
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Otávio Augusto Leitão dos Santos | -- | 3775 | 2023-01-31 17:57:52 | | | |
| 2 | Lindsay Dong | Meta information modification | 3775 | 2023-02-01 02:54:59 | | |
Video Upload Options
Nanotechnology involves materials at a scale ranging from 1 to 100 nm in one of its dimensions. It has brought advances in several areas such as electronics, pharmaceuticals, biotechnology, agriculture, cosmetics, and food. Nanostructures have a higher surface-to-volume ratio compared to bulk materials aside from exhibiting enhanced catalytic, mechanical, optical, electrical, tribological, thermal, and other properties. For this reason, nanomaterials have been widely studied and applied for the production of different products such as textiles, food coloring, pharmaceuticals, and cosmetics. Furthermore, the interaction of nanomaterials with biological systems and the environment still needs to be clarified. Moreover, some issues such as toxicity, bioaccumulation, and physicochemical transformations are found to be dependent on several factors such as size, capping agent, and shape, making the comparisons even more complex.
1. Synthesis of Nanomaterials
2. Applications
A variety of products containing NMs are already on the market and under development. However, through their production and utilization, these NMs can reach the environment and may cause contamination and pollution.
3. Environment Transformations
4. Toxicity
4.1. Aquatic Environments
4.2. Terrestrial Environments
3.3. Human and Animal Health
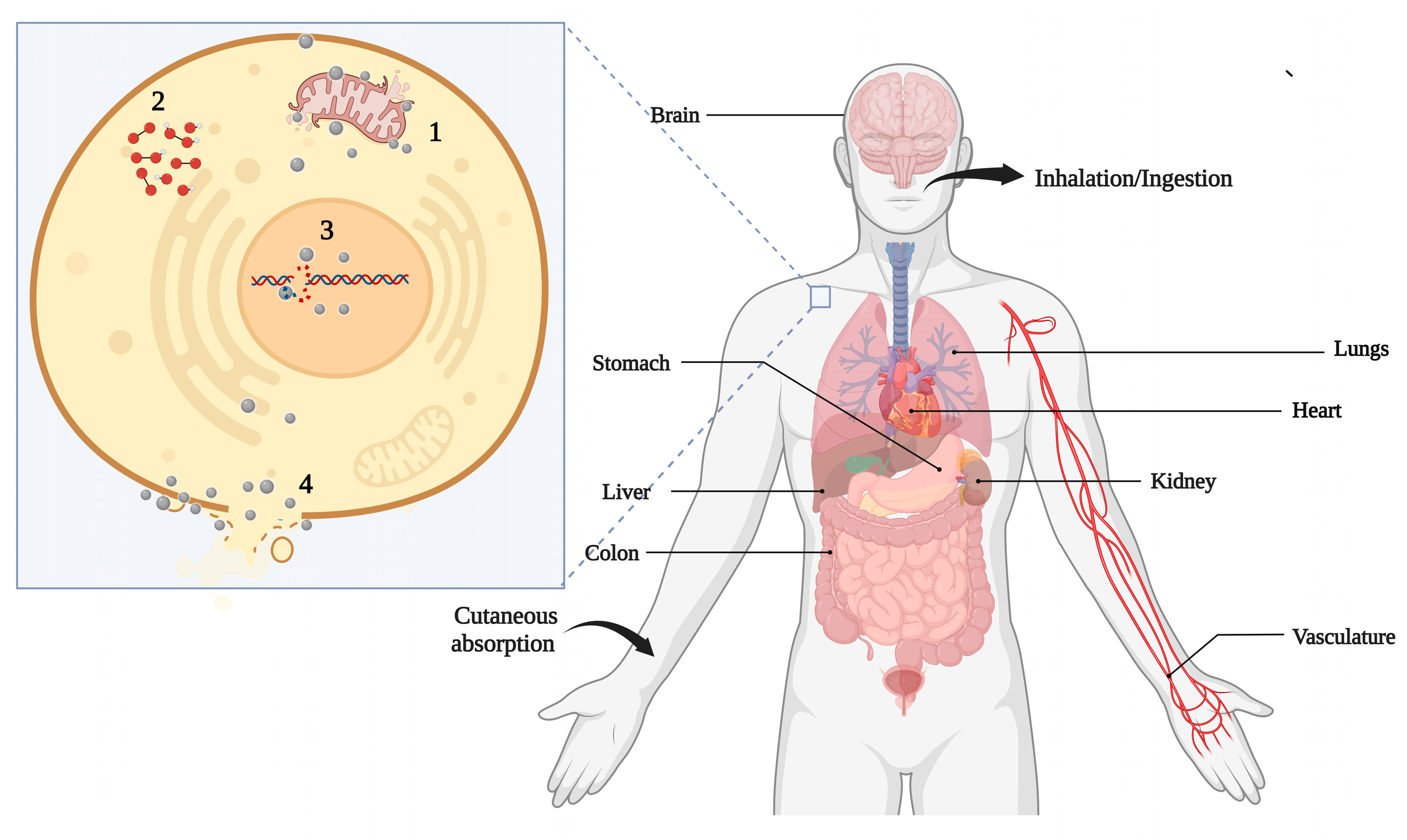
3.4. Impacts on the Food Chain
References
- Paschoalino, M.P.; Marcone, G.P.S.; Jardim, W.F. Os Nanomateriais e a Questão Ambiental. Quim. Nova 2010, 33, 421–430.
