
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ryuji Hiramatsu | -- | 3617 | 2023-01-30 09:02:38 | | | |
| 2 | Lindsay Dong | Meta information modification | 3617 | 2023-01-31 09:23:03 | | |
Video Upload Options
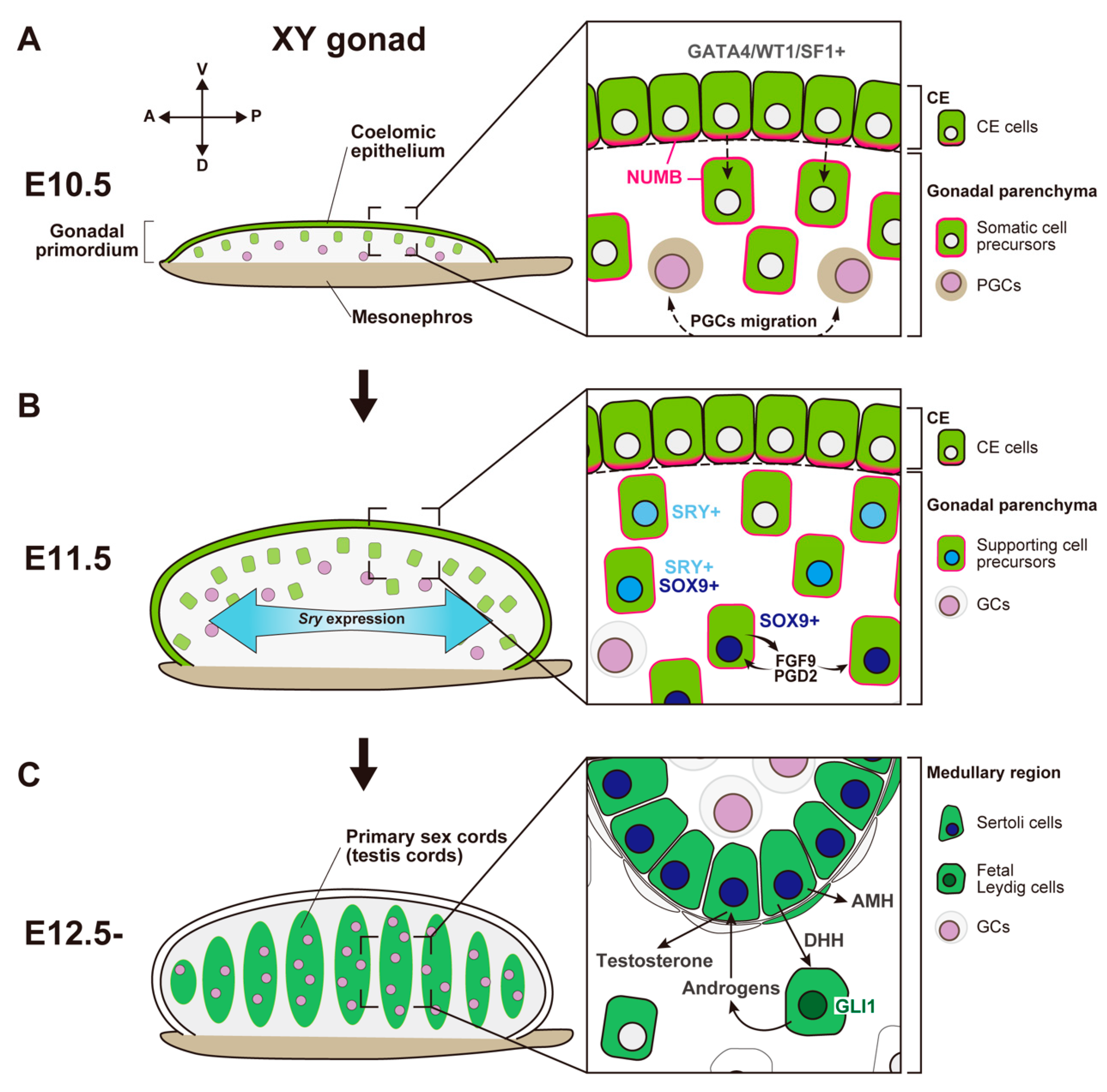
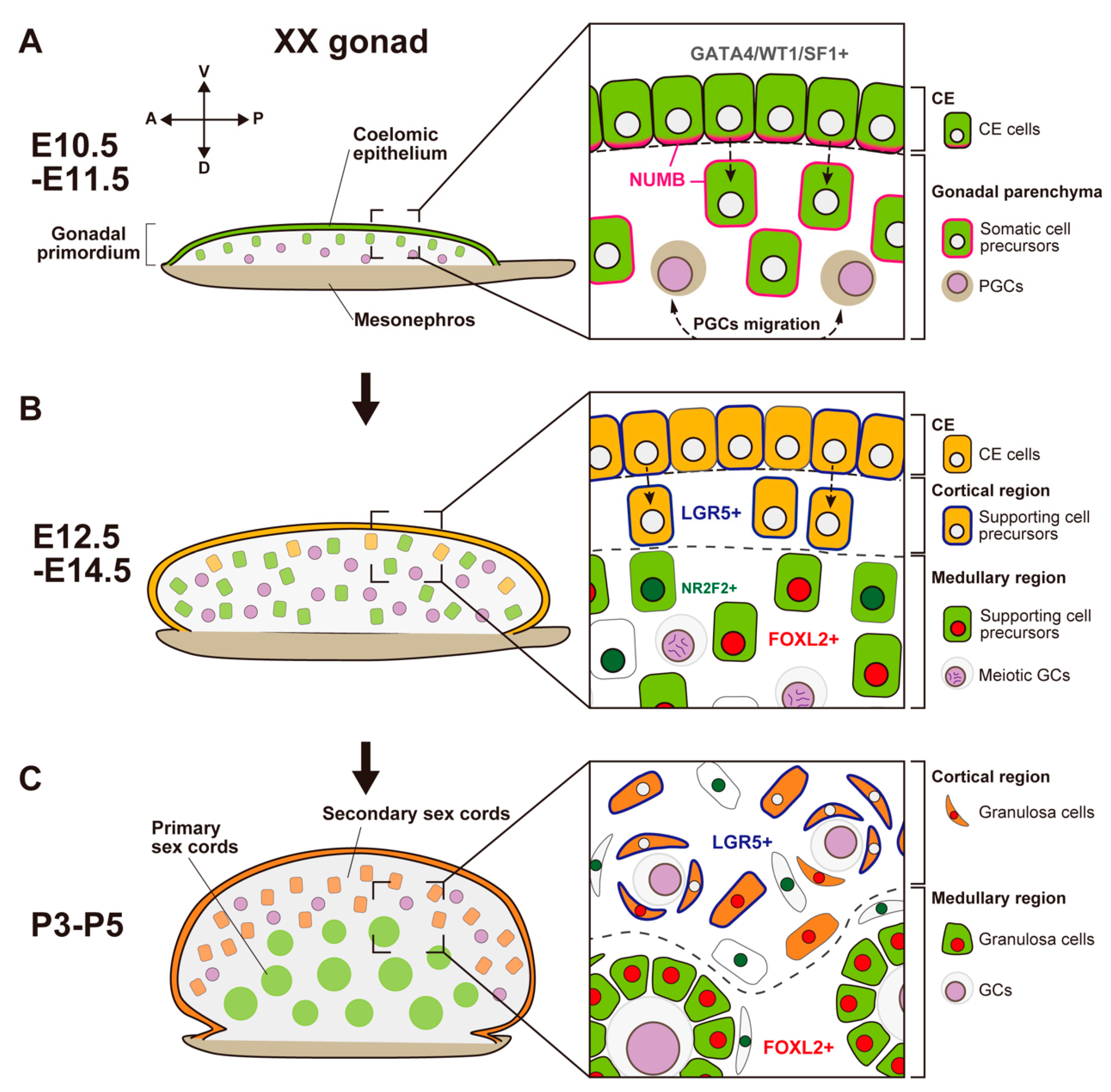
In most mammals, the sex of the gonads is based on the fate of the supporting cell lineages, which arises from the proliferation of coelomic epithelium (CE) that surfaces on the bipotential genital ridge in both XY and XX embryos. Genetic studies and single-cell transcriptome analyses in mice have revealed the cellular and molecular events in the two-wave proliferation of the CE that produce the supporting cells. This proliferation contributes to the formation of the primary sex cords in the medullary region of both the testis and the ovary at the early phase of gonadal sex differentiation, as well as to that of the secondary sex cords in the cortical region of the ovary at the perinatal stage. To support gametogenesis, the testis forms seminiferous tubules in the medullary region, whereas the ovary forms follicles mainly in the cortical region. The medullary region in the ovary exhibits morphological and functional diversity among mammalian species that ranges from ovary-like to testis-like characteristics.
