
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nahdia Afiifah Abdul Jalil | -- | 2959 | 2023-02-01 04:43:39 | | | |
| 2 | Lindsay Dong | -1 word(s) | 2958 | 2023-02-02 01:31:44 | | |
Video Upload Options
Honey and propolis have recently become the key target of attention for treating certain diseases and promoting overall health and well-being. A high content of flavonoids and phenolic acids found in both honey and propolis contributes to the antioxidant properties to scavenge free radicals. Honey and propolis also exhibited antibacterial effects where they act in two ways, namely the production of hydrogen peroxide (H2O2) and gluconic acids following the enzymatic activities of glucose oxidase, which exerts oxidative damage on the bacteria. Additionally, the anti-inflammatory effects of honey and propolis are mainly by reducing proinflammatory factors such as interleukins and tumor necrosis factor alpha (TNF-α). Their effects on pain were discovered through modulation at a peripheral nociceptive neuron or binding to an opioid receptor in the higher center. The aforementioned properties of honey have been reported to possess potential therapeutic topical application on the exterior parts of the eyes, particularly in treating conjunctivitis, keratitis, blepharitis, and corneal injury. In contrast, most of the medicinal values of propolis are beneficial in the internal ocular area, such as the retina, optic nerve, and uvea.
1. Introduction
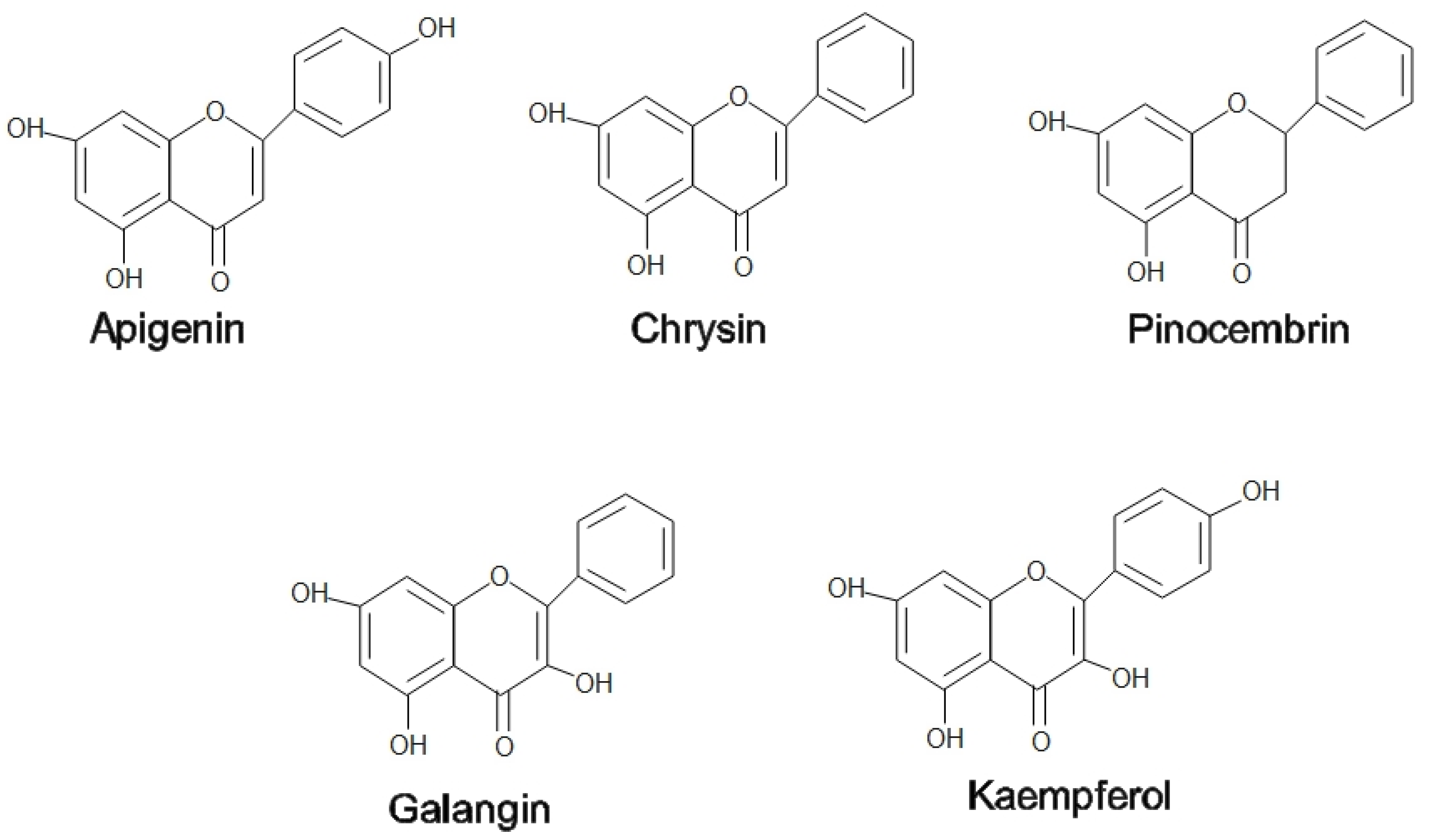
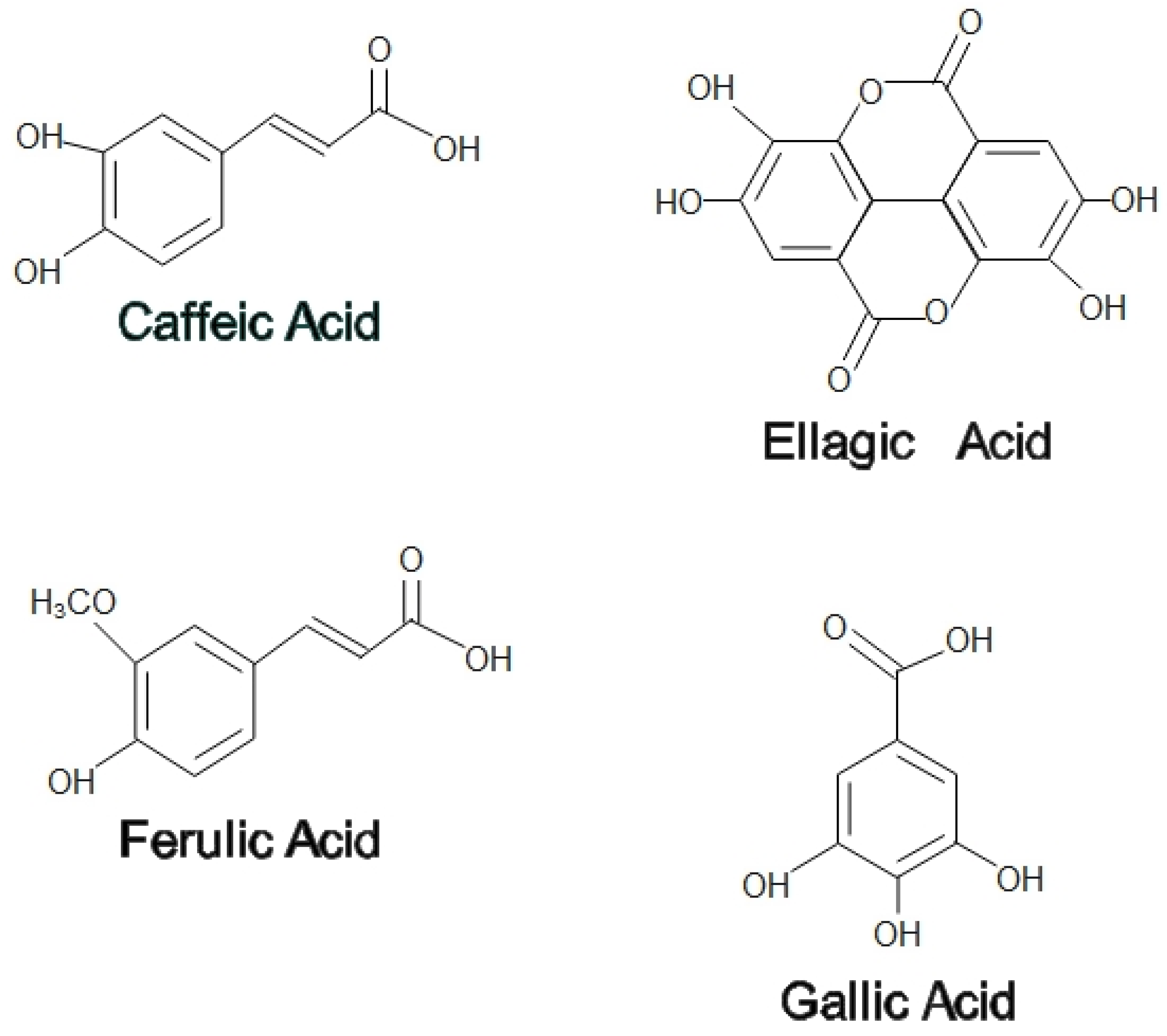
2. Medicinal Properties of Honey
2.1. Antioxidant
2.2. Antibacterial
2.3. Anti-Inflammatory
2.4. Anti-Nociceptive
3. Medicinal Properties of Propolis
3.1. Antioxidants
3.2. Antibacterial
3.3. Anti-Inflammatory
3.4. Anti-Nociceptive
The analgesic effect of propolis has been studied extensively in several animal models. In a tail flick experiment, a water extract of Anatolian green propolis produced a strong analgesic effect. Supplementation of this propolis to the toothpaste formulations has been shown to have analgesic activity and could be used as a component in treating periodontal disease [34].
