated Base Editing in Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/40814. Accessed February 07, 2026.
Li, Yizhen, Jing Liang, Bufang Deng, Yingli Jiang, Jingyan Zhu, Like Chen, Min Li, Juan Li. "CRISPR/Cas9-Mediated Base Editing in Plants" Encyclopedia, https://encyclopedia.pub/entry/40814 (accessed February 07, 2026).
Li, Y., Liang, J., Deng, B., Jiang, Y., Zhu, J., Chen, L., Li, M., & Li, J. (2023, February 03). CRISPR/Cas9-Mediated Base Editing in Plants. In Encyclopedia. https://encyclopedia.pub/entry/40814
Li, Yizhen, et al. "CRISPR/Cas9-Mediated Base Editing in Plants." Encyclopedia. Web. 03 February, 2023.
Copy Citation
The clustered regularly interspaced, short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) is a third-generation gene editing technology following ZFNs and TALENs. It has the advantages of being highly efficient, simple, inexpensive, and easily usable. In the CRISPR/Cas9 system, a Cas9-single guide RNA (sgRNA) complex binds to a specific nucleotide sequence with the guidance of the sgRNA and cleaves the target DNA strand, causing a double-strand break (DSB).
base editing
CRISPR/Cas9
plant
genome editing
crop improvement
1. Introduction
The clustered regularly interspaced, short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) is a third-generation gene editing technology following ZFNs and TALENs. It has the advantages of being highly efficient, simple, inexpensive, and easily usable [1][2][3].
In the CRISPR/Cas9 system, a Cas9-single guide RNA (sgRNA) complex binds to a specific nucleotide sequence with the guidance of the sgRNA and cleaves the target DNA strand, causing a double-strand break (DSB) [4][5][6][7]. These DSBs can be corrected by nonhomologous end-joining (NHEJ) or the homology-directed repair (HDR) mechanism [8][9]. NHEJ is a method of repair in which the ends of DSBs are directly linked by DNA ligase and do not depend on homologous DNA sequences; therefore, NHEJ repair is rapid but not exact. The homologous repair process is complex and precise but requires a homologous DNA sequence template and can occur only in the G2/S phase of a cell [10][11][12][13].
Due to the genetic basis underlying the diversity of many important crop species and single-nucleotide variations [14][15], it is necessary to develop a technique that allows precise and effective single-base substitutions. Base editing technology is a novel target gene modification technique developed based on the CRISPR/Cas system, by its utilization of a tethered deaminase domain or nickase Cas9 for base conversion from A > G or C > T or C > G without the donor DNA and a DSB introduction in the genome. Recent studies have utilized Base-editors to create single and multiple nucleotide modifications in cells.
2. Base Editing
Base editors (BEs) enable single-nucleotide targeted mutations without severing the nucleic acid backbone and enable direct chemical modification of target nucleobases. The original base editor used a single-stranded DNA-specific cytidine deaminase combined with an inactivated Cas9 (dCas9) to convert a cytosine (C)-guanine (G) base pair to thymine (T)-adenine (A) in the target region, called the cytosine base editor (CBE). Later researchers developed the Adenine Base Editor and the Guanine Base Editor based on the CBE. Currently available base editing systems include cytosine (C) base editors (CBEs), adenine (A) base editors (ABEs), and guanine (G) base editors (CGBEs) (Figure 1) [16][17][18][19]. Each of these categories is discussed below.

