Antifungal resistance, antifungal drug tolerance, and biofilm formation directly contribute to rising cases of fungal morbidity and mortality. As with all of the infectious diseases, prevention is the optimal way to mitigate disease outbreak and transmission. The application of effective disinfection and sterilization regimes, particularly in hospital settings, is vitally important, where a focus on fungal biofilm formation on indwelling medical devices is important. Preventing the growth of mycotoxin-producing fungi on foods through the performance of appropriate end-to-end processes is advisable, as mycotoxins are recalcitrant and challenging to eliminate once they have been formed. Adopting the OneHealth approach will support and enable solutions to address this complex societal challenge.
- fungi
- antifungal drug resistance
- mycotoxins
1. Introduction
Cutaneous Infection
Cutaneous mycoses are superficial fungal infections of the skin, hair, or nails (onychomycosis), which are the most important causative agents of disease, including dermatophytes species (Microsporum, Trichophyton, and Epidermophyton), Malassezia furfur, and Candida (albicans and non-albicans Candida). The prevalence rate of cutaneous or superficial fungal infections (SFI) is approximately 20–25% globally [36][9]. Immunocompromised persons are high risk of cutaneous infections, where homeless persons are an often-overlooked high risk group due to malnutrition, lack of healthcare, injury, and co-morbidities [37][10]. Homeless patients are also high risk for cutaneous fungal infection related complications including cellulitis and osteomyelitis [37][10]. Importantly, deep cutaneous fungal infections (DCFIs) have high rates of morbidity and mortality, particularly amongst immunocompromised patients, with mortality rates of 4% to 10% in localized infections and ca. 83% to 94% in disseminated cases [38][11]. Clinically, the diagnosis of cutaneous etiological agents involves both mycological and histological findings [39][12]. Aspergillus, Cryptococcus, and Candida spp. are the main fungal species associated with invasive fungal infections of the lungs, brain, and bloodstream, respectively [12][6]. Disseminated infections are typically caused by Blastomyces, Coccidioides, Paracoccidioides, Histoplasma, and Cryptococcus spp. [44][13]. The pulmonary system (lungs) are the most common site of IFIs [45][14]. Triazole-resistant A. fumigatus and MDR yeast including Candida glabrata and Candida auris are of particular concern [46][15]. IFIs are separated from superficial mycoses due to the involvement of blood and other sterile body tissues or organs, and they are categorized as serious, deep, deep-seated, disseminated, and systemic fungal infections [47][16]. To cause an IFI in a patient, the fungi must have the ability to grow at or above 37 °C to reach internal tissues, the ability to lyse tissues and absorb their components, and they must be able to evade the host’s immune system [7][8]. Clinically invasive fungal diseases affect many organs and deep tissues, causing endocarditis, meningitis, and respiratory infections, and they are not often detected in blood cultures [46,48][15][17]. Furthermore, the insertion of venous catheters and intravascular devices and medical interventions allow for infections with nosocomial IFDs [7][8]. Cryptococcal meningitis caused by Cryptococcus neoformans or Cryptococcus gattii is common in HIV patients, where both of the species possess an innate resistance to fluconazole, where a combination therapy with flucytosine is implemented to improve