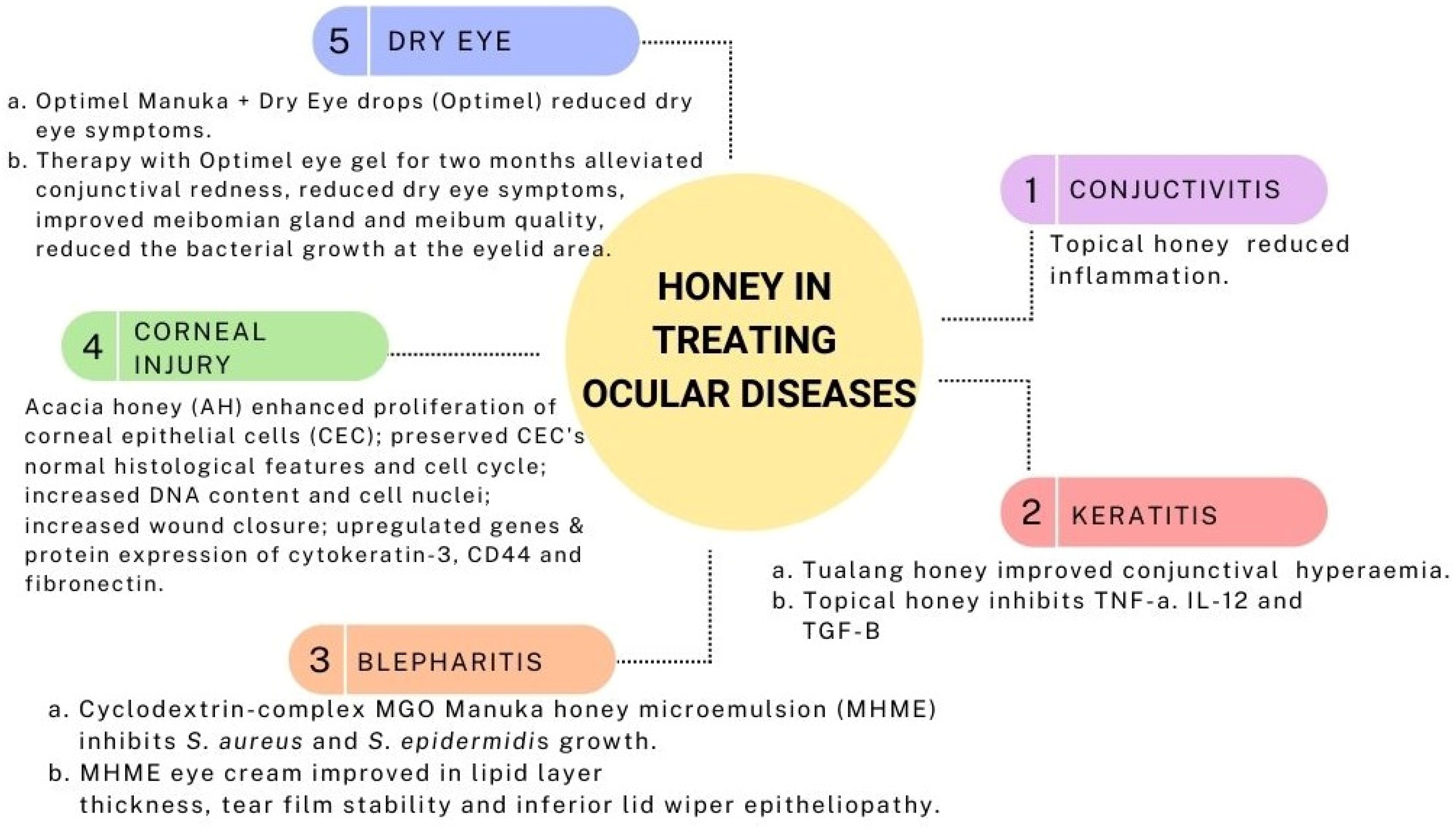
4. Medicinal Values of Honey-Related Products on Ocular Diseases
4.1. Conjunctivitis
4.2. Keratitis
In a rabbit model of Pseudomonas-induced keratitis, topical Tualang honey 30% displayed similar results to topical gentamicin and a combination of both in terms of clinical and antimicrobial effects. Clinically, all three treatments (Tualang honey, gentamicin, and mixed of both) improved conjunctival hyperemia though no apparent effects on corneal edema. Slit lamp examination score, a collective score of corneal infiltrates, corneal ulcer, hypopyon, and corneal perforation, were almost similar. Additionally, the Tualang honey and gentami
4.3. Blepharitis
A preclinical study on the cyclodextrin-complexed MGO Manuka honey microemulsion (MHME) has shown to be effective in inhibiting bacterial growth in blepharitis. The in vitro study found that S. aureus and S. epidermidis growth were suppressed at doses of 400 mg/kg and 550 mg/kg of MGO. Subsequent instillation of either diluted MGO MHME or saline control in rabbit eyes revealed the safety and tolerability of the MHME. During this in vivo phase, no significant immediate or cumulative harmful effects were identified upon the evaluation of tear film osmolarity, lipid layer grade, tear evaporation rate, fluorescein staining, phenol red thread, corneal opacity, conjunctival hyperemia and iris appearance grades [38]. Therefore, MHME was further tested on human subjects in the form of eye cream for periocular application for a two-weeks duration. The MHME eye cream caused no significant changes in clinical (visual acuity, eyelid irritation, ocular surface characteristics) and impression cytology evaluation (matrix metalloproteinase-9, IL-6, and MUC5AC) [39], implying the safety and tolerability of MHME eye cream in the clinical study despite transient ocular stinging, which disappeared after water irrigation.