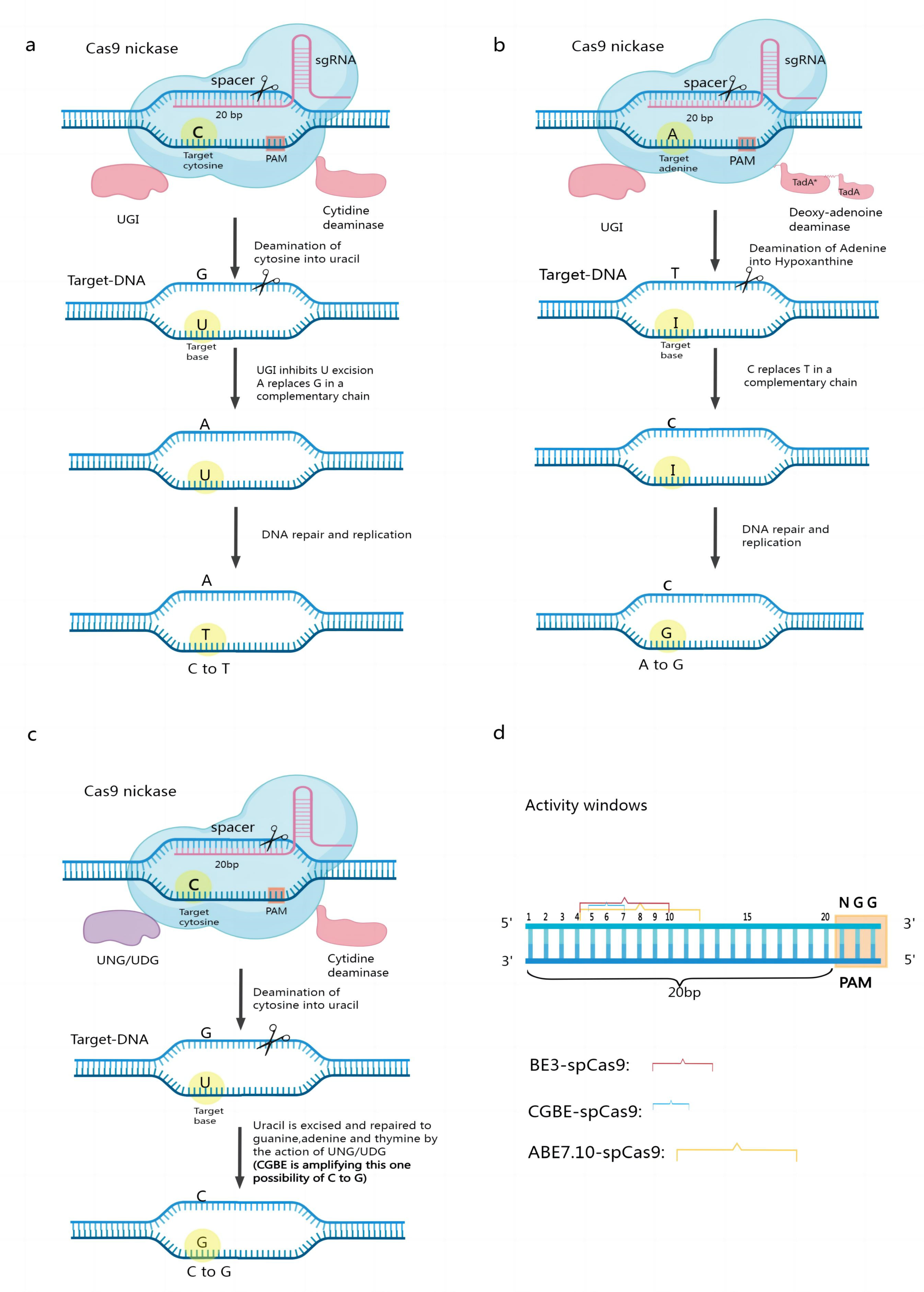
Figure 1. Mechanism of base editing work. (a) Structure and working mechanism of the cytosine base editor; (b) Structure and working mechanism of the adenine base editor; (c) Structure and editing mechanism of CGBE; (d) Window of activity for some typical CBEs, ABEs, and CGBEs.
