
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Neil Rowan | -- | 3299 | 2023-01-30 19:29:10 | | | |
| 2 | Lindsay Dong | + 6 word(s) | 3305 | 2023-01-31 10:49:08 | | |
Video Upload Options
Antifungal resistance, antifungal drug tolerance, and biofilm formation directly contribute to rising cases of fungal morbidity and mortality. As with all of the infectious diseases, prevention is the optimal way to mitigate disease outbreak and transmission. The application of effective disinfection and sterilization regimes, particularly in hospital settings, is vitally important, where a focus on fungal biofilm formation on indwelling medical devices is important. Preventing the growth of mycotoxin-producing fungi on foods through the performance of appropriate end-to-end processes is advisable, as mycotoxins are recalcitrant and challenging to eliminate once they have been formed. Adopting the OneHealth approach will support and enable solutions to address this complex societal challenge.
1. Introduction
Cutaneous Infection
2. Efficacy of Disinfection Strategies for Addressing Fungal Pathogens
2.1. Reusable Medical Devices and Surgical Instruments
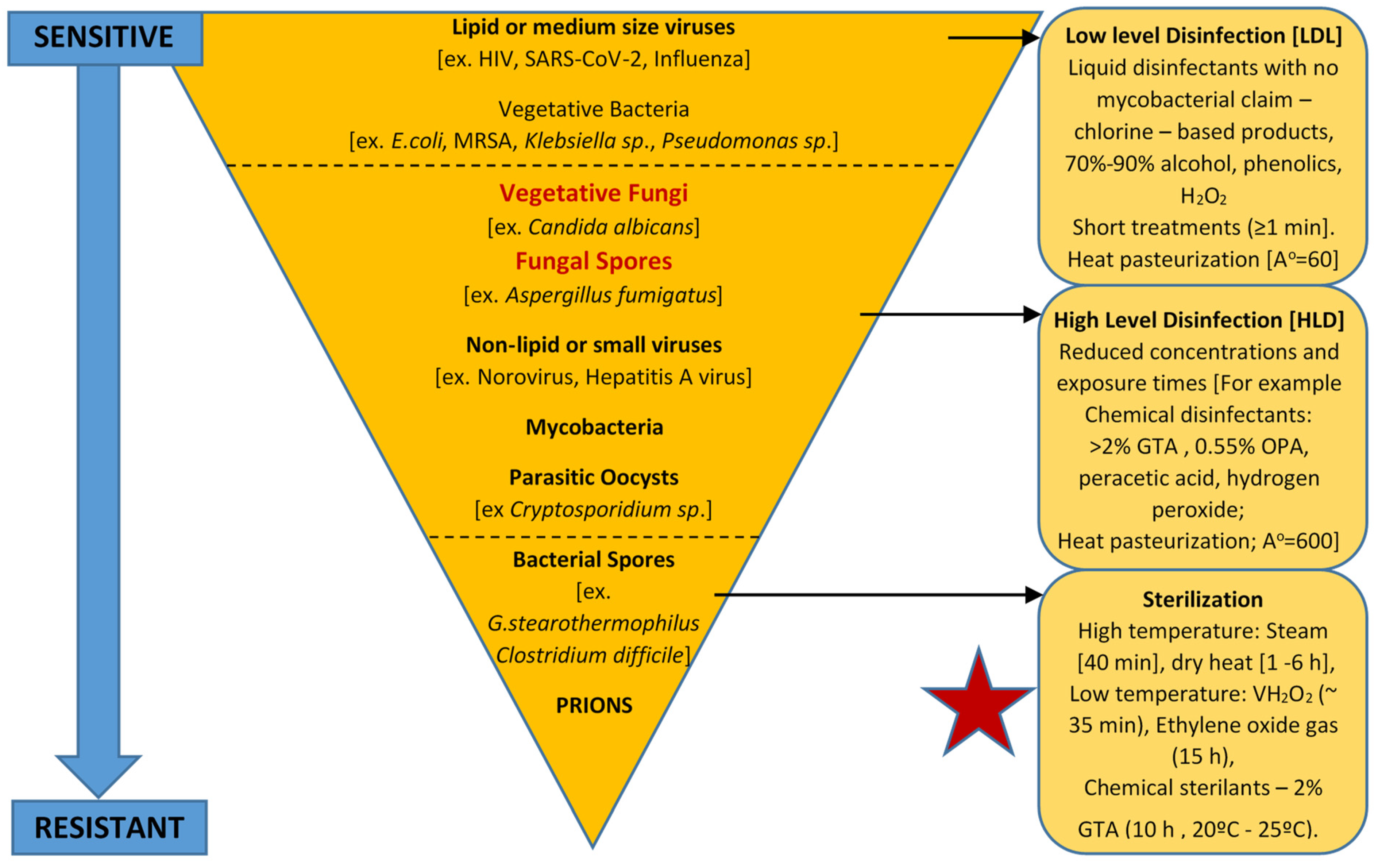
2.2. Central Venous and Urinary Catheters
Modern technology has allowed us to use a wider and newer variety of medical devices. The combination of an increasingly aging population and a consistently growing number of inserted devices is likely to escalate the occurrence of infectious complications related to medical devices [34]. The number of indwelling medical devices is increasing, and an increasing proportion of device-related infections are being caused by Candida spp. Candida spp. produce biofilms on synthetic materials, which facilitates the adhesion of the organisms to the devices and renders them relatively refractory to medical therapy. The management of device-related Candida infections can be challenging. The removal of the infected device is generally needed to cure the Candida infections caused by the medical devices. However, since the pathogenesis of Candida bloodstream infection is complicated, more studies are necessary to determine the role of catheter exchange in patients with both gastrointestinal tract mucositis and indwelling catheters. Kojaic and coworkers [34] noted that C. albicans biofilm formation has three developmental phases: the adherence of yeast cells to the device’s surface (early phase), the formation of a matrix with dimorphic switching from yeast to hyphal forms (intermediate phase), and the increase in the amount of the matrix material, taking on a three-dimensional