The present overview concentrates on recent developments of tautomerism of β-diketones and β-thioxoketones, both in solution and in the solid state. In particular, the latter has been a matter of debate and unresolved problems. Measurements of 13C, 17O, and 2H chemical shifts have been used. Deuterium isotope effects on chemical shifts are proposed as a tool in the study of this problem. Photoconversion of β-diketones and β-thioxoketones are discussed in detail, and the incorporation of β-diketones into molecules with fluorescent properties is assessed. Finally, docking studies of β-diketones are scrutinized with an emphasis on correct tautomeric structures and knowledge about barriers to interconversion of tautomers.
β-Diketones
β-diketones is a very broad subject. The present paper concentrates on some recent developments, primarily since 2008. The β-diketones (see Scheme 1) are a very versatile group of molecules that easily can be synthesized [
1], and hence be tailored to fulfill different purposes. In addition, they can be isolated as natural products often with extraordinary structures [
2]. β-Diketones are very good synthons [
1,
3]; e.g., they can be converted into β-thioxoketones (Scheme 2). In addition, they are known to have useful biological effects [
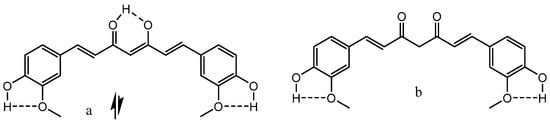
2]. A well know example is curcumin and derivatives thereof (
Figure 1) [
4,
5,
6]. The physical properties are related to uptake [
7]. β-Diketones can form a large number of metal complexes [
8]. A group of compounds with similar properties is the β-trioxoketones [
9], and a well-studied case is usnic acid [
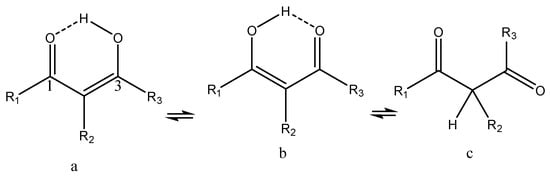
9]. Linear β-diketones may exist as different tautomers (enol and keto- forms, Scheme 1). The key features of the enol forms are strong intramolecular hydrogen bonds, tautomeric forms (see Scheme 1), and a very low barrier to interconversion between the enolic forms.
Scheme 1. Tautomers of β-diketones. a and b are enol forms, c is the diketo-form.
Figure 1. Curcumin. The second enolic tautomer is similar to acetylacetone (see Scheme 1). (a) is the enol form, (b) the diketo-form.
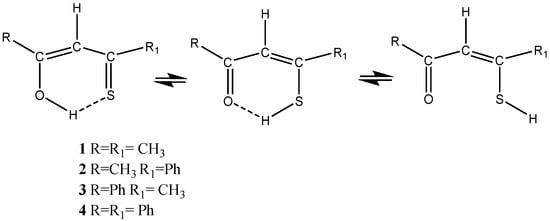
Scheme 2. Tautomers of β-thioxoketones plus the open form.
β-Thioxoketones
β-Thioxoketones (see Scheme 2) and β-diketones are in many ways complementary. Both types display strong intramolecular hydrogen bonds, show tautomerism, and have low barriers to interconversion [
10]. The β-thioxoketones are by nature asymmetric and may actually show an open form (Scheme 2). β-Thioxoketones are colored, and they provide a way of exciting the molecules with visible light sources. β-thioxoketones form, by their nature, very good metal complexes [
11], some of which also show biological effects [
12]. Β-thioxoketones can be synthesized from β-diketones [
13] and also from salicylaldehyde [
14].
The present review will concentrate on structural studies, including tautomers in the liquid and solid state. The importance of low barrier hydrogen bonds (LBHB) will be discussed and hydrogen bond strength, docking studies, and photoconversion will be assessed. The primary experimental methods treated are NMR and isotope effects on chemical shifts, X-ray structures, and infra-red spectroscopy. The experimental techniques are supplemented by theoretical calculations, including Density Functional Theory (DFT) calculations [
15,
16]. In particular, the low barriers call for advanced calculations both in the liquid and in the solid state.
This entry is adapted from the peer-reviewed paper 10.3390/encyclopedia3010013