the fungal clearance [12][6]. Additionally, ca. 7% of systemic Candida infections display reduced azole susceptibility [44][13]. For invasive aspergillosis, voriconazole is typically administered, and amphotericin B (AMPB) and the echinocandins also show anti-aspergillus activity, whereas the Aspergillus species possess a resistance to fluconazole [41][18]. The effective treatment of IFIs is also impacted by the lack of an accurate diagnosis. The diagnosis of IFIs is challenging, as the tests are slow, with limited sensitivity and specificity, and they are typically quite expensive [46][15]. The number of bloodstream infections (BSIs) with fungal etiological agents has increased in recent years. Candida species are responsible for 90% of the fungal BSIs, and they result in late-onset sepsis etiologies amongst neonates [50][19]. Candida BSIs have a mortality rate of 30–40%, regardless of the therapeutic treatments [51][20]. Interestingly, studies describe higher mortality rates among countries and regions, where Latin American has a rate of ca. 60% compared to 20% in Spain [52][21]. Additionally, 80% of Candida BSIs occur in immunocompetent patients with nosocomial co-morbidities [7][8]. Studies have demonstrated the risk factors including diabetes, neoplasm, neutropenia, renal insufficiency, immunosuppression, cardiovascular disease, surgery, and age for fungal BSIs [51][20]. The incidence rates of BSIs with fungal pathogens are ca. 4.1% and ca. 0.69% in ICU patients in developing and developed countries, respectively [53][22], and this is directly related to the use of antifungal drugs, immunosuppression, steroids, placement of central venous catheters, and the low immunity of patients [51][20].2. Efficacy of Disinfection Strategies for Addressing Fungal Pathogens
2.1. Reusable Medical Devices and Surgical Instruments
Despite advances in medicine and innovations in many underpinning fields including disease prevention and control, the Spaulding classification system, which was originally proposed in 1957, remains widely used for defining the disinfection and sterilization of contaminated re-usable medical devices and surgical instruments [58][23]. Medical devices are a common source of hospital acquired infections (HAIs), and they have accounted for between 60% and 80% of all bloodstream-, urinary tract-, and pneumonia-related HAIs [59][24]. However, there is a marked lack of published information on the relevance of priority WHO fungal pathogens and the contamination of reusable medical devices in terms of transmission post-processing and sterilization. Notably, fungal infections cause over 1.5 million deaths per year, and a quarter million of these deaths are caused by the genus Candida [62][25]. The mortality rate of invasive candidiasis (infections by Candida) can be greater than 40% due to there being limited treatment options and increased antifungal resistance [4,62][4][25]. To mitigate the risk of HAIs, the current methods for the safe processing of medical devices still rely upon the guiding classification system of Dr E. H. Spaulding, which was originally conceived and published over 50 years ago [61][26]. Spaulding’s underpinning hypothesis was that healthcare facilities should apply appropriate disinfection and sterilization methods to process medical devices and surgical instruments based on the degree of the patients’ risk of acquiring an infection due to their use. Spaulding’s system divides all of the medical devices into three discrete categories based on the severity of the perceived risk to the patients of acquiring