
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yury V. Zhernov | -- | 1902 | 2023-02-02 12:38:13 | | | |
| 2 | Peter Tang | Meta information modification | 1902 | 2023-02-03 10:54:41 | | |
Video Upload Options
Micro ribonucleic acids (microRNAs or miRNAs) form a distinct subtype of non-coding RNA and are widely recognized as one of the most significant gene expression regulators in mammalian cells. Mechanistically, the regulation occurs through microRNA binding with its response elements in the 3’-untranslated region of target messenger RNAs (mRNAs), resulting in the post-transcriptional silencing of genes, expressing target mRNAs. Compared to small interfering RNAs, microRNAs have more complex regulatory patterns, making them suitable for fine-tuning gene expressions in different tissues. Dysregulation of microRNAs is well known as one of the causative factors in malignant cell growth. Breast cancer (BCa) is the most common cancer in women worldwide and seriously impairs patients’ physical health. Its incidence has been predicted to rise further. Mounting evidence indicates that microRNAs play key roles in tumorigenesis and development. Prostate cancer (PCa) is one of the most commonly diagnosed cancers in men. Different microRNAs play an important role in PCa. Early diagnosis of BCa and PCa using microRNAs is very useful for improving individual outcomes in the framework of predictive, preventive, and personalized (3P) medicine, thereby reducing the economic burden.
1. Introduction
|
microRNA |
siRNA |
|
|---|---|---|
|
Structure |
small (18–25 nucleotides) non-coding single-stranded molecules |
small (21–23 nucleotides) non-coding double-stranded molecules |
|
Target |
Many DNA/mRNA molecules |
One mRNA molecule |
|
Sites |
Cytoplasm, nucleus |
Cytoplasm |
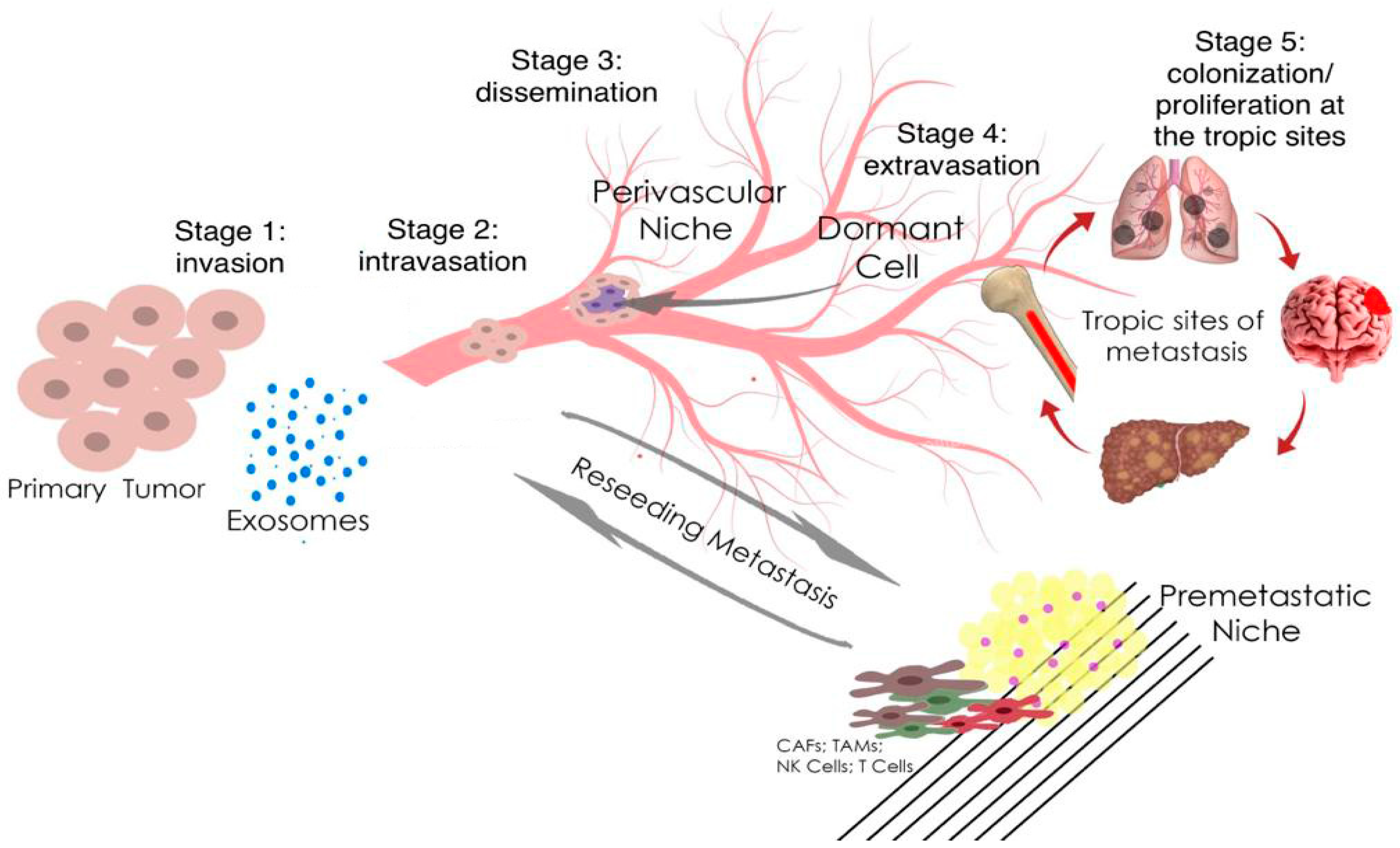
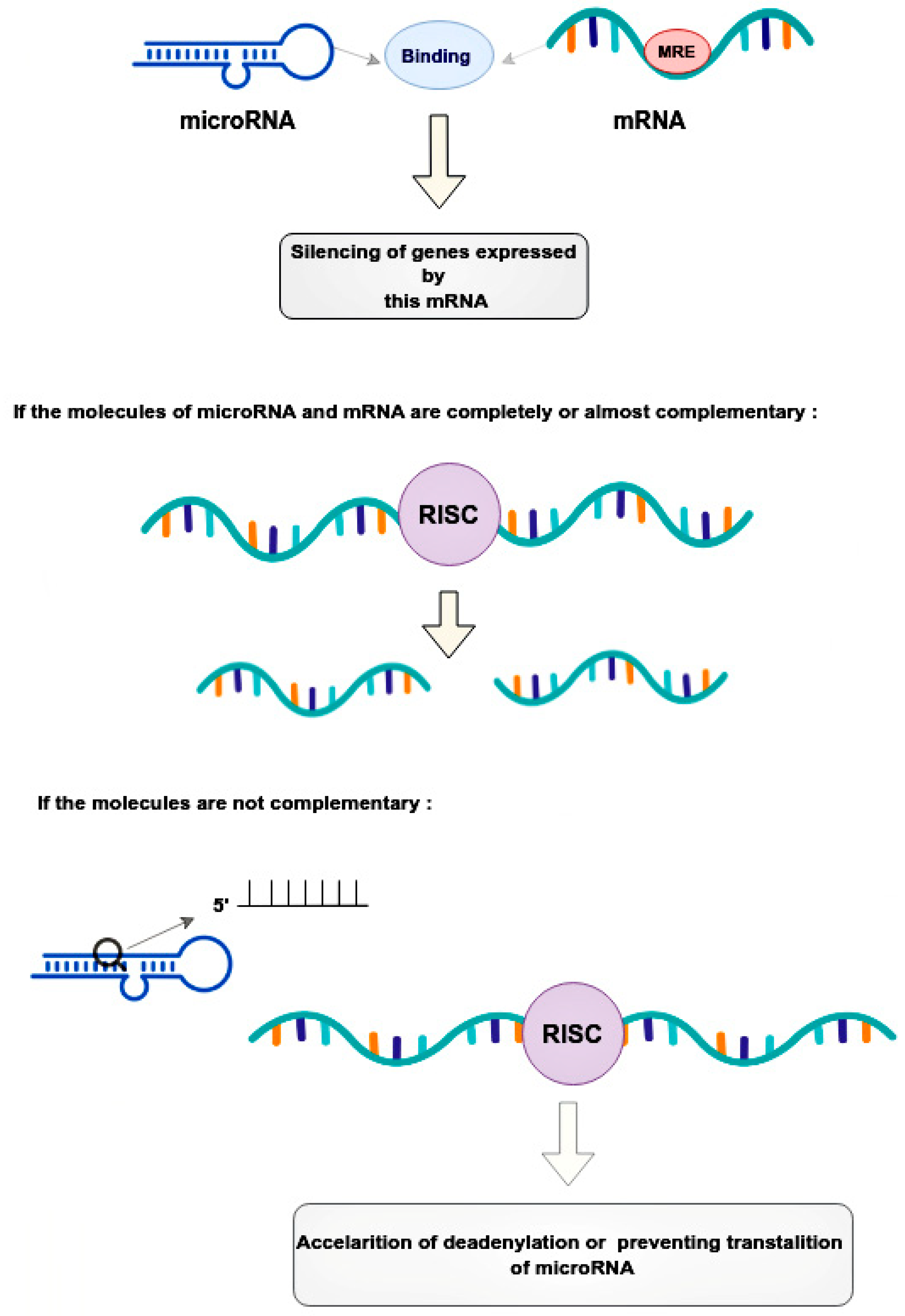
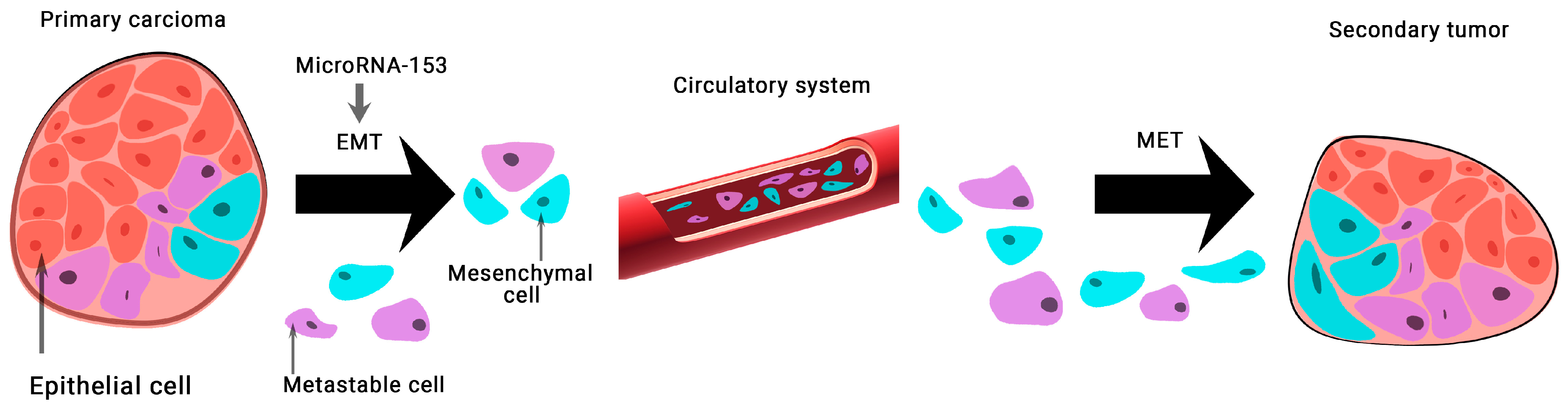
2. The Role microRNA in Prostate and Breast Cancer
