Micro ribonucleic acids (microRNAs or miRNAs) form a distinct subtype of non-coding RNA and are widely recognized as one of the most significant gene expression regulators in mammalian cells. Mechanistically, the regulation occurs through microRNA binding with its response elements in the 3’-untranslated region of target messenger RNAs (mRNAs), resulting in the post-transcriptional silencing of genes, expressing target mRNAs. Compared to small interfering RNAs, microRNAs have more complex regulatory patterns, making them suitable for fine-tuning gene expressions in different tissues. Dysregulation of microRNAs is well known as one of the causative factors in malignant cell growth. Breast cancer (BCa) is the most common cancer in women worldwide and seriously impairs patients’ physical health. Its incidence has been predicted to rise further. Mounting evidence indicates that microRNAs play key roles in tumorigenesis and development. Prostate cancer (PCa) is one of the most commonly diagnosed cancers in men. Different microRNAs play an important role in PCa. Early diagnosis of BCa and PCa using microRNAs is very useful for improving individual outcomes in the framework of predictive, preventive, and personalized (3P) medicine, thereby reducing the economic burden.
1. Introduction
According to the Global Cancer Observatory’s (GLOBOCAN) estimates for 2020, breast cancer (BCa) is the most common cancer among women worldwide and accounts for nearly 2.3 million reported cases [
1]. The five-year survival rate is less than 30%, despite all the successes of modern treatment approaches. Median incidence is 61 years [
1]. Prostate cancer (PCa) is a commonly diagnosed disease worldwide. It has amounted 1,414,249 new cases of PCa and 375,000 deaths from this disease in 2020 [
1]. In a third of patients with PCa, the tumor progresses (perineural and stromal invasion) after initial regression in response to androgen deprivation therapy [
2]. Despite modern advances in surgical, chemotherapeutic, and radiation treatments, the five-year survival rate in castration-resistant patients is approximately 31.0% [
3]. Most of the deaths associated with PCa are due to the failure of existing treatments to prevent tumor spread [
4].
This rate of increase in the incidence of BCa and PCa compels the use of the latest screening programs and strengthens the need for preventive measures aimed at reducing the incidence of the disease. Thus, primary, secondary, and tertiary care, which is provided within the framework of predictive, preventive, and personalized (3P) medicine, is a promising strategy for the development of new approaches in therapy and shows excellent results both in terms of individual results and economic costs. A paradigm shift from reactive treatment of symptomatic PCa to a prognostic approach and personalized prevention is essential [
5,
6].
One of the latest developments is the detection of microRNAs. These molecules have been shown to contribute significantly to carcinogenesis, disease progression, and the acquisition of resistance to traditional antitumor drugs.
Micro ribonucleic acids (microRNAs or miRNAs) were isolated in the early 1990s from
Caenorhabditis elegans [
7]. They are small (18–25 nucleotides) non-encoded single-stranded molecules whose main function is to regulate the expression of a particular gene at the transcription stage and in the post-transcriptional period. Compared to small interfering RNAs (siRNAs), microRNAs have more complex regulatory patterns, making them suitable for fine-tuning gene expression in different tissues (
Table 1). A microRNA binds to the non-coding part of the target RNA of the desired gene and manifests its functions through silencing systems, preventing the above steps of gene expression, and can also inhibit biological processes, such as cell differentiation, proliferation and development, depending on the nucleotide sequence [
8,
9].
Table 1. The differences between microRNA and siRNA.
An interesting aspect is that the same microRNA in different systems can have opposite functions, depending on the pathological or physiological conditions, or the kind of connections and with which genes the connections are established. According to other data, the predominant number of targets in different tissues does not differ much, but different isoforms of the 3’-untranslated region (3’-UTR) and landscape may affect the resulting functions [
7].
The regulation of gene expression is of great importance as part of the cellular response to negative environmental influences, such as hypoxia, starvation, and DNA damage [
10]. MicroRNA acts as a control unit for the work of proteins responsible for DNA repair, therefore microRNAs can function as oncogenes (oncomirs) or be tumor growth suppressors (oncosuppressors) [
11].
Different types of cancer may share aberrant microRNAs, although most microRNAs are specific to each type of cancer. Thus, there are a number of specific microRNAs for BCa, but they differ in their mechanism of action and role in the development of BCa. Differential microRNA expression is strongly associated with specific tumor stages, lymph node metastases, poor prognosis, and response to specific therapies [
7].