4.4. Corneal Injury
Proliferation is one of the primary steps in wound healing to repopulate the injured area [40]. In a study by Ker-woon et al. (2014), Acacia honey (AH), which is a monofloral honey yielded by Apis mellifera honeybees, has been studied for its proliferative capacity on corneal epithelial cells (CEC). AH enhanced the proliferation of CEC while preserving its normal histological features and cell cycle, accompanied by increased DNA content and cell nuclei [41]. In subsequent in vitro corneal abrasion models, AH accelerated the wound closure, likely attributed to the additional ATP supply of AH. Moreover, AH upregulated both genes and proteins expression of cytokeratin-3, a cluster of differentiation 44 (CD44), and fibronectin [42]. Fibronectin and CD44 expressions were enhanced during the initial and middle phases of the experiment, respectively. This is in accordance with the function of fibronectin in the early phase of wound healing as a temporary extracellular matrix to aid cellular migration [43]. Similarly, CD44 aids the migratory phase by providing adhesive durability for cell-to-cell and cell-to-matrix interactions [42].
4.5. Dry Eye
Optimel Manuka+ Dry Eye drops (Optimel) is an MGH containing a proprietary mix of 16% Leptospermum spp. (manuka) honey and other Australian and New Zealand honey, which have been approved to treat chronic dry eye conditions and blepharitis [44]. A study was carried out to investigate the effects of Optimel eye drop on contact lens-related dry eye by comparing two ways of treatment. One group received the Optimel eye drop for two weeks, followed by another two weeks of conventional lubricant (Systane Ultra), while the other group received the reverse pattern of the treatment. Following Optimel eye drop treatment, dry eye symptoms were significantly alleviated. However, the overall signs of dry eyes seemed to have no significant difference, probably due to the limited time of treatment. However, a majority of the subjects reported good compliance with Optimel eye drop treatment, indicating its safety in reducing dry eye symptoms in contact lens wearers [44].
5. Medicinal Values of Propolis on Ocular Diseases
Propolis is a natural product that has valuable pharmacological and pharmaceutical properties. With more than 300 biologically active components, propolis has thus far been shown to be effective in treating various ocular diseases in animal and in vitro models [48]. Its efficacy in ocular diseases is likely attributable to its antiglaucoma, antiangiogenic, antioxidant, anti-inflammatory, wound healing agent, and neuroprotective properties [48][49][50][51].
References
- Ajibola, A. Novel Insights into the Health Importance of Natural Honey. Malays. J. Med. Sci. 2015, 22, 7–22.
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323.
- Shehu, A.; Ismail, S.; Rohin, M.; Harun, A.; Aziz, A.; Haque, M. Antifungal Properties of Malaysian Tualang Honey and Stingless Bee Propolis against Candida Albicans and Cryptococcus Neoformans. J. Appl. Pharm. Sci. 2016, 6, 44–50.
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047.
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360.
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510.
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846.
- Nagai, T.; Sakai, M.; Inoue, R.; Inoue, H.; Suzuki, N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001, 75, 237–240.
- Silva, C.F.; Rosalen, P.L.; Soares, J.C.; Massarioli, A.P.; Campestrini, L.H.; Semarini, R.A.; Ikegaki, M.; Alencar, S.M. Polyphenols in Brazilian organic honey and their scavenging capacity against reactive oxygen and nitrogen species. J. Apic. Res. 2020, 59, 136–145.
- Cheng, N.; Cao, W.; Wang, Y. The Protective Effect of Whole Honey and Phenolic Extract on Oxidative DNA Damage in Mice Lymphocytes Using Comet Assay. Mater. Veg. 2017, 72, 388–395.
- Brudzynski, K. A current perspective on hydrogen peroxide production in honey. A review. Food Chem. 2020, 332, 127229.
- Radan, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V.; Goudarzi, G. In vivo and in vitro evidence for the involvement of Nrf2-antioxidant response element signaling pathway in the inflammation and oxidative stress induced by particulate matter (PM10): The effective role of gallic acid. Free Radic. Res. 2019, 53, 210–225.