an infection from their use. Critical use items: Where a device enters the sterile tissue and must be sterile, which is defined as being free from viable microorganisms [58][23]. Items contaminated with any microorganism (including fungal species) are referred to as high risk to the patients if they are contaminated and enter the sterile tissue or vascular system, and they have a high potential for causing disease transmission [61][26]. Such items should be sterile, such as by using steam sterilization where it is possible. The examples include surgical instruments. Given that many items contain heat-sensitive materials, other appropriate sterilization modalities should be applied, including vaporized hydrogen peroxide (VH2O2), VH2O2 gas plasma, and ethylene oxide gas [63][27]. The use of liquid chemical sterilants may also be considered appropriate, such as formulations based on glutaraldehyde (GTA), peracetic acid (PA), hydrogen peroxide (HP), or ortho-phthalaldehyde (OPA) [61,63][26][27]. Semi-critical use items: When a device only comes into contact with the intact mucus membranes or nonintact skin, it should also be subjected to sterilization, or if this is not feasible due to its sensitive material composition or complex design features, then a high-level disinfection (HLD) process must be deployed at a minimum level that would be expected to kill all of the microorganisms, except for the bacterial endospores [63][27]. The examples of semi-critical items including “respiratory therapy, anaesthesia equipment, some endoscopes, laryngoscope blades and handles, esophageal manometry probes, endocavitary probes, nasopharyngoscopes, prostate biopsy probes, infrared coagulation devices, anorectal manometry catheters, cystoscopies, and diaphragm fitting rings” [61][26]. Depending on the regional claim requirements, disinfectants should demonstrate a broad spectrum of antimicrobial activity, and typically, the ability to eliminate at least 106 (or 6-logs) of the mycobacterial cells on the contaminated surfaces of the medical devices. For the fungi of concern, mycobacteria are typically deemed to exhibit greater resistance to high-quality disinfectants, thus, mycobacterial cells are recognized as being representative (or bio-indicators) of the HLD process efficacy. The examples of chemical disinfectants authorized in the USA for HLD use include biocides such as GTA HP, OPA, hypochlorite, and PA with HP [64][28]. Non-critical use items: Which includes devices that are in contact with intact skin (but not mucous membranes), requiring low-level-to-intermediate-level disinfection [64][28]. The skin contains intact integumentary layers, and as such, it provides a natural barrier to the microorganisms. There remains a risk to the skin as a result of cross-contamination from the devices, but this risk is considered to be low. These risks can be practically reduced by the combination of physical removal and disinfection [63][27]. The examples of non-critical use items include blood pressure cuffs, bed surfaces and rails, patient furniture, and so forth [61][26]. Figure 1 illustrates the microbial resistance profile to applied disinfection and sterilization modalities. It should be noted that the overall pattern of resistance to applied lethal technologies may vary depending on the modality. Microorganisms with higher resistance are widely used to challenge and test the effectiveness of disinfection and sterilization methods. Mycobacterial cells and Bacillus endospores have been used as indicators of HLD and sterilization, respectively [61][26]. Fungi