- Chaturvedi, V.; Verma, P. Fabrication of Silver Nanoparticles from Leaf Extract of Butea Monosperma (Flame of Forest) and Their Inhibitory Effect on Bloom-Forming Cyanobacteria. Bioresour. Bioprocess 2015, 2, 18.
- Shemer, G.; Krichevski, O.; Markovich, G.; Molotsky, T.; Lubitz, I.; Kotlyar, A.B. Chirality of Silver Nanoparticles Synthesized on DNA. J. Am. Chem. Soc. 2006, 128, 11006–11007.
- Gao, X.; Wei, L.; Yan, H.; Xu, B. Green Synthesis and Characteristic of Core-Shell Structure Silver/Starch Nanoparticles. Mater. Lett. 2011, 65, 2963–2965.
- Syed, A.; Ahmad, A. Extracellular Biosynthesis of Platinum Nanoparticles Using the Fungus Fusarium Oxysporum. Colloids Surf. B Biointerfaces 2012, 97, 27–31.
- Prabhawathi, V.; Sivakumar, P.M.; Doble, M. Green Synthesis of Protein Stabilized Silver Nanoparticles Using Pseudomonas Fluorescens, a Marine Bacterium, and Its Biomedical Applications When Coated on Polycaprolactam. Ind. Eng. Chem. Res. 2012, 51, 5230–5239.
- Oluwafemi, O.S.; Vuyelwa, N.; Scriba, M.; Songca, S.P. Green Controlled Synthesis of Monodispersed, Stable and Smaller Sized Starch-Capped Silver Nanoparticles. Mater. Lett. 2013, 106, 332–336.
- Chowdhury, S.; Basu, A.; Kundu, S. Green Synthesis of Protein Capped Silver Nanoparticles from Phytopathogenic Fungus Macrophomina Phaseolina (Tassi) Goid with Antimicrobial Properties against Multidrug-Resistant Bacteria. Nanoscale Res. Lett. 2014, 9, 365.
- Maliszewska, I.; Juraszek, A.; Bielska, K. Green Synthesis and Characterization of Silver Nanoparticles Using Ascomycota Fungi Penicillium Nalgiovense AJ12. J. Clust. Sci. 2014, 25, 989–1004.
- Roni, M.; Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Nicoletti, M.; Madhiyazhagan, P.; Dinesh, D.; Suresh, U.; Khater, H.F.; Wei, H.; et al. Characterization and Biotoxicity of Hypnea Musciformis-Synthesized Silver Nanoparticles as Potential Eco-Friendly Control Tool against Aedes Aegypti and Plutella Xylostella. Ecotoxicol. Environ. Saf. 2015, 121, 31–38.
- Asghar, M.A.; Zahir, E.; Shahid, S.M.; Khan, M.N.; Asghar, M.A.; Iqbal, J.; Walker, G. Iron, Copper and Silver Nanoparticles: Green Synthesis Using Green and Black Tea Leaves Extracts and Evaluation of Antibacterial, Antifungal and Aflatoxin B1 Adsorption Activity. LWT 2018, 90, 98–107.
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green Synthesis of Gold Nanoparticles by Thermophilic Filamentous Fungi. Sci. Rep. 2018, 8, 3943.