3. CBEs
The first-generation CBE, BE1, consists of rat C deaminase (rAPOBEC1) and dCas9, whose cleavage activity is completely lost [20][21]. When the fusion protein targets genomic DNA under the guidance of sgRNA, C deaminase can bind to the ssDNA in the R-loop region formed by the Cas9 protein, sgRNA, and genomic DNA and deaminate C to uracil (U) within a certain range along the ssDNA. During DNA replication, U is read by DNA polymerase as thymine (T). The final substitution of C/G to T/A base pairs then occurs (Figure 1a). Later researchers developed a second-generation cytosine base editor, BE2, by incorporating a uracil DNA glycosylase inhibitor (UGI) from phage PBS on top of BE1. Because UGI can inhibit the action of uracil DNA glycosylase (UDG) in the organism, BE2 is three times more efficient at editing than BE1. CBEs have undergone several generations of updates; notably, BE3 has replaced dCas9 in BE2 with nCas9(D10A) [22][23][24][25]. nCas9(D10A) specifically creates a gap in the nonedited strand, which in turn stimulates the intracellular base mismatch repair pathway (MMR) [26][27], which uses the editing strand containing U as a template for repair, resulting in increased editing efficiency. These optimized CBEs can better serve precision breeding [18][24][28]. However, existing CBEs/CGBEs rely on the natural cytosine deaminase AID/APOBEC and often produce high insertional deletion by-products and off-target effects due to the activation of the base excision repair pathway by cytosine deamination. Recently, researchers have transformed the adenine deaminase TadA-8e into a non-natural cytosine deaminase using only cytosine as a substrate and constructed the first novel CGBE/CBE family of base editors—Td-CGBE/Td-CBEs—that do not rely on the AID/APOBEC deaminase family, demonstrating lower off-target effects and very low indels events. Constructing a corresponding base editor in plants will facilitate crop improvement [29].
4. ABEs
Three main components compose ABEs: synthetic A deaminase, nCas9 (D10A), and sgRNA [30]. The A deaminase protein binds to ssDNA and deaminates A into inosine I, which is then read and replicated as G at the DNA level. This enables the instant exchange of A–T base pairs with G–C base pairs when the fusion protein targets genomic DNA under the guidance of sgRNA [31] (Figure 1b). Using ABEs eliminates the limitation that CBEs can edit only C or G and opens up a wider range of base transformation possibilities. In contrast to CBEs, ABEs do not require the suppression of alkyl adenine DNA glycosylase (AAG) activity [32][33][34].
5. CGBEs
C-to-G base editors (CGBEs) were constructed by adapting existing CBE tools to generate a new tool suitable for mediating C–G base reversals [35].
Combining a Cas9 nickase (nCas9-D10A), cytidine deaminase, and Uracil-N-glycosylase (UNG) leads to the production of CGBEs. Cytidine deaminase causes the conversion of a target C to U under the guidance of RNA. UNG locates U in the DNA and eliminates it, resulting in the formation of an AP site [36]. When nCas9 creates the AP site and binds the nonedited strand, DNA repair and replication mechanisms are triggered, preferentially inserting a G at the AP site. In contrast to CBEs, which contain a UNG inhibitor, CGBEs contain UNG [37][38] (Figure 1c).
In the base editor, the single-stranded DNA in the R-loop is exposed during base editing. This single-stranded DNA binds to the 20 bp of the sgRNA, but there is a preference for the action of cytidine deaminase on this 20 bp fragment so that different base editors have specific BE activity windows. The Activity Window of the Classical Cytosine Base Editor BE3-SpCas9 is bases 4–10. Bases 4–12 of the classical adenine is the base editor ABE7.10-SpCas9 activity window. Bases 5–7 of the classical guanine base editor is the CGBE-SpCas9 activity window. The different base editor activity windows depend on various factors, such as Cas proteins, deaminases, and variant connectors [39][40][41] (Figure 1d).
6. Application of Base Editors in Plants
In the long history of breeding, several major strategies have been used, such as crossbreeding, mutation breeding, etc. Gene editing and transgenics are an important part of the new breeding era [42][43][44][45][46][47]. Crossbreeding can only introduce known good traits [48][49]. Mutation breeding is a longer breeding process in which researchers create random mutations throughout the plant genome through physical and chemical mutagenesis. Transgenic breeding techniques allow for the direct introduction of good genes specific to a crop or genes from other species to obtain crop varieties with higher yields and better nutritional quality [50][51][52][53]. However, this breeding method requires the integration of exogenous genes into the plant genome and is, therefore, subject to strict controls. BE technology is an effective complement to the above three breeding methods. BEs allow for targeted modification of the plant genome without introducing exogenous genes to obtain the target variety quickly [54][55][56][57].