architecture (maturation phase). Fully mature Candida biofilms have a mixture of morphological forms, and they consist of a dense network of yeasts, hyphae, and pseudohyphae in a matrix of polysaccharides, carbohydrate, protein, and unknown components. The organisms in biofilms behave differently from freely suspended cells with respect to antimicrobial resistance. Both the bacteria and Candida cells within biofilms are markedly resistant to antimicrobial agents [34].
C. auris has become a global threat as it can colonize the skin, medical devices, and hospital environments, causing nosocomial outbreaks of blood and urinary tract infections worldwide [25]. Candida auris can spread among patients in hospitals, and it is intrinsically resistant to one or more classes of antifungals, which makes it particularly difficult to treat in healthcare settings. Comparative genomics has demonstrated that C. auris has expanded families of transporters and lipases, as well as mutations and copy number variants, in genes/enzymes linked to increased resistance and virulence [2]. Understanding the evolution of emerging fungal pathogens such as C. auris will be useful for the design of antifungal drugs and therapies for susceptible patients, potentially improving the clinical outcomes.
3. Knowledge Gap in Molecular and Cellular Mechanisms Underpinning Disinfection of Fungal Pathogens including AMR Post-Treatment Modalities
4. Need for Improved In Vitro and In Vivo Compatibility Tests for Medical Devices Encompassing Antifungal and Disinfection Efficacy
5. Mycotoxins and Appropriate Decontamination Strategies
Mycotoxins are secondary metabolites of mold and fungi; they are generally toxic to living organisms. This term, by general consensus, is used almost exclusively for fungi associated with food products and animal feed, excluding the toxins produced by mushrooms. Mycotoxins are secondary metabolites with no apparent function in the normal metabolism of fungi [39]. They are produced mainly, although not exclusively, when the fungus reaches maturity [39]. They are molecules with structures which vary from simple heterocyclic rings with molecular weights of up to 50 Da to groups with 6–8 irregularly arranged heterocylic rings with a total molecular weight of >500 Da, and they do not show immunogenicity. Studies have revealed the existence of at least around 400 different mycotoxins [40]. Hundreds of mycotoxins have been identified thus far, with some of them, such as aflatoxins, ochratoxins, trichothecenes, zearalenone, fumonisins, and patulin, being considered agro-economically important [39].