- Pizzorno Backx, B.; Rech Pedrosa, B.; Delazare, T.; Carmo Damasceno, F.R.D.; Leitao Dos Santos, O.A. Green Synthesis of Silver Nanoparticles: A Study of the Dispersive Efficiency and Antimicrobial Potential of the Extracts of Plinia Cauliflora for Application in Smart Textiles Materials for Healthcare. J. Nanomater. Mol. Nanotechnol. 2018, 7.
- Dos Santos, O.A.L.; Backx, B.P. Estudo Da Eficiência Da Síntese De Nanopartículas De Prata Em Extrato De Beta Vulgaris Para Aplicação Em Têxteis Com Atividade Antimicrobiana. In Ciências da Saúde; Antonella Carvalho de Oliveira; Atena Editora: Ponta Grossa, Brazil, 2019; pp. 143–157.
- Dos Santos, O.A.L.; de Araujo, I.; Dias da Silva, F.; Sales, M.N.; Christoffolete, M.A.; Backx, B.P. Surface Modification of Textiles by Green Nanotechnology against Pathogenic Microorganisms. Curr. Res. Green Sustain. Chem. 2021, 4, 100206.
- Nath, D.; Banerjee, P. Green Nanotechnology—A New Hope for Medical Biology. Environ. Toxicol. Pharm. 2013, 36, 997–1014.
- Sujitha, V.; Murugan, K.; Paulpandi, M.; Panneerselvam, C.; Suresh, U.; Roni, M.; Nicoletti, M.; Higuchi, A.; Madhiyazhagan, P.; Subramaniam, J.; et al. Green-Synthesized Silver Nanoparticles as a Novel Control Tool against Dengue Virus (DEN-2) and Its Primary Vector Aedes Aegypti. Parasitol. Res. 2015, 114, 3315–3325.
- Prasad, R. Synthesis of Silver Nanoparticles in Photosynthetic Plants. J. Nanopart. 2014, 2014, 963961.
- Hyeon, T.; Manna, L.; Wong, S.S. Sustainable Nanotechnology. Chem. Soc. Rev. 2015, 44, 5755–5757.
- Dos Santos, O.A.L.; Backx, B.P. Green Nanotechnology: The Influence of Intermolecular and Supramolecular Interactions. J. Nanotechnol. Nanomater. 2020, 1, 104–108.
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 9780128184899.
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green Synthesis, Characterization and Anti-Inflammatory Activity of Silver Nanoparticles Using European Black Elderberry Fruits Extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777.
- Mariselvam, R.; Ranjitsingh, A.J.A.; Usha Raja Nanthini, A.; Kalirajan, K.; Padmalatha, C.; Mosae Selvakumar, P. Green Synthesis of Silver Nanoparticles from the Extract of the Inflorescence of Cocos Nucifera (Family: Arecaceae) for Enhanced Antibacterial Activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 537–541.
- Puišo, J.; Jonkuvienė, D.; Mačionienė, I.; Šalomskienė, J.; Jasutienė, I.; Kondrotas, R. Biosynthesis of Silver Nanoparticles Using Lingonberry and Cranberry Juices and Their Antimicrobial Activity. Colloids Surf. B Biointerfaces 2014, 121, 214–221.
- Ramesh, P.S.; Kokila, T.; Geetha, D. Plant Mediated Green Synthesis and Antibacterial Activity of Silver Nanoparticles Using Emblica Officinalis Fruit Extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 339–343.
- Bindhu, M.R.; Umadevi, M. Antibacterial and Catalytic Activities of Green Synthesized Silver Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 373–378.
- Arokiyaraj, S.; Vincent, S.; Saravanan, M.; Lee, Y.; Oh, Y.K.; Kim, K.H. Green Synthesis of Silver Nanoparticles Using Rheum Palmatum Root Extract and Their Antibacterial Activity against Staphylococcus Aureus and Pseudomonas Aeruginosa. Artif. Cells Nanomed. Biotechnol. 2017, 45, 372–379.
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana Peel Extract Mediated Novel Route for the Synthesis of Silver Nanoparticles. Colloids Surf. A Phys. Eng. Asp. 2010, 368, 58–63.
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; Pyne, S.; Misra, A. Green Synthesis of Silver Nanoparticles Using Seed Extract of Jatropha Curcas. Colloids Surf. A Phys. Eng. Asp. 2009, 348, 212–216.