In recent years, public investment in research has been used to sequence, assemble and annotate the genomes of major crops, and a wealth of functional genetic information on plants have been gained [54][55][56][57][58]. Base editing allows for precise genome editing, and its successful operation in breeding has opened up new opportunities for crop improvement. Since 2016, BEs have been used to edit the genomes of various plant species, including rice, maize, cotton, oilseed rape, tomato, strawberry, and watermelon [59][60][61][62][63][64][65][66][67][68][69].
References
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405.
- Khalil, A.M. The genome editing revolution: Review. J. Genet. Eng. Biotechnol. 2020, 18, 68.
- Gupta, D.; Bhattacharjee, O.; Mandal, D.; Sen, M.K.; Dey, D.; Dasgupta, A.; Kazi, T.A.; Gupta, R.; Sinharoy, S.; Acharya, K.; et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. 2019, 232, 116636.
- Doench, J.G.; Fusi, N.; Sullender, M.; Hegde, M.; Vaimberg, E.W.; Donovan, K.F.; Smith, I.; Tothova, Z.; Wilen, C.; Orchard, R.; et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat. Biotechnol. 2016, 34, 184–191.
- Liu, G.; Lin, Q.; Jin, S.; Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell 2022, 82, 333–347.
- Chapman, J.R.; Taylor, M.R.; Boulton, S.J. Playing the end game: DNA double-strand break repair pathway choice. Mol. Cell 2012, 47, 497–510.
- Kakarougkas, A.; Jeggo, P.A. DNA DSB repair pathway choice: An orchestrated handover mechanism. Br. J. Radiol. 2014, 87, 20130685.
- Frit, P.; Ropars, V.; Modesti, M.; Charbonnier, J.B.; Calsou, P. Plugged into the Ku-DNA hub: The NHEJ network. Prog. Biophys. Mol. Biol. 2019, 147, 62–76.
- Di Stazio, M.; Foschi, N.; Athanasakis, E.; Gasparini, P.; d’Adamo, A.P. Systematic analysis of factors that improve homologous direct repair (HDR) efficiency in CRISPR/Cas9 technique. PLoS ONE 2021, 16, e0247603.
- Bennett, E.P.; Petersen, B.L.; Johansen, I.E.; Niu, Y.; Yang, Z.; Chamberlain, C.A.; Met, O.; Wandall, H.H.; Frodin, M. INDEL detection, the ‘Achilles heel’ of precise genome editing: A survey of methods for accurate profiling of gene editing induced indels. Nucleic Acids Res. 2020, 48, 11958–11981.
- van de Kooij, B.; Kruswick, A.; van Attikum, H.; Yaffe, M.B. Multi-pathway DNA-repair reporters reveal competition between end-joining, single-strand annealing and homologous recombination at Cas9-induced DNA double-strand breaks. Nat. Commun. 2022, 13, 5295.
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714.
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478.
- Ravi, S.; Campagna, G.; Della Lucia, M.C.; Broccanello, C.; Bertoldo, G.; Chiodi, C.; Maretto, L.; Moro, M.; Eslami, A.S.; Srinivasan, S.; et al. SNP Alleles Associated With Low Bolting Tendency in Sugar Beet. Front. Plant Sci. 2021, 12, 693285.
- McCarthy, J.J.; Hilfiker, R. The use of single-nucleotide polymorphism maps in pharmacogenomics. Nat. Biotechnol. 2000, 18, 505–508.
- Sretenovic, S.; Liu, S.; Li, G.; Cheng, Y.; Fan, T.; Xu, Y.; Zhou, J.; Zheng, X.; Coleman, G.; Zhang, Y.; et al. Exploring C-To-G Base Editing in Rice, Tomato, and Poplar. Front. Genome Ed. 2021, 3, 756766.
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA Base-Editing and Prime-Editing. Int. J. Mol. Sci. 2020, 21, 6240.