1. Early Gonadal Supporting Cell Development in Mice
- (1)
-
Origin of Gonadal Supporting Cells from the Coelomic Epithelium
- (2)
-
SRY-Mediated Primary Sex Determination
2. Molecular and Cellular Events in Ovarian Somatic Cells
- (1)
-
Female Fate Determination in Somatic Supporting Cells in the Early Phase of Ovarian Development
- (2)
-
Secondary Population of Granulosa Cells in the Cortical Region of the Ovary
- (3)
-
Cortical–Medullary Regionality of Folliculogenesis Waves
3. Diversity of Ovarian Organogenesis along the Cortical–Medullary Axis

References
- Russell, L.D.; Ettlin, R.A.; Sinha hikim, A.P.; Clegg, E.D. Histological and Histopathological Evaluation of the Testis, 1st ed.; Cache River Press: Clearwater, FL, USA, 1990.
- Dellmann, H.; Brown, E.M. Textbook of Veterinary Histology; LEA & FEBIGER: Philadelphia, PA, USA, 1976.
- Capel, B. The battle of the sexes. Mech. Dev. 2000, 92, 89–103.
- Hatano, O.; Takakusu, A.; Nomura, M.; Morohashi, K. Identical origin of adrenal cortex and gonad revealed by expression profiles of Ad4BP/SF-1. Genes Cells 1996, 1, 663–671.
- Ikeda, Y.; Shen, W.H.; Ingraham, H.A.; Parker, K.L. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 1994, 8, 654–662.
- Harikae, K.; Miura, K.; Kanai, Y. Early gonadogenesis in mammals: Significance of long and narrow gonadal structure. Dev. Dyn. 2013, 242, 330–338.
- Svingen, T.; Koopman, P. Building the mammalian testis: Origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013, 27, 2409–2426.
- Wilhelm, D.; Yang, J.X.; Thomas, P. Mammalian sex determination and gonad development. Curr. Top. Dev. Biol. 2013, 106, 89–121.
- Wainwright, E.N.; Svingen, T.; Ng, E.T.; Wicking, C.; Koopman, P. Primary cilia function regulates the length of the embryonic trunk axis and urogenital field in mice. Dev. Biol. 2014, 395, 342–354.
- Karl, J.; Capel, B. Sertoli Cells of the Mouse Testis Originate from the Coelomic Epithelium. Dev. Biol. 1998, 203, 323–333.
- Lin, Y.; Barske, L.; Defalco, T.; Capel, B. Numb regulates somatic cell lineage commitment during early gonadogenesis in mice. Development 2017, 144, 1607–1618.
- Kanai, Y.; Hiramatsu, R.; Matoba, S.; Kidokoro, T. From SRY to SOX9: Mammalian Testis Differentiation. J. Biochem. 2005, 138, 13–19.
- Kashimada, K.; Koopman, P. Sry: The master switch in mammalian sex determination. Development 2010, 137, 3921–3930.
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244.
- Koopman, P.; Gubbay, J.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 1991, 351, 117–121.
- Miyawaki, S.; Kuroki, S.; Maeda, R.; Okashita, N.; Koopman, P.; Tachibana, M. The mouse Sry locus harbors a cryptic exon that is essential for male sex determination. Science 2020, 370, 121–124.
