Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agronomy
The clustered regularly interspaced, short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) is a third-generation gene editing technology following ZFNs and TALENs. It has the advantages of being highly efficient, simple, inexpensive, and easily usable. In the CRISPR/Cas9 system, a Cas9-single guide RNA (sgRNA) complex binds to a specific nucleotide sequence with the guidance of the sgRNA and cleaves the target DNA strand, causing a double-strand break (DSB).
- base editing
- CRISPR/Cas9
- plant
- genome editing
- crop improvement
1. Introduction
The clustered regularly interspaced, short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) is a third-generation gene editing technology following ZFNs and TALENs. It has the advantages of being highly efficient, simple, inexpensive, and easily usable [1,2,3].
In the CRISPR/Cas9 system, a Cas9-single guide RNA (sgRNA) complex binds to a specific nucleotide sequence with the guidance of the sgRNA and cleaves the target DNA strand, causing a double-strand break (DSB) [4,5,6,7]. These DSBs can be corrected by nonhomologous end-joining (NHEJ) or the homology-directed repair (HDR) mechanism [8,9]. NHEJ is a method of repair in which the ends of DSBs are directly linked by DNA ligase and do not depend on homologous DNA sequences; therefore, NHEJ repair is rapid but not exact. The homologous repair process is complex and precise but requires a homologous DNA sequence template and can occur only in the G2/S phase of a cell [10,11,12,13].
Due to the genetic basis underlying the diversity of many important crop species and single-nucleotide variations [14,15], it is necessary to develop a technique that allows precise and effective single-base substitutions. Base editing technology is a novel target gene modification technique developed based on the CRISPR/Cas system, by its utilization of a tethered deaminase domain or nickase Cas9 for base conversion from A > G or C > T or C > G without the donor DNA and a DSB introduction in the genome. Recent studies have utilized Base-editors to create single and multiple nucleotide modifications in cells.
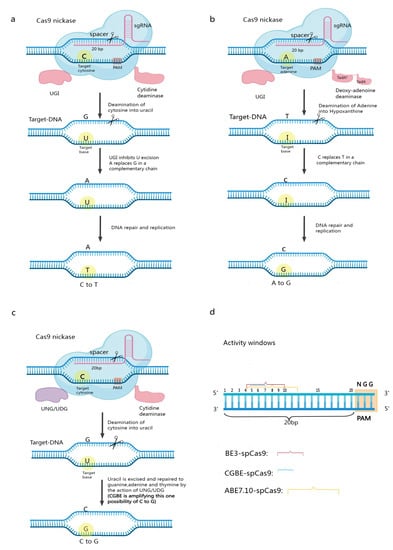
2. Base Editing
Base editors (BEs) enable single-nucleotide targeted mutations without severing the nucleic acid backbone and enable direct chemical modification of target nucleobases. The original base editor used a single-stranded DNA-specific cytidine deaminase combined with an inactivated Cas9 (dCas9) to convert a cytosine (C)-guanine (G) base pair to thymine (T)-adenine (A) in the target region, called the cytosine base editor (CBE). Later researchers developed the Adenine Base Editor and the Guanine Base Editor based on the CBE. Currently available base editing systems include cytosine (C) base editors (CBEs), adenine (A) base editors (ABEs), and guanine (G) base editors (CGBEs) (Figure 1) [16,17,18,19]. Each of these categories is discussed below.

Figure 1. Mechanism of base editing work. (a) Structure and working mechanism of the cytosine base editor; (b) Structure and working mechanism of the adenine base editor; (c) Structure and editing mechanism of CGBE; (d) Window of activity for some typical CBEs, ABEs, and CGBEs.