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Tran, N.L.; Nagle, R.B.; Cress, A.E.; Heimark, R.L. N-Cadherin Expression in Human Prostate Carcinoma Cell Lines: An Epithelial-Mesenchymal Transformation Mediating Adhesion with Stromal Cells. Am. J. Pathol. 1999, 155, 787–798.
- Pakzad, R.; Mohammadian-Hafshejani, A.; Ghoncheh, M.; Pakzad, I.; Salehiniya, H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int. 2015, 3, 135–140.
- Grant, C.M.; Kyprianou, N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl. Androl. Urol. 2013, 2, 202–211.
- Mazurakova, A.; Koklesova, L.; Samec, M.; Kudela, E.; Kajo, K.; Skuciova, V.; Csizmár, S.H.; Mestanova, V.; Pec, M.; Adamkov, M.; et al. Anti-breast cancer effects of phytochemicals: Primary, secondary, and tertiary care. EPMA J. 2022, 13, 315–334.
- Mazurakova, A.; Samec, M.; Koklesova, L.; Biringer, K.; Kudela, E.; Al-Ishaq, R.K.; Pec, M.; Giordano, F.A.; Büsselberg, D.; Kubatka, P.; et al. Anti-prostate cancer protection and therapy in the framework of predictive, preventive and personalised medicine—comprehensive effects of phytochemicals in primary, secondary and tertiary care. EPMA J. 2022, 13, 461–486.