- Albrecht, K.H.; Eicher, E.M. Evidence That Sry Is Expressed in Pre-Sertoli Cells and Sertoli and Granulosa Cells Have a Common Precursor. Dev. Biol. 2001, 240, 92–107.
- Bullejos, M.; Koopman, P. Spatially dynamic expression of Sry in mouse genital ridges. Dev. Dyn. 2001, 221, 201–205.
- Lovell-Badge, R.; Robertson, E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 1990, 109, 635–646.
- Sekido, R.; Lovell-Badge, R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 2008, 453, 930–934.
- Sekido, R.; Bar, I.; Narváez, V.; Penny, G.; Lovell-Badge, R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev. Biol. 2004, 274, 271–279.
- Kidokoro, T.; Matoba, S.; Hiramatsu, R.; Fujisawa, M.; Kanai-Azuma, M.; Taya, C.; Kurohmaru, M.; Kawakami, H.; Hayashi, Y.; Kanai, Y.; et al. Influence on spatiotemporal patterns of a male-specific Sox9 activation by ectopic Sry expression during early phases of testis differentiation in mice. Dev. Biol. 2005, 278, 511–525.
- Barrionuevo, F.; Bagheri-Fam, S.; Klattig, J.; Kist, R.; Taketo, M.M.; Englert, C.; Scherer, G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol. Reprod. 2006, 74, 195–201.
- Kim, Y.; Kobayashi, A.; Sekido, R.; Dinapoli, L.; Brennan, J.; Chaboissier, M.; Poulat, F.; Behringer, R.R.; Lovell-Badge, R.; Capel, B. Fgf9 and Wnt4 Act as Antagonistic Signals to Regulate Mammalian Sex Determination. PLoS Biol. 2006, 4, e187.
- Hiramatsu, R.; Harikae, K.; Tsunekawa, N.; Kurohmaru, M.; Matsuo, I.; Kanai, Y. FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development 2010, 137, 303–312.
- Wilhelm, D.; Hiramatsu, R.; Mizusaki, H.; Widjaja, L.; Combes, A.N.; Kanai, Y.; Koopman, P. SOX9 Regulates Prostaglandin D Synthase Gene Transcription in Vivo to Ensure Testis Development. J. Biol. Chem. 2007, 282, 10553–10560.
- Moniot, B.; Declosmenil, F.; Barrionuevo, F.; Scherer, G.; Aritake, K.; Malki, S.; Marzi, L.; Cohen-Solal, A.; Georg, I.; Klattig, J.; et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development 2009, 136, 1813–1821.
- Stévant, I.; Neirjinck, Y.; Borel, C.; Escoffier, J.; Smith, L.B.; Antonarakis, S.E.; Dermitzakis, E.T.; Nef, S. Deciphering cell lineage specification during male sex determination with single-cell RNA sequencing. Cell Rep. 2018, 22, 1589–1599.
- Stévant, I.; Kühne, F.; Greenfield, A.; Chaboissier, M.; Dermitzakis, E.T.; Nef, S. Dissecting Cell Lineage Specification and Sex Fate Determination in Gonadal Somatic Cells Using Single-Cell Transcriptomics. Cell Rep. 2019, 26, 3272–3283.e3.
- Mayère, C.; Regard, V.; Perea-Gomez, A.; Bunce, C.; Neirijnck, Y.; Djari, C.; Bellido-Carreras, N.; Sararols, P.; Reeves, R.; Greenaway, S.; et al. Origin, specification and differentiation of a rare supporting-like lineage in the developing mouse gonad. Sci. Adv. 2022, 8, eabm0972.
- Major, A.T.; Estermann, M.A.; Smith, C.A. Anatomy, Endocrine Regulation, and Embryonic Development of the Rete Testis. Endocrinology 2021, 162, bqab04.
- McKey, J.; Anbarci, D.N.; Bunce, C.; Ontiveros, A.E.; Behringer, R.R.; Capel, B. Integration of mouse ovary morphogenesis with developmental dynamics of the oviduct, ovarian ligaments, and rete ovarii. bioRxiv 2022, 11, e81088.