exhibit greater biocidal resistance to enveloped viruses (such as HIV and SARS-COV-2) and to Gram-positive and -negative vegetative bacterial cells. Fungi present in vegetative- and spore-forming morphologies can be further differentiated based upon these morphologies with increasing exposure to these applied lethal stresses. For example, Aspergillus spores are more tolerant to higher doses of UV-irradiation due to the protective peak absorption of pigments at a similar UV-C wavelength to that of DNA (ca. from 250 to 260 nm) [65,66][29][30]. However, fungi are considered to be more susceptible to high-level disinfection (HLD) compared to similarly treated non-enveloped viruses (such as norovirus), mycobacterial cells, and parasitic oocysts (Cryptosporidium species), or cysts (Giardia species) [67,68,69][31][32][33].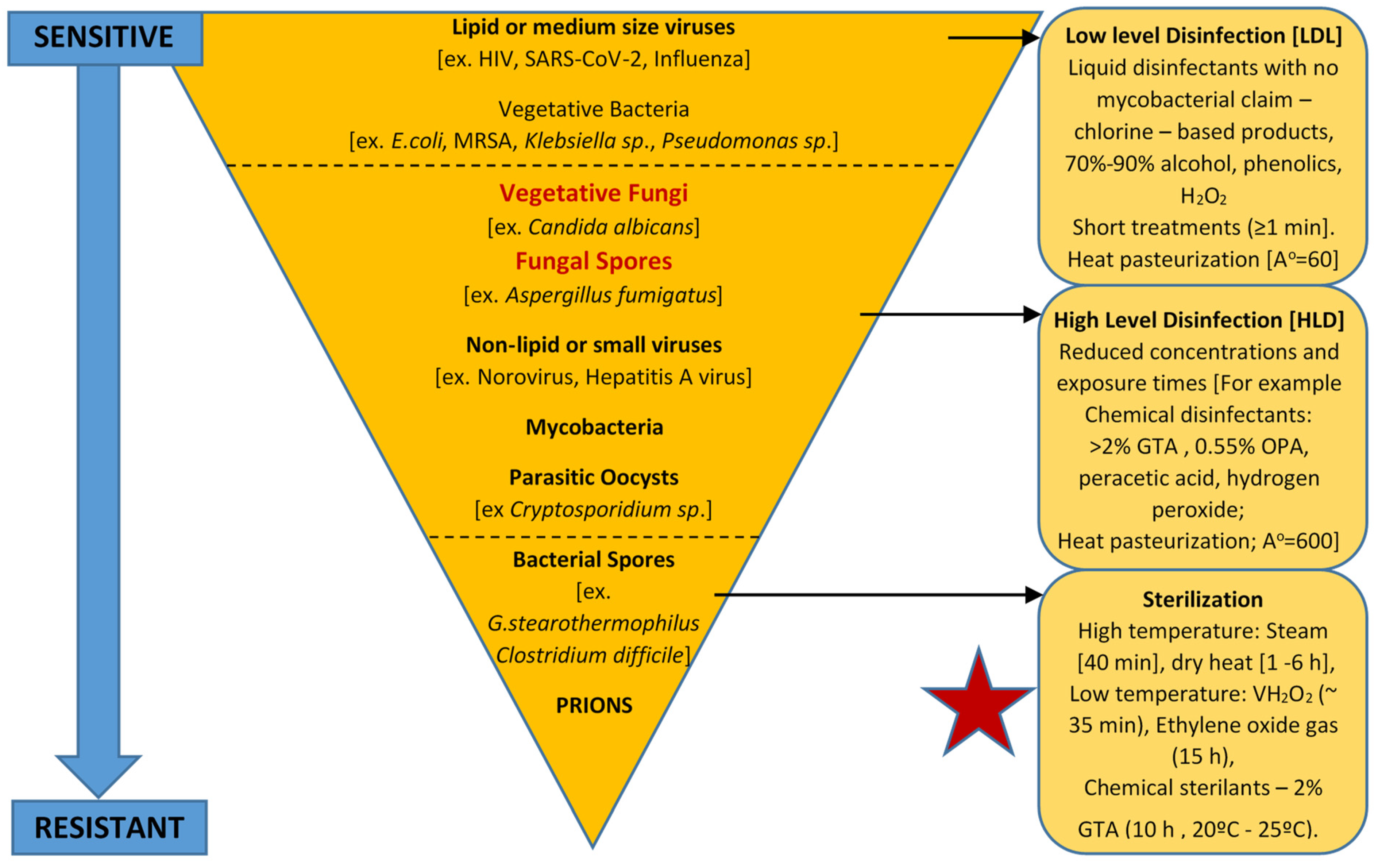
2.2. Central Venous and Urinary Catheters
Modern technology has allowed us to use a wider and newer variety of medical devices. The combination of an increasingly aging population and a consistently growing number of inserted devices is likely to escalate the occurrence of infectious complications related to medical devices [86][34]. The number of indwelling medical devices is increasing, and an increasing proportion of device-related infections are being caused by Candida spp. Candida spp. produce biofilms on synthetic materials, which facilitates the adhesion of the organisms to the devices and renders them relatively refractory to medical therapy. The management of device-related Candida infections can be challenging. The removal of the infected device is generally needed to cure the Candida infections caused by the medical devices. However, since the pathogenesis of Candida bloodstream infection is complicated, more studies are necessary to determine the role of catheter exchange in patients with both gastrointestinal tract mucositis and indwelling catheters. Kojaic and coworkers [86][34] noted that C. albicans biofilm formation has three developmental phases: the adherence of yeast cells to the device’s surface (early phase), the formation of a matrix with dimorphic switching from yeast to hyphal forms (intermediate phase), and the increase in the amount of the matrix material, taking on a three-dimensional architecture (maturation phase). Fully mature Candida biofilms have a mixture of morphological forms, and they consist of a dense network of yeasts, hyphae, and pseudohyphae in a matrix of polysaccharides, carbohydrate, protein, and unknown components. The organisms in biofilms behave differently from freely suspended cells with respect to antimicrobial resistance. Both the bacteria and Candida cells within biofilms are markedly resistant to antimicrobial agents [86][34].
C. auris has become a global threat as it can colonize the skin, medical devices, and hospital environments, causing nosocomial outbreaks of blood and urinary tract infections worldwide [62][25]. Candida auris can spread among patients in hospitals, and it is intrinsically resistant to one or more classes of antifungals, which makes it particularly difficult to treat in healthcare settings. Comparative genomics has demonstrated that C. auris has expanded families of transporters and lipases, as well as mutations and copy number variants, in genes/enzymes linked to increased resistance and virulence [2]. Understanding the evolution of emerging fungal pathogens such as C. auris will be useful for the design of antifungal drugs and therapies for susceptible patients, potentially improving the clinical outcomes.