- Thuronyi, B.W.; Koblan, L.W.; Levy, J.M.; Yeh, W.H.; Zheng, C.; Newby, G.A.; Wilson, C.; Bhaumik, M.; Shubina-Oleinik, O.; Holt, J.R.; et al. Continuous evolution of base editors with expanded target compatibility and improved activity. Nat. Biotechnol. 2019, 37, 1070–1079.
- Negishi, K.; Kaya, H.; Abe, K.; Hara, N.; Saika, H.; Toki, S. An adenine base editor with expanded targeting scope using SpCas9-NGv1 in rice. Plant Biotechnol. J. 2019, 17, 1476–1478.
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424.
- Huang, T.P.; Newby, G.A.; Liu, D.R. Precision genome editing using cytosine and adenine base editors in mammalian cells. Nat. Protoc. 2021, 16, 1089–1128.
- Matsoukas, I.G. Commentary: Programmable base editing of A.T to G.C in genomic DNA without DNA cleavage. Front. Genet. 2018, 9, 21.
- Komor, A.C.; Zhao, K.T.; Packer, M.S.; Gaudelli, N.M.; Waterbury, A.L.; Koblan, L.W.; Kim, Y.B.; Badran, A.H.; Liu, D.R. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci. Adv. 2017, 3, eaao4774.
- Koblan, L.W.; Doman, J.L.; Wilson, C.; Levy, J.M.; Tay, T.; Newby, G.A.; Maianti, J.P.; Raguram, A.; Liu, D.R. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 2018, 36, 843–846.
- Kim, Y.B.; Komor, A.C.; Levy, J.M.; Packer, M.S.; Zhao, K.T.; Liu, D.R. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 2017, 35, 371–376.
- Olave, M.C.; Graham, R.P. Mismatch repair deficiency: The what, how and why it is important. Genes Chromosomes Cancer 2022, 61, 314–321.
- Sameer, A.S.; Nissar, S.; Fatima, K. Mismatch repair pathway: Molecules, functions, and role in colorectal carcinogenesis. Eur. J. Cancer. Prev. 2014, 23, 246–257.
- Villiger, L.; Rothgangl, T.; Witzigmann, D.; Oka, R.; Lin, P.J.C.; Qi, W.; Janjuha, S.; Berk, C.; Ringnalda, F.; Beattie, M.B.; et al. In vivo cytidine base editing of hepatocytes without detectable off-target mutations in RNA and DNA. Nat. Biomed. Eng. 2021, 5, 179–189.
- Chen, L.; Zhu, B.; Ru, G.; Meng, H.; Yan, Y.; Hong, M.; Zhang, D.; Luan, C.; Zhang, S.; Wu, H.; et al. Re-engineering the adenine deaminase TadA-8e for efficient and specific CRISPR-based cytosine base editing. Nat. Biotechnol. 2022, 10.
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A*T to G*C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471.
- Grunewald, J.; Zhou, R.; Garcia, S.P.; Iyer, S.; Lareau, C.A.; Aryee, M.J.; Joung, J.K. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors. Nature 2019, 569, 433–437.
- Rees, H.A.; Wilson, C.; Doman, J.L.; Liu, D.R. Analysis and minimization of cellular RNA editing by DNA adenine base editors. Sci. Adv. 2019, 5, eaax5717.
- Kim, H.S.; Jeong, Y.K.; Hur, J.K.; Kim, J.S.; Bae, S. Adenine base editors catalyze cytosine conversions in human cells. Nat. Biotechnol. 2019, 37, 1145–1148.
- Montaldo, N.P.; Bordin, D.L.; Brambilla, A.; Rosinger, M.; Fordyce Martin, S.L.; Bjoras, K.O.; Bradamante, S.; Aas, P.A.; Furrer, A.; Olsen, L.C.; et al. Alkyladenine DNA glycosylase associates with transcription elongation to coordinate DNA repair with gene expression. Nat. Commun. 2019, 10, 5460.
