
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Poul Erik Hansen | -- | 488 | 2023-01-31 06:55:57 | | | |
| 2 | Sirius Huang | Meta information modification | 488 | 2023-01-31 06:57:10 | | |
Video Upload Options
The present overview concentrates on recent developments of tautomerism of β-diketones and β-thioxoketones, both in solution and in the solid state. In particular, the latter has been a matter of debate and unresolved problems. Measurements of 13C, 17O, and 2H chemical shifts have been used. Deuterium isotope effects on chemical shifts are proposed as a tool in the study of this problem. Photoconversion of β-diketones and β-thioxoketones are discussed in detail, and the incorporation of β-diketones into molecules with fluorescent properties is assessed. Finally, docking studies of β-diketones are scrutinized with an emphasis on correct tautomeric structures and knowledge about barriers to interconversion of tautomers.
β-Diketones
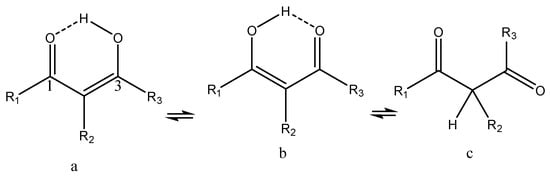
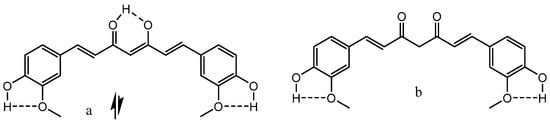
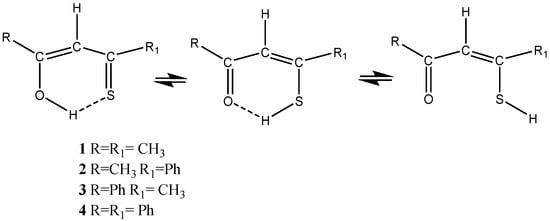
β-Thioxoketones
References
- De Gonzalo, G.; Alcántara, A.R. Recent Development in the Synthesis of β-diketones. Pharmaceuticals 2021, 14, 1043.
- Hansen, P.E. Structural Studies of β-diketones and its implications on Biological Effects. Pharmaceuticals 2021, 14, 1189.
- Kljun, J.; Ture, I. β-Diketones as Scaffolds for Anticancer Drug Design–From Organic Building Blocks to Natural Products and Metallodrug Components. Eur. J. Inorg. Chem. 2017, 12, 1655–1666.
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N. Biological activities of curcumin and its analogues (Congeners) made by man and Mother. Nat. Biochem. Pharmacol. 2008, 76, 1590–1611.
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637.
- Priyadarsini, K.I. Chemical and Structural Features Influencing the Biological Activity of Curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100.
- Slika, L.; Patra, D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2020, 17, 61–75.
- Malekshah, R.E.; Salehi, M.; Kubicki, M.; Khaleghian, A.J. Biological studies and computational modeling of two new copper complexes derived from β-diketones and their nano-complexes. Coord. Chem. 2019, 72, 1697–1714.
- Hansen, P.E.; Mortensen, J.; Kamounah, F.S. The importance of correct tautomeric structures for biological molecules. JSM Chem. 2015, 3, 1014–1019.
- Jezierska, A.; Panek, J.J. Investigation of an O-H….S hydrogen bond via Carr-Parrinello and Path Integral molecular dynamics. J. Comput. Chem. 2009, 30, 1241–1250.
- Mayoral, M.J.; Cornago, P.; Claramunt, R.; Cano, M. Pyridyl and pyridiniumyl β-diketonates as building blocks for palladium(III) and allyl-platinium(II) isomers. Multinuclear NMR structural elucidation and liquid crystal behavior. New. J. Chem. 2011, 35, 1020–1030.
- Andrews, P.C.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Kedzierski, L.; Peiris, R.M. Bismuth(III) beta-thioxoketonates as antibiotics against Helicobacter pylori and as anti-leishmanial agents. Dalton Trans. 2014, 43, 1279–1291.
- Duus, F.; Antonsen, J.W. Thioxoketones. I. Preparation and Structure of Thioacetylacetone. Acta Chem. Scand. 1977, B 31, 40–46.
- Semenova, I.S.; Yarovenko, V.N.; Levchenko, K.S.; Krayushkin, M.M. Synthesis of 1,3-thioxoketones from salicylaldehyde. Russ. Chem. Bull. 2013, 62, 1022–1025.
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652.
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789.