- Brennan, J.; Tilmann, C.; Capel, B. Pdgfr-alpha mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003, 17, 800–810.
- Behringer, R.R.; Cate, R.L.; Froelick, G.J.; Palmiter, R.D.; Brinster, R.L. Abnormal sexual development in transgenic mice chronically expressing Müllerian inhibiting substance. Nature 1990, 345, 167–170.
- Behringer, R.R.; Finegold, M.J.; Cate, R.L. Müllerian-inhibiting substance function during mammalian sexual development. Cell 1994, 79, 415–425.
- Mishina, Y.; Rey, R.; Finegold, M.J.; Matzuk, M.M.; Josso, N.; Cate, R.L.; Behringer, R.R. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996, 10, 2577–2587.
- Imbeaud, S.; Carré-Eusèbe, D.; Rey, R.; Belville, C.; Josso, N.; Picard, J.Y. Molecular genetics of the persistent müllerian duct syndrome: A study of 19 families. Hum. Mol. Genet. 1994, 3, 125–131.
- Imbeaud, S.; Faure, E.; Lamarre, I.; Mattéi, M.G.; di Clemente, N.; Tizard, R.; Carré-Eusèbe, D.; Belville, C.; Tragethon, L.; Tonkin, C.; et al. Insensitivity to anti-müllerian hormone due to a mutation in the human anti-müllerian hormone receptor. Nat. Genet. 1995, 11, 382–388.
- Knebelmann, B.; Boussin, L.; Guerrier, D.; Legeai, L.; Kahn, A.; Josso, N.; Picard, J.Y. Anti-Müllerian hormone Bruxelles: A nonsense mutation associated with the persistent Müllerian duct syndrome. Proc. Natl. Acad. Sci. USA 1991, 88, 3767–3771.
- Yao, H.H.C.; Whoriskey, W.; Capel, B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002, 16, 1433–1440.
- Li, Y.; Zheng, M.; Lau, Y.C.C. The Sex-Determining Factors SRY and SOX9 Regulate Similar Target Genes and Promote Testis Cord Formation during Testicular Differentiation. Cell Rep. 2014, 8, 723–733.
- Chassot, A.; Bradford, S.T.; Auguste, A.; Gregoire, E.P.; Pailhoux, E.; De Rooij, D.G.; Schedl, A.; Chaboissier, M. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development 2012, 139, 4461–4472.
- Carmon, K.S.; Gong, X.; Lin, Q.; Thomas, A.; Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 11452–11457.
- Munger, S.C.; Aylor, D.L.; Syed, H.A.; Magwene, P.M.; Threadgill, D.W.; Capel, B. Elucidation of the transcription network governing mammalian sex determination by exploiting strain-specific susceptibility to sex reversal. Genes Dev. 2009, 23, 2521–2536.
- Munger, S.C.; Natarajan, A.; Looger, L.L.; Ohler, U.; Capel, B. Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination. PLoS Genet. 2013, 9, e1003630.
- Jameson, S.A.; Natarajan, A.; Cool, J.; Defalco, T.; Maatouk, D.M.; Mork, L.; Munger, S.C.; Capel, B. Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad. PLoS Genet. 2012, 8, e1002575.
- Mizusaki, H.; Kawabe, K.; Mukai, T.; Ariyoshi, E.; Kasahara, M.; Yoshioka, H.; Swain, A.; Morohashi, K. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by Wnt4 in the female developing gonad. Mol. Endocrinol. 2003, 17, 507–519.
- Yao, H.H.C.; Matzuk, M.M.; Jorgez, C.J.; Menke, D.B.; Page, D.C.; Swain, A.; Capel, B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev. Dyn. 2004, 230, 210–215.
- Kim, B.; Kim, Y.; Cooke, P.S.; Rüther, U.; Jorgensen, J.S. The fused toes locus is essential for somatic-germ cell interactions that foster germ cell maturation in developing gonads in mice. Biol. Reprod. 2011, 84, 1024–1032.