- Kurt, I.C.; Zhou, R.; Iyer, S.; Garcia, S.P.; Miller, B.R.; Langner, L.M.; Grunewald, J.; Joung, J.K. CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat. Biotechnol. 2021, 39, 41–46.
- Cortizas, E.M.; Zahn, A.; Safavi, S.; Reed, J.A.; Vega, F.; Di Noia, J.M.; Verdun, R.E. UNG protects B cells from AID-induced telomere loss. J. Exp. Med. 2016, 213, 2459–2472.
- Cordoba-Canero, D.; Dubois, E.; Ariza, R.R.; Doutriaux, M.P.; Roldan-Arjona, T. Arabidopsis uracil DNA glycosylase (UNG) is required for base excision repair of uracil and increases plant sensitivity to 5-fluorouracil. J. Biol. Chem. 2010, 285, 7475–7483.
- Assefa, N.G.; Niiranen, L.; Johnson, K.A.; Leiros, H.K.; Smalas, A.O.; Willassen, N.P.; Moe, E. Structural and biophysical analysis of interactions between cod and human uracil-DNA N-glycosylase (UNG) and UNG inhibitor (Ugi). Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 2093–2100.
- Molla, K.A.; Yang, Y. CRISPR/Cas-Mediated Base Editing: Technical Considerations and Practical Applications. Trends Biotechnol. 2019, 37, 1121–1142.
- Porto, E.M.; Komor, A.C.; Slaymaker, I.M.; Yeo, G.W. Base editing: Advances and therapeutic opportunities. Nat. Rev. Drug Discov. 2020, 19, 839–859.
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788.
- Allier, A.; Teyssedre, S.; Lehermeier, C.; Moreau, L.; Charcosset, A. Optimized breeding strategies to harness genetic resources with different performance levels. BMC Genom. 2020, 21, 349.
- Till, B.J.; Reynolds, S.H.; Greene, E.A.; Codomo, C.A.; Enns, L.C.; Johnson, J.E.; Burtner, C.; Odden, A.R.; Young, K.; Taylor, N.E.; et al. Large-scale discovery of induced point mutations with high-throughput TILLING. Genome Res. 2003, 13, 524–530.
- Qutub, M.; Chandran, S.; Rathinavel, K.; Sampathrajan, V.; Rajasekaran, R.; Manickam, S.; Adhimoolam, K.; Muniyandi, S.J.; Natesan, S. Improvement of a Yairipok Chujak Maize Landrace from North Eastern Himalayan Region for beta-Carotene Content through Molecular Marker-Assisted Backcross Breeding. Genes 2021, 12, 762.
- Sserumaga, J.P.; Kayondo, S.I.; Kigozi, A.; Kiggundu, M.; Namazzi, C.; Walusimbi, K.; Bugeza, J.; Molly, A.; Mugerwa, S. Genome-wide diversity and structure variation among lablab accessions and their implication in a Forage breeding program. Genet. Resour. Crop Evol. 2021, 68, 2997–3010.
- Hill, R.C.; Fast, B.J.; Herman, R.A. Transgenesis affects endogenous soybean allergen levels less than traditional breeding. Regul. Toxicol. Pharmacol. 2017, 89, 70–73.
- Beans, C. Inner Workings: Crop researchers harness artificial intelligence to breed crops for the changing climate. Proc. Natl. Acad. Sci. USA 2020, 117, 27066–27069.
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91.
- Kumlehn, J.; Pietralla, J.; Hensel, G.; Pacher, M.; Puchta, H. The CRISPR/Cas revolution continues: From efficient gene editing for crop breeding to plant synthetic biology. J. Integr. Plant Biol. 2018, 60, 1127–1153.
- Schindele, A.; Dorn, A.; Puchta, H. CRISPR/Cas brings plant biology and breeding into the fast lane. Curr. Opin. Biotechnol. 2020, 61, 7–14.
- Turner-Hissong, S.D.; Mabry, M.E.; Beissinger, T.M.; Ross-Ibarra, J.; Pires, J.C. Evolutionary insights into plant breeding. Curr. Opin. Plant Biol. 2020, 54, 93–100.