43. Knowledge Gap in Molecular and Cellular Mechanisms Underpinning Disinfection of Fungal Pathogens including AMR Post-Treatment Modalities
Advanced studies on cell survival following antimicrobial processes also are of interest [89][35]. As an example, Farrell et al. [90][36] highlighted the potential of addressing a single composite study to address the relationship between the use of pulsed UV light irradiation and the simultaneous occurrence of molecular and cellular damage in clinical strains of C. albicans. This is particularly relevant, and it showed that the occurrence of late apoptotic and necrotic cell phonotypes can be detected in real-time using specific representative biomarkers. This coincides with the occurrence of irreversible fungal cell death, which may potentially supplement or replace the lengthy standard culture-based methods where there was good agreement between these indirect biomarker and direct culture-base enumeration approaches. Notably, this constituted the first study to investigate the mechanisms of cell destruction caused by pulsed UV using a sequential and simultaneous microbial protein leakage assay and the lipid hydroperoxidation in the cell membranes, specific patterns of reactive oxygen species generation, and nuclear damage of treated microbial cells using a Comet assay, along with the detection of specific apoptotic and necrotic stages. Design, testing, and validating the real-time markers to demonstrate irreversible fungal death will prove the effectiveness of the disinfection modalities.54. Need for Improved In Vitro and In Vivo Compatibility Tests for Medical Devices Encompassing Antifungal and Disinfection Efficacy
Researchers have noted that the limitations of in vitro and animal models of chronic device-related infections are important in the context of advancing the med-tech sector, with implications for pressing research and clinical practice [74][37]. Ramstedt et al. [75][38] evaluated the efficacy of antimicrobial and antifouling materials for a urinary tract medical device that also enabled the transmission of fungal infections. These authors addressed the challenges of antimicrobial material testing, including surface characterization, biocompatibility, cytotoxicity, in vitro and in vivo tests, microbial strain selection, and hydrodynamic conditions, from the perspective of complying to the complex pathology of device-associated urinary tract infections. Standard assays should be developed that enable us to make comparisons between the inter-laboratory generated results of industries and academia to perform harmonized assessments of the antimicrobial properties of urinary tract devices in a reliable way that includes improving in vitro and in vivo biocompatibility testing. Moreover, the high risk of infection and its associated costs clearly underlines the need to provide patients with devices with the lowest possible risk of infection, and it emphasizes the need for innovative products that reduce the incidence rate of device-associated UTIs. Although standards are available for guiding the development of new devices with respect to biocompatibility (ISO 10993) and material characterization, no such guidance exists for the development of antimicrobial devices [75][38].65. Mycotoxins and Appropriate Decontamination Strategies
Mycotoxins are secondary metabolites of mold and fungi; they are generally toxic to living organisms. This term, by general consensus, is used almost exclusively for fungi associated with food products and animal feed, excluding the toxins produced by mushrooms. Mycotoxins are secondary metabolites with no apparent function in the normal metabolism of fungi [91][39]. They are produced mainly, although not exclusively, when the fungus reaches maturity [91][39]. They are molecules with structures which vary from simple heterocyclic rings with molecular weights of up to 50 Da to groups with 6–8 irregularly arranged heterocylic rings with a total molecular weight of >500 Da, and they do not show immunogenicity. Studies have revealed the existence of at least around 400 different mycotoxins [92][40]. Hundreds of mycotoxins have been identified thus far, with some of them, such as aflatoxins, ochratoxins, trichothecenes, zearalenone, fumonisins, and patulin, being considered agro-economically important [91][39].