The consumption of mycotoxins-contaminated feed causes a plethora of harmful responses from acute toxicity to many persistent health disorders with lethal outcomes, such as mycotoxicosis when it is ingested by animals. Therefore, the main task for feed producers is to minimize the concentration of mycotoxins by applying different strategies that are aimed at minimizing the risk of the mycotoxin effects on animal and human health. However, once the mycotoxins enter the production chain, it is hard to eliminate or inactivate them [41]. Notably, mycotoxin-producing fungi are readily destroyed by moderate levels of disinfection. However, given the recalcitrant nature of mycotoxins, emphasis should be placed on ensuring appropriate end-to-end food production and management to prevent the growth of mycotoxin-producing organisms, such as cleaning the grains and removing the kernels that harbor molds. The use of feed additives or supplements that decrease the risk of animal exposure to mycotoxins can be viewed as a means of enhancing animal welfare. These feed supplements are referred to as the substances blended into feed (e.g., mineral clay, microorganism, and yeast cell wall), adsorbing or detoxifying the mycotoxins in the digestive tract of animals (biological detoxification) [41]. In general, mycotoxins are mainly stable compounds under thermal processing conditions used in feed and food [42]. However, the different thermal food and feed treatments that can have different impacts on the mycotoxins include extrusion, cooking, frying, baking, canning, crumbling, pelleting, roasting, flaking, and alkaline cooking. Among the thermal treatments, the utilization of high-temperature processes demonstrates the greatest potential for mycotoxin reduction [41]. Kabak [43] noted that the application of extrusion at a temperature that is higher than 150 °C has a significant impact on the reduction of zearalenone and fumonisins, while the same conditions led to the moderate reduction of aflatoxins and deoxynivalenol. Oxidizing agents such as ozone and hydrogen peroxide have been used to decontaminate mycotoxin-contaminated raw feed and compound feed [44]. The application of microorganisms or enzyme systems to contaminated feeds can detoxify the mycotoxins by metabolism or degradation in their gastrointestinal tract. This process is an irreversible and environmentally friendly method of detoxification, as it does not leave toxic residues or unwanted by-products [41]. However, the levels of particular mycotoxins in feeds have been reduced, but so far, no single technique has been established that is equally efficient against the broad variety of mycotoxins that can co-occur in various commodities [41]. Furthermore, the procedures of detoxication that appear to be efficient in vitro will not necessarily maintain their effectiveness in an in vivo test.
References
- Huang, J.; Liu, C.; Zheng, X. Clinical features of invasive fungal disease in children with no underlying disease. Sci. Rep. 2022, 12, 208.
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438.
- Petrasch, S.; Knapp, S.J.; van Kan, J.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892.
- Garvey, M.; Meade, E.; Rowan, N.J. Effectiveness of front line and emerging fungal disease prevention and control interventions and opportunities to address appropriate eco-sustainable solutions. Sci. Total Environ. 2022, 851, 158284.
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416.
- Gow, N.A.R.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M.; et al. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 2022, 13, 5352.
- Muderris, T.; Kaya, S.; Ormen, B.; Aksoy Gokmen, A.; Varer Akpinar, C.; Yertsever Gul, S. Mortality and risk factor analysis for Candida blood stream infection: A three year retrospective study. J. Mycol. Méd. 2020, 30, 101008.
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430.
- Khodadadi, H.; Zomorodian, K.; Nouraei, H.; Zareshahrabadi, Z.; Barzegar, S.; Zare, M.R.; Pakshir, K. Prevalence of superficial-cutaneous fungal infections in Shiraz, Iran: A five-year retrospective study (2015–2019). J. Clin. Lab. Anal. 2021, 35, e23850.
- Rasul, T.F.; Gamret, A.C.; Morgan, O.; Bergholz, D.R.; Eachus, E.; Mathew, M.; Faiz, A.; Elkhadem, A.; Dahl, V.; Motoa, G.; et al. Cutaneous Fungal Infections in Patients Experiencing Homelessness and Treatment in Low-Resource Settings: A Scoping Review. Cureus 2022, 14, e30840.
- Gonzalez Santiago, T.M.; Pritt, B.; Gibson, L.E.; Comfere, N.I. Diagnosis of deep cutaneous fungal infections: Correlation between skin tissue culture and histopathology. J. Am. Acad. Dermatol. 2014, 71, 293–301.
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J. Fungi 2016, 2, 4.
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737.
- Zhang, H.; Zhu, A. Emerging Invasive Fungal Infections: Clinical Features and Controversies in Diagnosis and Treatment Processes. Infect. Drug Resist. 2020, 13, 607–615.
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877.
- Webb, B.J.; Ferraro, J.P.; Rea, S.; Kaufusi, S.; Goodman, B.E.; Spalding, J. Epidemiology and Clinical Features of Invasive Fungal Infection in a US Health Care Network. Open Forum Infect. Dis. 2018, 5, ofy187.
- Jenks, J.D.; Gangneux, J.-P.; Schwartz, I.S.; Alastruey-Izquierdo, A.; Lagrou, K.; Thompson III, G.R.; Lass-Flörl, C.; Hoenigl, M.; European Confederation of Medical Mycology (ECMM) Council Investigators. Diagnosis of Breakthrough Fungal Infections in the Clinical Mycology Laboratory: An ECMM Consensus Statement. J. Fungi 2020, 6, 216.
- Eldridge, M.L.; Chambers, C.J.; Sharon, V.R.; Thompson, G.R. Fungal infections of the skin and nail: New treatment options. Expert Rev. Anti-Infect. Ther. 2014, 12, 1389–1405.
- Kotey, F.C.; Dayie, N.T.; Tetteh-Uarcoo, P.B.; Donkor, E.S. Candida Bloodstream Infections: Changes in Epidemiology and Increase in Drug Resistance. Infect. Dis. Res. Treat. 2021, 14, 11786337211026927.