- Kiran Kumar, H.A.; Mandal, B.K.; Mohan Kumar, K.; Maddinedi, S.B.; Sai Kumar, T.; Madhiyazhagan, P.; Ghosh, A.R. Antimicrobial and Antioxidant Activities of Mimusops Elengi Seed Extract Mediated Isotropic Silver Nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 13–18.
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green Synthesis of Silver Nanoparticles Using Olive Leaf Extract and Its Antibacterial Activity. Arab. J. Chem. 2014, 7, 1131–1139.
- Veisi, H.; Azizi, S.; Mohammadi, P. Green Synthesis of the Silver Nanoparticles Mediated by Thymbra Spicata Extract and Its Application as a Heterogeneous and Recyclable Nanocatalyst for Catalytic Reduction of a Variety of Dyes in Water. J. Clean. Prod. 2018, 170, 1536–1543.
- Anandan, M.; Poorani, G.; Boomi, P.; Varunkumar, K.; Anand, K.; Chuturgoon, A.A.; Saravanan, M.; Gurumallesh Prabu, H. Green Synthesis of Anisotropic Silver Nanoparticles from the Aqueous Leaf Extract of Dodonaea Viscosa with Their Antibacterial and Anticancer Activities. Process Biochem. 2019, 80, 80–88.
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green Synthesis and Characterization of Silver Nanoparticles Using Fritillaria Flower Extract and Their Antibacterial Activity against Some Human Pathogens. Polyhedron 2019, 158, 8–14.
- Natsuki, J. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325.
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of Nanoparticles and Silver Nanoparticles. Bioresour. Bioprocess. 2015, 2, 47.
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic Silver Nanoparticles: Synthesis, Mechanism, and Applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593.
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28.
- Van Phu, D.; Lang, V.T.K.; Kim Lan, N.T.; Duy, N.N.; Chau, N.D.; Du, B.D.; Cam, B.D.; Hien, N.Q. Synthesis and Antimicrobial Effects of Colloidal Silver Nanoparticles in Chitosan by γ -Irradiation. J. Exp. Nanosci. 2010, 5, 169–179.
- Priyadarshini, B.; Rath, P.P.; Behera, S.S.; Panda, S.R.; Sahoo, T.R.; Parhi, P.K. Kinetics, Thermodynamics and Isotherm Studies on Adsorption of Eriochrome Black-T from Aqueous Solution Using Rutile TiO 2. IOP Conf. Ser. Mater. Sci. Eng. 2018, 310, 012051.
- Wright, P.F. Potential Risks and Benefits of Nanotechnology: Perceptions of Risk in Sunscreens. Med. J. Aust. 2016, 204, 369–370.
- Qian, Y.; Qin, C.; Chen, M.; Lin, S. Nanotechnology in Soil Remediation—Applications vs. Implications. Ecotoxicol. Environ. Saf. 2020, 201, 110815.
- Kanel, S.R.; Nepal, D.; Manning, B.; Choi, H. Transport of Surface-Modified Iron Nanoparticle in Porous Media and Application to Arsenic(III) Remediation. J. Nanopart. Res. 2007, 9, 725–735.
- Pala, I.R.; Brock, S.L. ZnS Nanoparticle Gels for Remediation of Pb 2+ and Hg 2+ Polluted Water. ACS Appl. Mater. Interfaces 2012, 4, 2160–2167.
- Murgueitio, E.; Cumbal, L.; Abril, M.; Izquierdo, A.; Debut, A.; Tinoco, O. Green Synthesis of Iron Nanoparticles: Application on the Removal of Petroleum Oil from Contaminated Water and Soils. J. Nanotechnol. 2018, 2018, 4184769.
- Perera, M.; Wijenayaka, L.A.; Siriwardana, K.; Dahanayake, D.; Nalin de Silva, K.M. Gold Nanoparticle Decorated Titania for Sustainable Environmental Remediation: Green Synthesis, Enhanced Surface Adsorption and Synergistic Photocatalysis. RSC Adv. 2020, 10, 29594–29602.
- Akinpelu, A.A.; Ali, M.E.; Johan, M.R.; Saidur, R.; Chowdhury, Z.Z.; Shemsi, A.M.; Saleh, T.A. Effect of the Oxidation Process on the Molecular Interaction of Polyaromatic Hydrocarbons (PAH) with Carbon Nanotubes: Adsorption Kinetic and Isotherm Study. J. Mol. Liq. 2019, 289, 111107.