Prostate carcinomas show biodiversity and can either be localized to the prostate or become highly invasive and metastasize to regional lymph nodes and other organs [
12]. Although localized prostate carcinoma is successfully treated, treatment efficacy is greatly reduced when prostate tumor cells metastasize outside the gland, mainly through perineural and stromal invasion. The process of metastasis is diverse, but the mechanisms that contribute to the spread of the metastatic phenotype in prostate carcinomas are not well understood at the molecular level. The epithelial-mesenchymal transition (EMT) is a key process for the transition from non-invasive to invasive PCa, in which polarized epithelial cells lose their tight intercellular junctions, which leads to an increase in their migratory ability, an increase in invasive properties, and the acquisition of a mesenchymal phenotype (
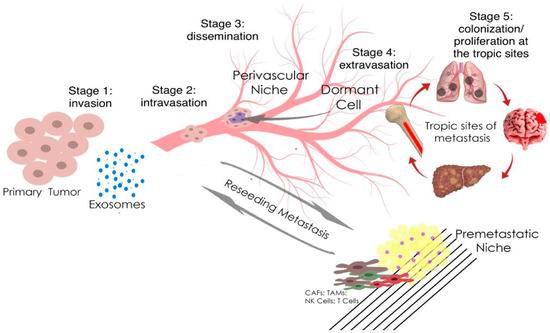
Figure 1) [
13].
Figure 1. Metastasis and tumor invasion. Tumor cells at the primary tumor sites leave their primary site by dint of invasion into the surrounding stroma (stage 1: invasion). Then they penetrate (stage 2: intravasation) and migrate through the blood or lymph vessels into the circulation (stage 3: dissemination). Some of tumor cells survive and migrate to the target organ parenchyma (tropic sites of metastasis), which is the “pre-metastatic niche” (stage 4: extravasation). Finally, cells adapt and proliferate to form metastasis with the subsequent formation of a clinically obvious tumor (stage 5: colonization/proliferation at the tropic sites). Myeloid cells, such as tumor-associated macrophages (TAMs), can promote metastatic spread through blood and lymphatic vessels. Transforming growth factor-β (TGF-β), produced by TAMs, and cancer-associated fibroblasts (CAFs) are regulators of the epithelial–mesenchymal transition and metastasis. In the pre-metastatic niche, natural killer cells (NK cells) and T cells inhibit tumor growth.
MicroRNAs (microRNAs) are involved in the regulation of up to 60% of the protein-coding genes [
14]; correspondingly, assessment of microRNA expression in various cancer diseases is a topical problem in molecular oncology in recent years. A considerable part of such studies is focused on the search for tumor-specific microRNAs potentially utile in designing the diagnostic systems for cancer diseases [
15].
MicroRNAs form a distinct non-coding RNA subtype and are widely recognized as one of the important regulators of gene expression in mammalian cells [
16].
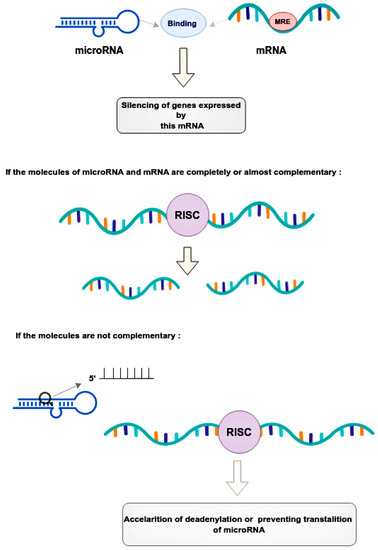
Mechanical regulation occurs through the binding of microRNAs to microRNA response elements (MREs) in the 3’-untranslated regions (3’-UTRs) of target messenger RNAs (mRNAs) [
17]. This leads to the post-transcriptional silencing of the genes expressing these mRNAs [
17]. The mode of silencing in the RNA-induced silencing complex (RISC) depends on the complementarity between the MRE mRNA and the guide microRNA [
18]. If the molecules are completely or nearly perfectly complementary, RISC cleaves the target mRNA [
18]. If microRNA is not fully complementary to mRNA, then RISC can bind to the MRE sequence only by the heptameric 5’-seed region of the guide microRNA, accelerating mRNA decay due to deadenylation or preventing translation at its various stages by blocking initiation factors and binding of ribosome subunits, slowing down elongation or contributing to premature termination [
18]. Thus, microRNAs, compared to small interfering RNAs, have more complex regulatory patterns that are suitable for fine-tuning gene expressions in different tissues (
Figure 2).