The consumption of mycotoxins-contaminated feed causes a plethora of harmful responses from acute toxicity to many persistent health disorders with lethal outcomes, such as mycotoxicosis when it is ingested by animals. Therefore, the main task for feed producers is to minimize the concentration of mycotoxins by applying different strategies that are aimed at minimizing the risk of the mycotoxin effects on animal and human health. However, once the mycotoxins enter the production chain, it is hard to eliminate or inactivate them [93][41]. Notably, mycotoxin-producing fungi are readily destroyed by moderate levels of disinfection. However, given the recalcitrant nature of mycotoxins, emphasis should be placed on ensuring appropriate end-to-end food production and management to prevent the growth of mycotoxin-producing organisms, such as cleaning the grains and removing the kernels that harbor molds. The use of feed additives or supplements that decrease the risk of animal exposure to mycotoxins can be viewed as a means of enhancing animal welfare. These feed supplements are referred to as the substances blended into feed (e.g., mineral clay, microorganism, and yeast cell wall), adsorbing or detoxifying the mycotoxins in the digestive tract of animals (biological detoxification) [93][41]. In general, mycotoxins are mainly stable compounds under thermal processing conditions used in feed and food [101][42]. However, the different thermal food and feed treatments that can have different impacts on the mycotoxins include extrusion, cooking, frying, baking, canning, crumbling, pelleting, roasting, flaking, and alkaline cooking. Among the thermal treatments, the utilization of high-temperature processes demonstrates the greatest potential for mycotoxin reduction [93][41]. Kabak [102][43] noted that the application of extrusion at a temperature that is higher than 150 °C has a significant impact on the reduction of zearalenone and fumonisins, while the same conditions led to the moderate reduction of aflatoxins and deoxynivalenol. Oxidizing agents such as ozone and hydrogen peroxide have been used to decontaminate mycotoxin-contaminated raw feed and compound feed [103][44]. The application of microorganisms or enzyme systems to contaminated feeds can detoxify the mycotoxins by metabolism or degradation in their gastrointestinal tract. This process is an irreversible and environmentally friendly method of detoxification, as it does not leave toxic residues or unwanted by-products [93][41]. However, the levels of particular mycotoxins in feeds have been reduced, but so far, no single technique has been established that is equally efficient against the broad variety of mycotoxins that can co-occur in various commodities [93][41]. Furthermore, the procedures of detoxication that appear to be efficient in vitro will not necessarily maintain their effectiveness in an in vivo test.
References
- Huang, J.; Liu, C.; Zheng, X. Clinical features of invasive fungal disease in children with no underlying disease. Sci. Rep. 2022, 12, 208.
- Blackwell, M. The Fungi: 1, 2, 3 … 5.1 million species? Am. J. Bot. 2011, 98, 426–438.
- Petrasch, S.; Knapp, S.J.; van Kan, J.; Blanco-Ulate, B. Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol. 2019, 20, 877–892.
- Garvey, M.; Meade, E.; Rowan, N.J. Effectiveness of front line and emerging fungal disease prevention and control interventions and opportunities to address appropriate eco-sustainable solutions. Sci. Total Environ. 2022, 851, 158284.
- Denning, D.W.; Bromley, M.J. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416.
- Gow, N.A.R.; Johnson, C.; Berman, J.; Coste, A.T.; Cuomo, C.A.; Perlin, D.S.; Bicanic, T.; Harrison, T.S.; Wiederhold, N.; Bromley, M.; et al. The importance of antimicrobial resistance in medical mycology. Nat. Commun. 2022, 13, 5352.
- Muderris, T.; Kaya, S.; Ormen, B.; Aksoy Gokmen, A.; Varer Akpinar, C.; Yertsever Gul, S. Mortality and risk factor analysis for Candida blood stream infection: A three year retrospective study. J. Mycol. Méd. 2020, 30, 101008.
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Mem. Inst. Oswaldo Cruz 2020, 115, e200430.
- Khodadadi, H.; Zomorodian, K.; Nouraei, H.; Zareshahrabadi, Z.; Barzegar, S.; Zare, M.R.; Pakshir, K. Prevalence of superficial-cutaneous fungal infections in Shiraz, Iran: A five-year retrospective study (2015–2019). J. Clin. Lab. Anal. 2021, 35, e23850.