- Sohrabi, F.; Dianat, M.; Badavi, M.; Radan, M.; Mard, S.A. Gallic acid suppresses inflammation and oxidative stress through modulating Nrf2-HO-1-NF-κB signaling pathways in elastase-induced emphysema in rats. Environ. Sci. Pollut. Res. 2021, 28, 56822–56834.
- Movaffagh, J.; Bazzaz, B.S.F.; Yazdi, A.T.; Sajadi-Tabassi, A.; Azizzadeh, M.; Najafi, E.; Amiri, N.; Taghanaki, H.B.; Ebrahimzadeh, M.H.; Moradi, A. Wound Healing and Antimicrobial Effects of Chitosan-hydrogel/Honey Compounds in a Rat Full-thickness Wound Model. Wounds 2019, 31, 228–235.
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89.
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196.
- Masoura, M.; Passaretti, P.; Overton, T.W.; Lund, P.A.; Gkatzionis, K. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci. Rep. 2020, 10, 17692.
- Vallianou, N.G.; Gounari, P.; Skourtis, A.; Panagos, J.; Kazazis, C. Honey and its Anti-Inflammatory, Anti-Bacterial and Anti-Oxidant Properties. Gen. Med. 2014, 2, 1–5.
- Nolan, V.C.; Harrison, J.; Cox, J.A. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251.
- Rehman, K.; Akash, M.S.H. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87.
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236.
- Mandal, P.; Khan, M.I.; Shah, S. Drugs—Do we need them? Applications of non-pharmaceutical therapy in anterior eye disease: A review. Contact Lens Anterior Eye 2017, 40, 360–366.
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164.
- Ahmed, S.; Othman, N.H. Honey as a Potential Natural Anticancer Agent: A Review of Its Mechanisms. Evid. Based Complement. Altern. Med. 2013, 2013, 829070.
- Zhang, C.; Shen, X.; Chen, J.; Jiang, X.; Hu, F. Identification of Free Radical Scavengers from Brazilian Green Propolis Using Off-Line HPLC-DPPH Assay and LC-MS. J. Food Sci. 2017, 82, 1602–1607.
- Tomasin, R.; Gomes-Marcondes, M.C.C. Oral administration of Aloe vera and honey reduces walker tumour growth by decreasing cell proliferation and increasing apoptosis in tumour tissue. Phytother. Res. 2011, 25, 619–623.
- Šuran, J.; Cepanec, I.; Mašek, T.; Radić, B.; Radić, S.; Gajger, I.T.; Vlainić, J. Propolis extract and its bioactive compounds—From traditional to modern extraction technologies. Molecules 2021, 26, 2930.
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed. Pharmacother. 2018, 98, 469–483.
- Okińczyc, P.; Paluch, E.; Franiczek, R.; Widelski, J.; Wojtanowski, K.K.; Mroczek, T.; Krzyżanowska, B.; Skalicka-Woźniak, K.; Sroka, Z. Antimicrobial activity of Apis mellifera L. and Trigona sp. propolis from Nepal and its phytochemical analysis. Biomed. Pharmacother. 2020, 129, 110435.
- Orsi, R.; Fernandes, A.; Bankova, V.; Sforcin, J. The effects of Brazilian and Bulgarian propolis in vitro against Salmonella Typhi and their synergism with antibiotics acting on the ribosome. Nat. Prod. Res. 2012, 26, 430–437.
- Búfalo, M.C.; Ferreira, I.; Costa, G.; Francisco, V.; Liberal, J.; Cruz, M.T.; Lopes, M.C.; Batista, M.T.; Sforcin, J.M. Propolis and its constituent caffeic acid suppress LPS-stimulated pro-inflammatory response by blocking NF-κB and MAPK activation in macrophages. J. Ethnopharmacol. 2013, 149, 84–92.