3. CBEs
The first-generation CBE, BE1, consists of rat C deaminase (rAPOBEC1) and dCas9, whose cleavage activity is completely lost [20,21]. When the fusion protein targets genomic DNA under the guidance of sgRNA, C deaminase can bind to the ssDNA in the R-loop region formed by the Cas9 protein, sgRNA, and genomic DNA and deaminate C to uracil (U) within a certain range along the ssDNA. During DNA replication, U is read by DNA polymerase as thymine (T). The final substitution of C/G to T/A base pairs then occurs (Figure 1a). Later researchers developed a second-generation cytosine base editor, BE2, by incorporating a uracil DNA glycosylase inhibitor (UGI) from phage PBS on top of BE1. Because UGI can inhibit the action of uracil DNA glycosylase (UDG) in the organism, BE2 is three times more efficient at editing than BE1. CBEs have undergone several generations of updates; notably, BE3 has replaced dCas9 in BE2 with nCas9(D10A) [22,23,24,25]. nCas9(D10A) specifically creates a gap in the nonedited strand, which in turn stimulates the intracellular base mismatch repair pathway (MMR) [26,27], which uses the editing strand containing U as a template for repair, resulting in increased editing efficiency. These optimized CBEs can better serve precision breeding [18,24,28]. However, existing CBEs/CGBEs rely on the natural cytosine deaminase AID/APOBEC and often produce high insertional deletion by-products and off-target effects due to the activation of the base excision repair pathway by cytosine deamination. Recently, researchers have transformed the adenine deaminase TadA-8e into a non-natural cytosine deaminase using only cytosine as a substrate and constructed the first novel CGBE/CBE family of base editors—Td-CGBE/Td-CBEs—that do not rely on the AID/APOBEC deaminase family, demonstrating lower off-target effects and very low indels events. Constructing a corresponding base editor in plants will facilitate crop improvement [29].
4. ABEs
Three main components compose ABEs: synthetic A deaminase, nCas9 (D10A), and sgRNA [30]. The A deaminase protein binds to ssDNA and deaminates A into inosine I, which is then read and replicated as G at the DNA level. This enables the instant exchange of A–T base pairs with G–C base pairs when the fusion protein targets genomic DNA under the guidance of sgRNA [31] (Figure 1b). Using ABEs eliminates the limitation that CBEs can edit only C or G and opens up a wider range of base transformation possibilities. In contrast to CBEs, ABEs do not require the suppression of alkyl adenine DNA glycosylase (AAG) activity [32,33,34].
5. CGBEs
C-to-G base editors (CGBEs) were constructed by adapting existing CBE tools to generate a new tool suitable for mediating C–G base reversals [35].
Combining a Cas9 nickase (nCas9-D10A), cytidine deaminase, and Uracil-N-glycosylase (UNG) leads to the production of CGBEs. Cytidine deaminase causes the conversion of a target C to U under the guidance of RNA. UNG locates U in the DNA and eliminates it, resulting in the formation of an AP site [36]. When nCas9 creates the AP site and binds the nonedited strand, DNA repair and replication mechanisms are triggered, preferentially inserting a G at the AP site. In contrast to CBEs, which contain a UNG inhibitor, CGBEs contain UNG [37,38] (Figure 1c).
In the base editor, the single-stranded DNA in the R-loop is exposed during base editing. This single-stranded DNA binds to the 20 bp of the sgRNA, but there is a preference for the action of cytidine deaminase on this 20 bp fragment so that different base editors have specific BE activity windows. The Activity Window of the Classical Cytosine Base Editor BE3-SpCas9 is bases 4–10. Bases 4–12 of the classical adenine is the base editor ABE7.10-SpCas9 activity window. Bases 5–7 of the classical guanine base editor is the CGBE-SpCas9 activity window. The different base editor activity windows depend on various factors, such as Cas proteins, deaminases, and variant connectors [39,40,41] (Figure 1d).
6. Application of Base Editors in Plants
In the long history of breeding, several major strategies have been used, such as crossbreeding, mutation breeding, etc. Gene editing and transgenics are an important part of the new breeding era [42,43,44,45,46,47]. Crossbreeding can only introduce known good traits [48,49]. Mutation breeding is a longer breeding process in which researchers create random mutations throughout the plant genome through physical and chemical mutagenesis. Transgenic breeding techniques allow for the direct introduction of good genes specific to a crop or genes from other species to obtain crop varieties with higher yields and better nutritional quality [50,51,52,53]. However, this breeding method requires the integration of exogenous genes into the plant genome and is, therefore, subject to strict controls. BE technology is an effective complement to the above three breeding methods. BEs allow for targeted modification of the plant genome without introducing exogenous genes to obtain the target variety quickly [54,55,56,57].
In recent years, public investment in research has been used to sequence, assemble and annotate the genomes of major crops, and a wealth of functional genetic information on plants have been gained [54,55,56,57,58]. Base editing allows for precise genome editing, and its successful operation in breeding has opened up new opportunities for crop improvement. Since 2016, BEs have been used to edit the genomes of various plant species, including rice, maize, cotton, oilseed rape, tomato, strawberry, and watermelon [59,60,61,62,63,64,65,66,67,68,69].
This entry is adapted from the peer-reviewed paper 10.3390/cimb45020059
This entry is offline, you can click here to edit this entry!