- Saunders, T.L. The History of Transgenesis. Methods Mol. Biol. 2020, 2066, 1–26.
- Araki, M.; Ishii, T. Towards social acceptance of plant breeding by genome editing. Trends Plant Sci. 2015, 20, 145–149.
- Cubry, P.; Tranchant-Dubreuil, C.; Thuillet, A.C.; Monat, C.; Ndjiondjop, M.N.; Labadie, K.; Cruaud, C.; Engelen, S.; Scarcelli, N.; Rhone, B.; et al. The Rise and Fall of African Rice Cultivation Revealed by Analysis of 246 New Genomes. Curr. Biol. 2018, 28, 2274–2282 e2276.
- Eraslan, G.; Avsec, Z.; Gagneur, J.; Theis, F.J. Deep learning: New computational modelling techniques for genomics. Nat. Rev. Genet. 2019, 20, 389–403.
- Hufford, M.B.; Seetharam, A.S.; Woodhouse, M.R.; Chougule, K.M.; Ou, S.; Liu, J.; Ricci, W.A.; Guo, T.; Olson, A.; Qiu, Y.; et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 2021, 373, 655–662.
- International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Stein, N.; Feuillet, C.; Keller, B.; Rogers, J.; Pozniak, C.J.; Choulet, F.; Distelfeld, A.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191.
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.; Pikaard, C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006, 45, 616–629.
- Doman, J.L.; Raguram, A.; Newby, G.A.; Liu, D.R. Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat. Biotechnol. 2020, 38, 620–628.
- Li, J.; Sun, Y.; Du, J.; Zhao, Y.; Xia, L. Generation of Targeted Point Mutations in Rice by a Modified CRISPR/Cas9 System. Mol. Plant 2017, 10, 526–529.
- Qin, L.; Li, J.; Wang, Q.; Xu, Z.; Sun, L.; Alariqi, M.; Manghwar, H.; Wang, G.; Li, B.; Ding, X.; et al. High-efficient and precise base editing of C*G to T*A in the allotetraploid cotton (Gossypium hirsutum) genome using a modified CRISPR/Cas9 system. Plant Biotechnol. J. 2020, 18, 45–56.
- Wu, J.; Chen, C.; Xian, G.; Liu, D.; Lin, L.; Yin, S.; Sun, Q.; Fang, Y.; Zhang, H.; Wang, Y. Engineering herbicide-resistant oilseed rape by CRISPR/Cas9-mediated cytosine base-editing. Plant Biotechnol. J. 2020, 18, 1857–1859.
- Xu, R.; Liu, X.; Li, J.; Qin, R.; Wei, P. Identification of herbicide resistance OsACC1 mutations via in planta prime-editing-library screening in rice. Nat. Plants 2021, 7, 888–892.
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.L.; Wang, D.; Gao, C. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 438–440.
- Yan, D.; Ren, B.; Liu, L.; Yan, F.; Li, S.; Wang, G.; Sun, W.; Zhou, X.; Zhou, H. High-efficiency and multiplex adenine base editing in plants using new TadA variants. Mol. Plant 2021, 14, 722–731.
- Hunziker, J.; Nishida, K.; Kondo, A.; Kishimoto, S.; Ariizumi, T.; Ezura, H. Multiple gene substitution by Target-AID base-editing technology in tomato. Sci. Rep. 2020, 10, 20471.
- Xing, S.; Chen, K.; Zhu, H.; Zhang, R.; Zhang, H.; Li, B.; Gao, C. Fine-tuning sugar content in strawberry. Genome Biol. 2020, 21, 230.
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through CRISPR/Cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356.
- Zhang, R.; Chen, S.; Meng, X.; Chai, Z.; Wang, D.; Yuan, Y.; Chen, K.; Jiang, L.; Li, J.; Gao, C. Generating broad-spectrum tolerance to ALS-inhibiting herbicides in rice by base editing. Sci. China Life Sci. 2021, 64, 1624–1633.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
880
Revisions:
2 times
(View History)
Update Date:
06 Feb 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