- Grishina, K.A.; Khaylenko, V.A.; Khaylenko, D.V.; Karpukhin, A.V. Role of microRNAs in breast cancer development and their potential as biomarkers. Tumors Female Reprod. Syst. 2018, 14, 40–47. (In Russian)
- Ding, Y.; Wu, W.; Ma, Z.; Shao, X.; Zhang, M.; Wang, Z. Potential value of MicroRNA-21 as a biomarker for predicting the prognosis of patients with breast cancer. Medicine 2021, 100, e25964.
- Fridrichova, I.; Zmetakova, I. MicroRNAs Contribute to Breast Cancer Invasiveness. Cells 2019, 8, 1361.
- Ali Syeda, Z.; Langden, S.S.S.; Munkhzul, C.; Lee, M.; Song, S.J. Regulatory Mechanism of MicroRNA Expression in Cancer. Int. J. Mol. Sci. 2020, 21, 1723.
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59.
- Cussenot, O.; Valeri, A.; Berthon, P.; Fournier, G.; Mangin, P. Hereditary Prostate Cancer and Other Genetic Predispositions to Prostate Cancer. Urol. Int. 1998, 60 (Suppl. 2), 30–35.
- Brawer, M.K. Prostatic intraepithelial neoplasia: An overview. Rev. Urol. 2005, 7 (Suppl. 3), S11–S18.
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105.
- Patil, P.A.; Magi-Galluzzi, C. MicroRNA in prostate cancer: Practical aspects. Histol. Histopathol. 2015, 30, 1379–1396.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Gellrich, F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223.
- Pu, M.; Chen, J.; Tao, Z.; Miao, L.; Qi, X.; Wang, Y.; Ren, J. Regulatory network of miRNA on its target: Coordination between transcriptional and post-transcriptional regulation of gene expression. Cell. Mol. Life Sci. 2019, 76, 441–451.
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529.
- Cheng, L.; Lopez-Beltran, A.; Massari, F.; MacLennan, G.T.; Montironi, R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: A move toward precision medicine. Mod. Pathol. 2017, 31, 24–38.
- Ellinger, J.; Alajati, A.; Kubatka, P.; Giordano, F.A.; Ritter, M.; Costigliola, V.; Golubnitschaja, O. Prostate cancer treatment costs increase more rapidly than for any other cancer—How to reverse the trend? EPMA J. 2022, 13, 1–7.
- Golubnitschaja, O.; Kubatka, P.; Mazurakova, A.; Samec, M.; Alajati, A.; Giordano, F.A.; Costigliola, V.; Ellinger, J.; Ritter, M. Systemic Effects Reflected in Specific Biomarker Patterns Are Instrumental for the Paradigm Change in Prostate Cancer Management: A Strategic Paper. Cancers 2022, 14, 675.
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518.
- Brase, J.C.; Johannes, M.; Schlomm, T.; Fälth, M.; Haese, A.; Steuber, T.; Beißbarth, T.; Kuner, R.; Sültmann, H. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int. J. Cancer 2011, 128, 608–616.
- Huang, X.; Yuan, T.; Liang, M.; Du, M.; Xia, S.; Dittmar, R.; Wang, D.; See, W.; Costello, B.A.; Quevedo, F.; et al. Exosomal miR-1290 and miR-375 as Prognostic Markers in Castration-resistant Prostate Cancer. Eur. Urol. 2015, 67, 33–41.
- Nguyen, H.C.N.; Xie, W.; Yang, M.; Hsieh, C.-L.; Drouin, S.; Lee, G.-S.M.; Kantoff, P.W. Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low-risk, localized prostate cancer. Prostate 2013, 73, 346–354.
- Wang, J.; Liang, S.; Duan, X. Molecular mechanism of miR-153 inhibiting migration, invasion and epithelial-mesenchymal transition of breast cancer by regulating transforming growth factor beta (TGF-β) signaling pathway. J. Cell. Biochem. 2019, 120, 9539–9546.
- Wu, Z.; He, B.; He, J.; Mao, X. Upregulation of miR-153 promotes cell proliferation via downregulation of the PTEN tumor suppressor gene in human prostate cancer. Prostate 2012, 73, 596.
- Bertoli, G.; Panio, A.; Cava, C.; Gallivanone, F.; Alini, M.; Strano, G.; Molfino, F.; Brioschi, L.; Viani, P.; Porro, D. Secreted miR-153 Controls Proliferation and Invasion of Higher Gleason Score Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 6339.
- Bi, C.-W.; Zhang, G.-Y.; Bai, Y.; Zhao, B.; Yang, H. Increased expression of miR-153 predicts poor prognosis for patients with prostate cancer. Medicine 2019, 98, e16705.