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered Nanomaterials for Antimicrobial Applications: A Review. Appl. Mater. Today 2020, 18, 32.
- Backx, B.P.; dos Santos, M.S.; dos Santos, O.A.L.; Filho, S.A. The Role of Biosynthesized Silver Nanoparticles in Antimicrobial Mechanisms. Curr. Pharm. Biotechnol. 2021, 22, 762–772.
- Zhao, Y.; Chen, L.; Wang, Y.; Song, X.; Li, K.; Yan, X.; Yu, L.; He, Z. Nanomaterial-Based Strategies in Antimicrobial Applications: Progress and Perspectives. Nano Res. 2021, 14, 4417–4441.
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32.
- Franco-Molina, M.A.; Mendoza-Gamboa, E.; Sierra-Rivera, C.A.; Gómez-Flores, R.A.; Zapata-Benavides, P.; Castillo-Tello, P.; Alcocer-González, J.M.; Miranda-Hernández, D.F.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C. Antitumor Activity of Colloidal Silver on MCF-7 Human Breast Cancer Cells. J. Exp. Clin. Cancer Res. 2010, 29, 148.
- Faedmaleki, F.; Shirazi, F.H.; Salarian, A.A.; Ashtiani, H.A.; Rastegar, H. Toxicity Effect of Silver Nanoparticles on Mice Liver Primary Cell Culture and HepG2 Cell Line. Iran. J. Pharm. Res. 2014, 13, 235–242.
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335.
- Rao, P.V.; Nallappan, D.; Madhavi, K.; Rahman, S.; Jun Wei, L.; Gan, S.H. Phytochemicals and Biogenic Metallic Nanoparticles as Anticancer Agents. Oxid. Med. Cell. Longev. 2016, 2016, 3685671.
- Hassan, H.F.H.; Mansour, A.M.; Abo-Youssef, A.M.H.; Elsadek, B.E.M.; Messiha, B.A.S. Zinc Oxide Nanoparticles as a Novel Anticancer Approach; in Vitro and in Vivo Evidence. Clin. Exp. Pharm. Physiol. 2017, 44, 235–243.
- Jadhav, K.; Deore, S.; Dhamecha, D.; Hr, R.; Jagwani, S.; Jalalpure, S.; Bohara, R. Phytosynthesis of Silver Nanoparticles: Characterization, Biocompatibility Studies, and Anticancer Activity. ACS Biomater. Sci. Eng. 2018, 4, 892–899.
- Al-Rubaye, H.I.; Al-Rubaye, B.K.; Al-Abodi, E.E.; Yousif, E.I. Green Chemistry Synthesis of Modified Silver Nanoparticles. J. Phys. Conf. Ser. 2020, 1664, 012080.
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387.
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron Oxide Nanoparticles for Sustained Delivery of Anticancer Agents. Mol. Pharm. 2005, 2, 194–205.
- Brown, S.D.; Nativo, P.; Smith, J.A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold Nanoparticles for the Improved Anticancer Drug Delivery of the Active Component of Oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684.
- Hirst, S.M.; Karakoti, A.S.; Tyler, R.D.; Sriranganathan, N.; Seal, S.; Reilly, C.M. Anti-Inflammatory Properties of Cerium Oxide Nanoparticles. Small 2009, 5, 2848–2856.
- Hebeish, A.; El-Rafie, M.H.; EL-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial Wound Dressing and Anti-Inflammatory Efficacy of Silver Nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515.
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In Vitro Anti-Inflammatory Activity of Spherical Silver Nanoparticles and Monodisperse Hexagonal Gold Nanoparticles by Fruit Extract of Prunus Serrulata: A Green Synthetic Approach. Artif. Cells Nanomed. Biotechnol. 2017, 46, 2022–2032.
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362.
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green Synthesis of Gold Nanoparticles Using Curcuma Pseudomontana Isolated Curcumin: Its Characterization, Antimicrobial, Antioxidant and Anti- Inflammatory Activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124.
- Nagajyothi, P.C.; Cha, S.J.; Yang, I.J.; Sreekanth, T.V.M.; Kim, K.J.; Shin, H.M. Antioxidant and Anti-Inflammatory Activities of Zinc Oxide Nanoparticles Synthesized Using Polygala Tenuifolia Root Extract. J. Photochem. Photobiol. B 2015, 146, 10–17.