- Rasul, T.F.; Gamret, A.C.; Morgan, O.; Bergholz, D.R.; Eachus, E.; Mathew, M.; Faiz, A.; Elkhadem, A.; Dahl, V.; Motoa, G.; et al. Cutaneous Fungal Infections in Patients Experiencing Homelessness and Treatment in Low-Resource Settings: A Scoping Review. Cureus 2022, 14, e30840.
- Gonzalez Santiago, T.M.; Pritt, B.; Gibson, L.E.; Comfere, N.I. Diagnosis of deep cutaneous fungal infections: Correlation between skin tissue culture and histopathology. J. Am. Acad. Dermatol. 2014, 71, 293–301.
- Rouzaud, C.; Hay, R.; Chosidow, O.; Dupin, N.; Puel, A.; Lortholary, O.; Lanternier, F. Severe Dermatophytosis and Acquired or Innate Immunodeficiency: A Review. J. Fungi 2016, 2, 4.
- Hossain, C.M.; Ryan, L.K.; Gera, M.; Choudhuri, S.; Lyle, N.; Ali, K.A.; Diamond, G. Antifungals and Drug Resistance. Encyclopedia 2022, 2, 1722–1737.
- Zhang, H.; Zhu, A. Emerging Invasive Fungal Infections: Clinical Features and Controversies in Diagnosis and Treatment Processes. Infect. Drug Resist. 2020, 13, 607–615.
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877.
- Webb, B.J.; Ferraro, J.P.; Rea, S.; Kaufusi, S.; Goodman, B.E.; Spalding, J. Epidemiology and Clinical Features of Invasive Fungal Infection in a US Health Care Network. Open Forum Infect. Dis. 2018, 5, ofy187.
- Jenks, J.D.; Gangneux, J.-P.; Schwartz, I.S.; Alastruey-Izquierdo, A.; Lagrou, K.; Thompson III, G.R.; Lass-Flörl, C.; Hoenigl, M.; European Confederation of Medical Mycology (ECMM) Council Investigators. Diagnosis of Breakthrough Fungal Infections in the Clinical Mycology Laboratory: An ECMM Consensus Statement. J. Fungi 2020, 6, 216.
- Eldridge, M.L.; Chambers, C.J.; Sharon, V.R.; Thompson, G.R. Fungal infections of the skin and nail: New treatment options. Expert Rev. Anti-Infect. Ther. 2014, 12, 1389–1405.
- Kotey, F.C.; Dayie, N.T.; Tetteh-Uarcoo, P.B.; Donkor, E.S. Candida Bloodstream Infections: Changes in Epidemiology and Increase in Drug Resistance. Infect. Dis. Res. Treat. 2021, 14, 11786337211026927.
- Xiao, G.; Liao, W.; Zhang, Y.; Luo, X.; Zhang, C.; Li, G.; Yang, Y.; Xu, Y. Analysis of fungal bloodstream infection in intensive care units in the Meizhou region of China: Species distribution and resistance and the risk factors for patient mortality. BMC Infect. Dis. 2020, 20, 599.
- Agnelli, C.; Valerio, M.; Bouza, E.; Guinea, J.; Sukiennik, T.; Guimaraes, T.; Queiroz-Telles, F.; Munoz, P.; Colombo, A.L. Prognostic factors of Candida spp. bloodstream infection in adults: A nine-year retrospective cohort study across tertiary hospitals in Brazil and Spain. Lancet Reg. Health—Am. 2022, 6, 100117.
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Joseph, L.; Alfouzan, W.; Asadzadeh, M. Increasing prevalence, molecular characterization and antifungal drug susceptibility of serial Candida auris isolates in Kuwait. PLoS ONE 2018, 13 (Suppl. 4), e0195743.
- McDonnell, G.; Hansen, J. Block’s Disinfection, Sterilization, and Preservation, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2020.
- Gold, K.M.; Hitchins, V.M. Cleaning Assessment of Disinfectant Cleaning Wipes on an External Surface of a Medical Device Contaminated with Artificial Blood or Streptococcus pneumonia. Am. J. Infect. Control 2013, 41, 901–907.