Figure 2. Regulatory patterns of mRNA and microRNA interaction. MicroRNAs bind to the mRNA MRE complex in 3’-untranslated regions, resulting in the post-transcriptional silencing of genes. The mode of silencing in RISC depends whenever mRNA and microRNA are completely or partially complementary. In cases where molecules are perfectly complementary, the RISC simply cleaves the target mRNA. In cases where molecules are no perfectly complementary, the RISC binds to the MRE only by the heptameric 5’seed region of the guide microRNA, leading to acceleration of mRNA decay. This happens due to deadenylation or interruption translation at its different stages. RISC—the RNA-induced silencing complex, MRE—microRNA response element.
The role of microRNAs as one of the main causal factors in the growth of malignant cells was first proposed in 2002 [
19]. Currently, there is more and more data on the dysregulation of microRNAs in the transcriptomes of various types of cancer. MicroRNA expression profiles change during the development of most malignant tumors, which suggests that microRNAs can act as oncogenes, tumor suppressors, or drivers of malignant transformation [
20]. There is also growing evidence of microRNA dysregulation in PCa. Blood patterns play one of the key role in prognostic diagnosis, targeted prevention, and the development of personalized treatment algorithms [
21,
22].
2. The Role microRNA in Prostate and Breast Cancer
MicroRNA-141 was first mentioned as a potential diagnostic marker in 2008 [
24]. The authors found that miR-141 was overexpressed 46-fold in a patient sample and could distinguish between advanced cases of metastatic PCa and healthy individuals with a specificity of 60% and a sensitivity of 100% [
24].
MicroRNA-141, microRNA-375, and microRNA-200b showed the highest correlation with clinical parameters of PCa. Also, miR-375 expression showed the highest correlation with tumor stage, Gleason score, and has a considerable association with lymph-node metastasis [
25].
The potential of microRNA-375 was found as prognostic biomarker of PCa by reported significant association of high expression of microRNA-375 levels with shorter overall survival. Therefore, microRNA-375 expression correlates with clinicopathological parameters and can be a prognostic biomarker of PCa [
26].
Another study showed that the expression of microRNA-141 together with microRNA-375 helps to differentiate patients with metastatic PCa and patients with low-risk localized cancer [
27].
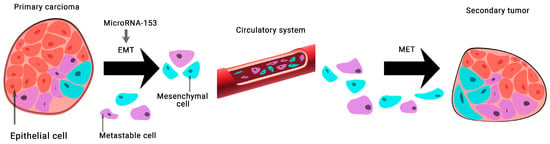
The expression of microRNA-153 in BCa tissue samples and 128 MDA-MB-231 cells was found to be significantly lower than normal. This group of scientists has shown that microRNA-153 inhibits migration, invasion, and epithelial-mesenchymal transition of BCa by regulating the signaling pathway of transforming growth factor beta (TGF-β) (
Figure 3) [
28].
Figure 3. The role of microRNA-153 in carcinogenesis. MicroRNA-153 is involved in epithelial-mesenchymal transition (EMT)-associated signaling pathways that stimulate tumorigenesis, cancer progression, and metastasis. A mesenchymal–epithelial transition (MET) is a reversible biological process that involves the transition from mesenchymal cells to polarized cells called epithelia.
Increased expression of microRNA-153 in PCa was discovered in 2013. It is known that microRNA-153 plays an important role in stimulating the proliferation of human PCa cells and represents a new mechanism for direct suppression of phosphatase and tensin homolog (PTEN) expression in PCa cells mediated through microRNA [
29].
MicroRNA-153 is up-regulated in PCa tissues and may play a major role in aggressive PCa by attacking potential target genes [
30].
So far, data on the clinical significance of microRNA-153 expression in PCa are scarce. Thus, it was found that the high expression of microRNA-153 in PCa tissues closely correlated with aggressive clinical pathological parameters, such as metastasis to the lymph nodes, to the bones, a high level on the Gleason score, and a high stage on the TNM Classification of Malignant Tumors (TNM). PCa patients with high levels of microRNA-153 expression had a clearly lower 5-year overall survival compared to patients with low microRNA-153 expression. Importantly, Cox’s multivariate regression analysis showed that microRNA-153 expression was an independent predictor of 5-year overall survival in patients with PCa [
31].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24031980