- Liu, C.F.; Liu, C.; Yao, H.H. Building pathways for ovary organogenesis in the mouse embryo. Curr. Top. Dev. Biol. 2010, 90, 263–290.
- Chassot, A.A.; Gregoire, E.P.; Lavery, R.; Taketo, M.M.; de Rooij, D.G.; Adams, I.R.; Chaboissier, M.C. RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS ONE 2011, 6, e25641.
- Nicol, B.; Yao, N.C. Gonadal Identity in the Absence of Pro-Testis Factor SOX9 and Pro-Ovary Factor Beta-Catenin in Mice. Biol. Reprod. 2015, 93, 35.
- Vainio, S.; Heikkilaè, M.; Kispert, A.; Chin, N.; Mcmahon, A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature 1999, 397, 405–409.
- Jeays-Ward, K.; Hoyle, C.; Brennan, J.; Dandonneau, M.; Alldus, G.; Capel, B.; Swain, A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 2003, 130, 3663–3670.
- Jordan, B.K.; Shen, J.H.; Olaso, R.; Ingraham, H.A.; Vilain, E. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/beta-catenin synergy. Proc. Natl. Acad. Sci. USA 2003, 100, 10866–10871.
- Chassot, A.; Ranc, F.; Gregoire, E.P.; Roepers-Gajadien, H.L.; Taketo, M.M.; Camerino, G.; De Rooij, D.G.; Schedl, A.; Chaboissier, M. Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008, 17, 1264–1277.
- Tomizuka, K.; Horikoshi, K.; Kitada, R.; Sugawara, Y.; Iba, Y.; Kojima, A.; Yoshitome, A.; Yamawaki, K.; Amagai, M.; Inoue, A.; et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum. Mol. Genet. 2008, 17, 1278–1291.
- Nef, S.; Schaad, O.; Stallings, N.R.; Cederroth, C.R.; Pitetti, J.; Schaer, G.; Malki, S.; Dubois-Dauphin, M.; Boizet-Bonhoure, B.; Descombes, P.; et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev. Biol. 2005, 287, 361–377.
- Beverdam, A.; Koopman, P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum. Mol. Genet. 2006, 15, 417–431.
- Chen, H.; Palmer, J.S.; Thiagarajan, R.D.; Dinger, M.E.; Lesieur, E.; Chiu, H.; Schulz, A.; Spiller, C.; Grimmond, S.M.; Little, M.H.; et al. Identification of Novel Markers of Mouse Fetal Ovary Development. PLoS ONE 2012, 7, e41683.
- Schmidt, D.; Ovitt, C.E.; Anlag, K.; Fehsenfeld, S.; Gredsted, L.; Treier, A.C.; Treier, M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 2004, 131, 933–942.
- Uda, M.; Ottolenghi, C.; Crisponi, L.; Garcia, J.E.; Deiana, M.; Kimber, W.; Forabosco, A.; Cao, A.; Schlessinger, D.; Pilia, G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum. Mol. Genet. 2004, 13, 1171–1181.
- Ottolenghi, C.; Pelosi, E.; Tran, J.; Colombino, M.; Douglass, E.; Nedorezov, T.; Cao, A.; Forabosco, A.; Schlessinger, D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 2007, 16, 2795–2804.
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation. Cell 2009, 139, 1130–1142.
- Maatouk, D.M.; Dinapoli, L.; Alvers, A.; Parker, K.L.; Taketo, M.M.; Capel, B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum. Mol. Genet. 2008, 17, 2949–2955.
- Boulanger, L.; Pannetier, M.; Gall, L.; Allais-Bonnet, A.; Elzaiat, M.; Le Bourhis, D.; Daniel, N.; Richard, C.; Cotinot, C.; Ghyselinck, N.B.; et al. FOXL2 is a female sex-determining gene in the goat. Curr. Biol. 2014, 24, 404–408.
- Pailhoux, E.; Vigier, B.; Chaffaux, S.; Servel, N.; Taourit, S.; Furet, J.P.; Fellous, M.; Grosclaude, F.; Cribiu, E.P.; Cotinot, C.; et al. A 11.7-kb deletion triggers intersexuality and polledness in goats. Nat. Genet. 2001, 29, 453–458.