- Sokeng, S.D.; Talla, E.; Sakava, P.; Tagne, M.A.F.; Henoumont, C.; Sophie, L.; Mbafor, J.T.; Fohouo, F.-N.T. Anti-Inflammatory and Analgesic Effect of Arachic Acid Ethyl Ester Isolated from Propolis. BioMed Res. Int. 2020, 2020, 8797284.
- Shimizu, Y.; Suzuki, T. Brazilian propolis extract reduces intestinal barrier defects and inflammation in a colitic mouse model. Nutr. Res. 2019, 69, 30–41.
- Okamoto, Y.; Tanaka, M.; Fukui, T.; Masuzawa, T. Brazilian propolis inhibits the differentiation of Th17 cells by inhibition of interleukin-6-induced phosphorylation of signal transducer and activator of transcription 3. Immunopharm. Immunot. 2012, 34, 803–809.
- Majtanova, N.; Cernak, M.; Majtan, J. Honey: A Natural Remedy for Eye Diseases. Complement. Med. Res. 2016, 23, 364–369.
- Ilechie, A.A.; Kwapong, P.K.; Kyei, S.; Mate-Kole, E.; Darko-Takyi, C. The efficacy of stingless bee honey for the treatment of bacteria-induced conjunctivitis in guinea pigs. J. Exp. Pharmacol. 2012, 4, 63–68.
- Salehi, A.; Jabarzare, S.; Neurmohamadi, M.; Kheiri, S.; Rafieian-Kopaei, M. A Double Blind Clinical Trial on the Efficacy of Honey Drop in Vernal Keratoconjunctivitis. Evid. Based Complement. Altern. Med. 2014, 2014, 287540.
- Craig, J.P.; Rupenthal, I.D.; Seyfoddin, A.; Cheung, I.M.Y.; Uy, B.; Wang, M.T.M.; A Watters, G.; Swift, S. Preclinical development of MGO Manuka Honey microemulsion for blepharitis management. BMJ Open Ophthalmol. 2016, 1, e000065.
- Craig, J.P.; Wang, M.T.M.; Ganesalingam, K.; Rupenthal, I.D.; Swift, S.; Loh, C.S.; Weehi, L.T.; Cheung, I.M.Y.; A Watters, G. Randomised masked trial of the clinical safety and tolerability of MGO Manuka Honey eye cream for the management of blepharitis. BMJ Open Ophthalmol. 2017, 1, e000066.
- Domingo, E.; Moshirfar, M.; Zabbo, C.P. Corneal Abrasion; StatPearls: Treasure Island, FL, USA, 2021.
- Ker-Woon, C.; Ghafar, N.A.; Hui, C.K.; Yusof, Y.A.M.; Luan, N.S. Proliferative Capacity of in Vitro Corneal Epithelium: Role of Acacia Honey in the Initial Step of Wound Healing. J. Med. Bioeng. 2014, 3, 107–112.
- Ker-Woon, C.; Ghafar, N.A.; Hui, C.K.; Yusof, Y.A.M.; Ngah, W.Z.W. The effects of acacia honey on in vitro corneal abrasion wound healing model. BMC Cell Biol. 2015, 16, 2.
- Hsiao, C.-T.; Cheng, H.-W.; Huang, C.-M.; Li, H.-R.; Ou, M.-H.; Huang, J.-R.; Khoo, K.-H.; Yu, H.W.; Chen, Y.-Q.; Wang, Y.-K.; et al. Fibronectin in cell adhesion and migration via N-glycosylation. Oncotarget 2017, 8, 70653–70668.
- Wong, D.; Albietz, J.M.; Tran, H.; Du Toit, C.; Li, A.H.; Yun, T.; Han, J.; Schmid, K.L. Treatment of contact lens related dry eye with antibacterial honey. Contact Lens Anterior Eye 2017, 40, 389–393.
- Perng, A.; Albietz, J.; Fung, K.; Ho, S.; Le, K.; Schmid, K. Treatment of Rhinosinusitis and Dry Eye with an Antibacterial Honey Nasal Spray. J. Apitherapy 2016, 1, 36–42.