- Xiao, G.; Liao, W.; Zhang, Y.; Luo, X.; Zhang, C.; Li, G.; Yang, Y.; Xu, Y. Analysis of fungal bloodstream infection in intensive care units in the Meizhou region of China: Species distribution and resistance and the risk factors for patient mortality. BMC Infect. Dis. 2020, 20, 599.
- Agnelli, C.; Valerio, M.; Bouza, E.; Guinea, J.; Sukiennik, T.; Guimaraes, T.; Queiroz-Telles, F.; Munoz, P.; Colombo, A.L. Prognostic factors of Candida spp. bloodstream infection in adults: A nine-year retrospective cohort study across tertiary hospitals in Brazil and Spain. Lancet Reg. Health—Am. 2022, 6, 100117.
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Joseph, L.; Alfouzan, W.; Asadzadeh, M. Increasing prevalence, molecular characterization and antifungal drug susceptibility of serial Candida auris isolates in Kuwait. PLoS ONE 2018, 13 (Suppl. 4), e0195743.
- McDonnell, G.; Hansen, J. Block’s Disinfection, Sterilization, and Preservation, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020.
- Gold, K.M.; Hitchins, V.M. Cleaning Assessment of Disinfectant Cleaning Wipes on an External Surface of a Medical Device Contaminated with Artificial Blood or Streptococcus pneumonia. Am. J. Infect. Control 2013, 41, 901–907.
- Barrett, T.M.; Tsui, C.K.M. Emerging fungal pathogen: Candida auris. Evol. Med. Public Health 2021, 9, 246–247.
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Antisepsis: An Overview. Am. J. Infect. Control 2019, 47, A3–A9.
- McDonnell, G.; OSMA Anti-Infective Working Group. Initiating Implant Infection Innovation. Orthopedic Design & Technology. September/October 2022. Available online: https://www.odtmag.com/issues/2023-09-01/view_features/initiating-implant-infection-innovation (accessed on 20 December 2022).
- Food and Drug Administration. FDA-Cleared Sterilants and High Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices. Available online: https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices-information-manufacturers/fda-cleared-sterilants-and-high-level-disinfectants-general-claims-processing-reusable-medical-and (accessed on 20 December 2022).
- Anderson, J.; Rowan, N.; MacGregor, S.; Fouracre, R.; Farish, O. Inactivation of food-borne enteropathogenic bacteria and spoilage fungi using pulsed-light. IEEE Trans. Plasma Sci. 2000, 28, 83–88.
- Rowan, N.J. Evidence that inimical food preservation barriers alter microbial resistance, cell morphology and virulence. Trends Food Sci. Technol. 1999, 10, 261–270.
- Hayes, J.; Kirf, D.; Garvey, M.; Rowan, N. Disinfection and toxicological assessments of pulsed UV and pulsed-plasma gas-discharge treated-water containing the waterborne protozoan enteroparasite Cryptosporidium parvum. J. Microbiol. Methods 2013, 94, 325–337.
- Garvey, M.; Farrell, H.; Cormican, M.; Rowan, N. Investigations of the relationship between use of in vitro cell culture-quantitative PCR and a mouse-based bioassay for evaluating critical factors affecting the disinfection performance of pulsed UV light for treating Cryptosporidium parvum oocysts in saline. J. Microbiol. Methods 2010, 80, 267–273.
- Garvey, M.; Stocca, A.; Rowan, N. Development of a combined in vitro cell culture--quantitative PCR assay for evaluating the disinfection performance of pulsed light for treating the waterborne enteroparasite Giardia lamblia. Exp. Parasitol. 2014, 144, 6–13.
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267.
- Masterson, K.; Meade, E.; Garvey, M.; Lynch, M.; Major, I.; Rowan, N.J. Development of a low-temperature extrusion process for production of GRAS bioactive-polymer loaded compounds for targeting antimicrobial-resistant (AMR) bacteria. Sci. Total. Environ. 2021, 800.
- Farrell, H.; Hayes, J.; Laffey, J.; Rowan, N. Studies on the relationship between pulsed UV light irradiation and the simultaneous occurrence of molecular and cellular damage in clinically-relevant Candida albicans. J. Microbiol. Methods 2013, 84, 317–326.
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038.
- Ramstedt, M.; Ribeiro, I.A.C.; Bujdakova, H.; Mergulhão, F.J.M.; Jordao, L.; Thomsen, P.; Alm, M.; Burmølle, M.; Vladkova, T.; Can, F.; et al. Evaluating Efficacy of Antimicrobial and Antifouling Materials for Urinary Tract Medical Devices: Challenges and Recommendations. Macromol. Biosci. 2019, 19, e1800384.
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7.
- de Rocha, M.E.B.; da Chagas Oliveira Freire, F.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165.
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617.
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146.
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619.
- Weltmann, K.-D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031.