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green Approach for Synthesis of Zinc Oxide Nanoparticles from Andrographis Paniculata Leaf Extract and Evaluation of Their Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30.
- El-Ghazaly, M.A.; Fadel, N.; Rashed, E.; El-Batal, A.; Kenawy, S.A. Anti-Inflammatory Effect of Selenium Nanoparticles on the Inflammation Induced in Irradiated Rats. Can. J. Physiol. Pharm. 2017, 95, 101–110.
- Rehman, M.U.; Yoshihisa, Y.; Miyamoto, Y.; Shimizu, T. The Anti-Inflammatory Effects of Platinum Nanoparticles on the Lipopolysaccharide-Induced Inflammatory Response in RAW 264.7 Macrophages. Inflamm. Res. 2012, 61, 1177–1185.
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113.
- Capaldi Arruda, S.C.; Diniz Silva, A.L.; Moretto Galazzi, R.; Antunes Azevedo, R.; Zezzi Arruda, M.A. Nanoparticles Applied to Plant Science: A Review. Talanta 2015, 131, 693–705.
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in Agriculture: Opportunities, Toxicological Implications, and Occupational Risks. Toxicol. Appl. Pharm. 2017, 329, 96–111.
- Chhipa, H.; Joshi, P. Nanofertilisers, Nanopesticides and Nanosensors in Agriculture. In Nanoscience in Food and Agriculture; Springer: Cham, Switzerland, 2016; pp. 247–282.
- Sanivada, S.K.; Pandurangi, V.S.; Challa, M.M. Nanofertilizers for Sustainable Soil Management. In Nanoscience in Food and Agriculture; Springer: Cham, Switzerland, 2017.
- Dos Santos, O.A.L.; Sneha, M.; Devarani, T.; Bououdina, M.; Backx, B.P.; Vijaya, J.J.; Bellucci, S. Review—Perovskite/Spinel Based Graphene Derivatives Electrochemical and Biosensors. J. Electrochem. Soc. 2021, 168, 067506.
- Vikesland, P.J. Nanosensors for Water Quality Monitoring. Nat. Nanotechnol. 2018, 13, 651–660.
- Mousavi, S.M.; Hashemi, S.A.; Zarei, M.; Amani, A.M.; Babapoor, A. Nanosensors for Chemical and Biological and Medical Applications. Med. Chem. 2018, 8, 205–217.
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for Diagnosis with Optical, Electric and Mechanical Transducers. RSC Adv. 2019, 9, 6793–6803.
- Yang, T.; Duncan, T.V. Challenges and Potential Solutions for Nanosensors Intended for Use with Foods. Nat. Nanotechnol. 2021, 16, 251–265.
- Nogueira, P.F.M.; Paino, I.M.M.; Zucolotto, V. Nanosilver: Propriedades, Aplicações e Impactos Na Saúde Pública e Meio Ambiente. Vigil. Sanit. Debate 2013, 1, 59–71.
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a New Generation of Nanoproduct in Biomedical Applications. Trends Biotechnol. 2010, 28, 580–588.
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2016, 11, 81–100.
- Zhang, J.; Guo, W.; Li, Q.; Wang, Z.; Liu, S. The Effects and the Potential Mechanism of Environmental Transformation of Metal Nanoparticles on Their Toxicity in Organisms. Environ. Sci. Nano 2018, 5, 2482–2499.
- Wheeler, K.E.; Chetwynd, A.J.; Fahy, K.M.; Hong, B.S.; Tochihuitl, J.A.; Foster, L.A.; Lynch, I. Environmental Dimensions of the Protein Corona. Nat. Nanotechnol. 2021, 16, 617–629.
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.; Lynch, I. Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials 2013, 6, 2295–2350.
- Liu, J.; Hurt, R.H. Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids. Environ. Sci. Technol. 2010, 44, 2169–2175.
- Jahan, S.; Alias, Y.B.; Bakar, A.F.B.A.; Yusoff, I.B. Toxicity Evaluation of ZnO and TiO2 Nanomaterials in Hydroponic Red Bean (Vigna Angularis) Plant: Physiology, Biochemistry and Kinetic Transport. J. Environ. Sci. 2018, 72.