- Alandejani, T.; Marsan, J.; Ferris, W.; Slinger, R.; Chan, F. Effectiveness of honey on Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Otolaryngol. Neck Surg. 2009, 141, 114–118.
- Cohen, M.; Kofonow, J.; Nayak, J.V.; Palmer, J.N.; Chiu, A.; Leid, J.G.; Cohen, N. Biofilms in Chronic Rhinosinusitis: A Review. Am. J. Rhinol. Allergy 2009, 23, 255–260.
- Scorza, F.A.; de Almeida, A.-C.G.; Fiorini, A.C.; Scorza, C.A.; Finsterer, J. Fighting eye diseases with Brazilian Green Propolis. Biomed. Pharmacother. 2021, 140, 111740.
- Mahmoud, S.S.; ElAbrak, E.S.; Aly, M.A.; Ali, E.M. Oculohypotensive effects of various acetozolamide nanopreparations for topical treatment of animal model-induced glaucoma and their impact on optic nerve. PLoS ONE 2019, 14, e0212588.
- Park, J.W.; Sung, M.S.; Ha, J.Y.; Guo, Y.; Piao, H.; Heo, H.; Park, S.W. Neuroprotective Effect of Brazilian Green Propolis on Retinal Ganglion Cells in Ischemic Mouse Retina. Curr. Eye Res. 2020, 45, 955–964.
- Bozkan, Z.; Belge, A.; Sarierler, M.; Tunca, R.; Yaygingül, R.; Ipek, E. Tavşanlarda Korneanın Subakut Alkali Yanıklarında Amniyotik Membran Transplantasyonu, Topikal Su Bazlı Propolis Ekstraktı, Kortikosteriod ve Antibiyotiğin Farklı Kombinasyonlarda Kullanımının Etkinliğinin Karşılaştırılması. Kafkas Univ. Vet. Fak. Derg. 2019, 25, 825–833.
- Skulachev, V.P.; Iomdina, E.N.; Maslova, I.P.K.; Robustova, O.V.; Averina, O.A.; Kovaleva, N.A.; Aliev, G.; Reddy, V.P.; Zamyatnin, A.A.; Skulachev, M.V.; et al. Mitochondria-targeted antioxidant SkQ1 reverses glaucomatous lesions in rabbits. Front. Biosci. 2015, 20, 892–901.
- Jia, Y.; Jiang, S.; Chen, C.; Lu, G.; Xie, Y.; Sun, X.; Huang, L. Caffeic acid phenethyl ester attenuates nuclear factor-κB-mediated inflammatory responses in Müller cells and protects against retinal ganglion cell death. Mol. Med. Rep. 2019, 19, 4863–4871.
- Demir, E.; Taysi, S.; Al, B.; Demir, T.; Okumus, S.; Saygili, O.; Saricicek, E.; Dirier, A.; Akan, M.; Tarakcioglu, M.; et al. The effects of Nigella sativa oil, thymoquinone, propolis, and caffeic acid phenethyl ester on radiation-induced cataract. Wien. Klin. Wochenschr. 2016, 128, 587–595.
- Sharma, N.; Singh, D.; Maharana, P.K.; Kriplani, A.; Velpandian, T.; Pandey, R.M.; Vajpayee, R.B. Comparison of Amniotic Membrane Transplantation and Umbilical Cord Serum in Acute Ocular Chemical Burns: A Randomized Controlled Trial. Am. J. Ophthalmol. 2016, 168, 157–163.
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204.
- Knollinger, A.M.; McDonald, J.E.; Carpenter, N.A.; Crook, E.K. Use of equine amniotic membrane free-island grafts for treatment of a midstromal corneal ulcer and descemetocele in a snow leopard (Panthera uncia). J. Am. Veter Med. Assoc. 2018, 253, 1623–1629.