- Barrett, T.M.; Tsui, C.K.M. Emerging fungal pathogen: Candida auris. Evol. Med. Public Health 2021, 9, 246–247.
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Antisepsis: An Overview. Am. J. Infect. Control 2019, 47, A3–A9.
- McDonnell, G.; OSMA Anti-Infective Working Group. Initiating Implant Infection Innovation. Orthopedic Design & Technology. September/October 2022. Available online: https://www.odtmag.com/issues/2023-09-01/view_features/initiating-implant-infection-innovation (accessed on 20 December 2022).
- Food and Drug Administration. FDA-Cleared Sterilants and High Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices. Available online: https://www.fda.gov/medical-devices/reprocessing-reusable-medical-devices-information-manufacturers/fda-cleared-sterilants-and-high-level-disinfectants-general-claims-processing-reusable-medical-and (accessed on 20 December 2022).
- Anderson, J.; Rowan, N.; MacGregor, S.; Fouracre, R.; Farish, O. Inactivation of food-borne enteropathogenic bacteria and spoilage fungi using pulsed-light. IEEE Trans. Plasma Sci. 2000, 28, 83–88.
- Rowan, N.J. Evidence that inimical food preservation barriers alter microbial resistance, cell morphology and virulence. Trends Food Sci. Technol. 1999, 10, 261–270.
- Hayes, J.; Kirf, D.; Garvey, M.; Rowan, N. Disinfection and toxicological assessments of pulsed UV and pulsed-plasma gas-discharge treated-water containing the waterborne protozoan enteroparasite Cryptosporidium parvum. J. Microbiol. Methods 2013, 94, 325–337.
- Garvey, M.; Farrell, H.; Cormican, M.; Rowan, N. Investigations of the relationship between use of in vitro cell culture-quantitative PCR and a mouse-based bioassay for evaluating critical factors affecting the disinfection performance of pulsed UV light for treating Cryptosporidium parvum oocysts in saline. J. Microbiol. Methods 2010, 80, 267–273.
- Garvey, M.; Stocca, A.; Rowan, N. Development of a combined in vitro cell culture--quantitative PCR assay for evaluating the disinfection performance of pulsed light for treating the waterborne enteroparasite Giardia lamblia. Exp. Parasitol. 2014, 144, 6–13.
- Kojic, E.M.; Darouiche, R.O. Candida infections of medical devices. Clin. Microbiol. Rev. 2004, 17, 255–267.
- Masterson, K.; Meade, E.; Garvey, M.; Lynch, M.; Major, I.; Rowan, N.J. Development of a low-temperature extrusion process for production of GRAS bioactive-polymer loaded compounds for targeting antimicrobial-resistant (AMR) bacteria. Sci. Total. Environ. 2021, 800.
- Farrell, H.; Hayes, J.; Laffey, J.; Rowan, N. Studies on the relationship between pulsed UV light irradiation and the simultaneous occurrence of molecular and cellular damage in clinically-relevant Candida albicans. J. Microbiol. Methods 2013, 84, 317–326.
- Stewart, P.S.; Bjarnsholt, T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect. 2020, 26, 1034–1038.
- Ramstedt, M.; Ribeiro, I.A.C.; Bujdakova, H.; Mergulhão, F.J.M.; Jordao, L.; Thomsen, P.; Alm, M.; Burmølle, M.; Vladkova, T.; Can, F.; et al. Evaluating Efficacy of Antimicrobial and Antifouling Materials for Urinary Tract Medical Devices: Challenges and Recommendations. Macromol. Biosci. 2019, 19, e1800384.
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of Mycotoxins and Their Consequences on Human Health. Toxicol. Res. 2019, 35, 1–7.
- de Rocha, M.E.B.; da Chagas Oliveira Freire, F.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165.
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617.
- Bullerman, L.B.; Bianchini, A. Stability of mycotoxins during food processing. Int. J. Food Microbiol. 2007, 119, 140–146.
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619.
- Weltmann, K.-D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031.