- Sohal, I.S.; Cho, Y.K.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Dissolution Behavior and Biodurability of Ingested Engineered Nanomaterials in the Gastrointestinal Environment. ACS Nano 2018, 12, 8115–8128.
- Quik, J.T.K.; Stuart, M.C.; Wouterse, M.; Peijnenburg, W.; Hendriks, A.J.; van de Meent, D. Natural Colloids Are the Dominant Factor in the Sedimentation of Nanoparticles. Environ. Toxicol. Chem. 2012, 31, 1019–1022.
- Lovern, S.B.; Klaper, R. Daphnia Magna Mortality When Exposed To Titanium Dioxide And Fullerene (C60) Nanoparticles. Environ. Toxicol. Chem. 2006, 25, 1132.
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental Transformation and Nano-Toxicity of Engineered Nano-Particles (ENPs) in Aquatic and Terrestrial Organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581.
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of Metal and Metal Oxide Nanoparticles on Growth and Physiology of Globally Important Food Crops: A Critical Review. J. Hazard. Mater. 2017, 322, 2–16.
- Hannah, W.; Thompson, P.B. Nanotechnology, Risk and the Environment: A Review. J. Environ. Monit. 2008, 10, 291.
- Yao, D.; Chen, Z.; Zhao, K.; Yang, Q.; Zhang, W. Limitation and Challenge Faced to the Researches on Environmental Risk of Nanotechnology. Procedia Environ. Sci. 2013, 18, 149–156.
- Lee, W.-M.; Kwak, J.I.; An, Y.-J. Effect of Silver Nanoparticles in Crop Plants Phaseolus Radiatus and Sorghum Bicolor: Media Effect on Phytotoxicity. Chemosphere 2012, 86, 491–499.
- Zhang, Z.; Ke, M.; Qu, Q.; Peijnenburg, W.J.G.M.; Lu, T.; Zhang, Q.; Ye, Y.; Xu, P.; Du, B.; Sun, L.; et al. Impact of Copper Nanoparticles and Ionic Copper Exposure on Wheat (Triticum aestivum L.) Root Morphology and Antioxidant Response. Environ. Pollut. 2018, 239, 689–697.
- Xiong, T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper Oxide Nanoparticle Foliar Uptake, Phytotoxicity, and Consequences for Sustainable Urban Agriculture. Environ. Sci. Technol. 2017, 51, 5242–5251.
- Iannone, M.F.; Groppa, M.D.; de Sousa, M.E.; Fernández van Raap, M.B.; Benavides, M.P. Impact of Magnetite Iron Oxide Nanoparticles on Wheat (Triticum aestivum L.) Development: Evaluation of Oxidative Damage. Environ. Exp. Bot. 2016, 131.
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the Toxicity of Silver Nanoparticles and Silver Ions on the Growth of Terrestrial Plant Model Arabidopsis Thaliana. J. Environ. Sci. 2013, 25, 1947–1956.
- Bagherzadeh Homaee, M.; Ehsanpour, A.A. Silver Nanoparticles and Silver Ions: Oxidative Stress Responses and Toxicity in Potato (Solanum tuberosum L.) Grown in Vitro. Hortic Environ. Biotechnol. 2016, 57, 544–553.
- Cvjetko, P.; Milošić, A.; Domijan, A.M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of Silver Ions and Differently Coated Silver Nanoparticles in Allium Cepa Roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28.
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-Coated Silver Nanoparticles in Biological and Environmental Conditions: Fate, Stability and Toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34.
- Balbus, J.M.; Florini, K.; Denison, R.A.; Walsh, S.A. Protecting Workers and the Environment: An Environmental NGO’s Perspective on Nanotechnology. J. Nanopart. Res. 2006, 9, 11–22.
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290.
- Kulandaivelu, B.; Gothandam, K.M. Cytotoxic Effect on Cancerous Cell Lines by Biologically Synthesized Silver Nanoparticles. Braz. Arch. Biol. Technol. 2016, 59.
- Abdolahpur Monikh, F.; Chupani, L.; Arenas-Lago, D.; Guo, Z.; Zhang, P.; Darbha, G.K.; Valsami-Jones, E.; Lynch, I.; Vijver, M.G.; van Bodegom, P.M.; et al. Particle Number-Based Trophic Transfer of Gold Nanomaterials in an Aquatic Food Chain. Nat. Commun. 2021, 12, 899.