- Ottolenghi, C.; Omari, S.; Garcia-Ortiz, J.E.; Uda, M.; Crisponi, L.; Forabosco, A.; Pilia, G.; Schlessinger, D. Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 2005, 14, 2053–2062.
- Mork, L.; Maatouk, D.M.; Mcmahon, J.A.; Guo, J.J.; Zhang, P.; Mcmahon, A.P.; Capel, B. Temporal Differences in Granulosa Cell Specification in the Ovary Reflect Distinct Follicle Fates in Mice. Biol. Reprod. 2012, 86, 37.
- Ng, A.; Tan, S.; Singh, G.; Rizk, P.; Swathi, Y.; Tan, T.Z.; Huang, R.Y.; Leushacke, M.; Barker, N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014, 16, 745–757.
- Rastetter, R.H.; Bernard, P.; Palmer, J.S.; Chassot, A.; Chen, H.; Western, P.S.; Ramsay, R.G.; Chaboissier, M.; Wilhelm, D. Marker genes identify three somatic cell types in the fetal mouse ovary. Dev. Biol. 2014, 394, 242–252.
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007.
- Jaks, V.; Barker, N.; Kasper, M.; van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgard, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291–1299.
- Barker, N.; Clevers, H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 2010, 138, 1681–1696.
- Plaks, V.; Brenot, A.; Lawson, D.A.; Linnemann, J.R.; Van Kappel, E.C.; Wong, K.C.; de Sauvage, F.; Klein, O.D.; Werb, Z. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep. 2013, 3, 70–78.
- Zheng, W.; Zhang, H.; Gorre, N.; Risal, S.; Shen, Y.; Liu, K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 2014, 23, 920–928.
- Tam, P.P.; Snow, M.H. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J. Embryol. Exp. Morphol. 1981, 64, 133–147.
- Lei, L.; Spradling, A.C. Mouse primordial germ cells produce cysts that partially fragment prior to meiosis. Development 2013, 140, 2075–2081.
- Bowles, J.; Knight, D.; Smith, C.; Wilhelm, D.; Richman, J.; Mamiya, S.; Yashiro, K.; Chawengsaksophak, K.; Wilson, M.J.; Rossant, J.; et al. Retinoid signaling determines germ cell fate in mice. Science 2006, 312, 596–600.
- Koubova, J.; Menke, D.B.; Zhou, Q.; Capel, B.; Griswold, M.D.; Page, D.C. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 2474–2479.
- Menke, D.B.; Koubova, J.; Page, D.C. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev. Biol. 2003, 262, 303–312.
- Koubova, J.; Hu, Y.C.; Bhattacharyya, T.; Soh, Y.Q.; Gill, M.E.; Goodheart, M.L.; Hogarth, C.A.; Griswold, M.D.; Page, D.C. Retinoic acid activates two pathways required for meiosis in mice. PLoS Genet. 2014, 10, e1004541.
- Pepling, M.E.; Spradling, A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001, 234, 339–351.
- Perez, G.I.; Robles, R.; Knudson, C.M.; Flaws, J.A.; Korsmeyer, S.J.; Tilly, J.L. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat. Genet. 1999, 21, 200–203.
- Alton, M.; Taketo, T. Switch from BAX-dependent to BAX-independent germ cell loss during the development of fetal mouse ovaries. J. Cell Sci. 2007, 120, 417–424.
- Greenfeld, C.R.; Pepling, M.E.; Babus, J.K.; Furth, P.A.; Flaws, J.A. BAX regulates follicular endowment in mice. Reproduction 2007, 133, 865–876.
- McGeady, T.A.; Quinn, P.J.; Fitzpatrick, E.S.; Ryan, M.T.; Kilroy, D.; Lonergan, P. Veterinary Embryology, 2nd ed.; John Wiley & Sons: Chichester, UK, 2017.
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy, 4th ed.; Saunders/Elsevier: St. Louis, MO, USA, 2010.
- Browne, P.; Place, N.J.; Vidal, J.D.; Moore, I.T.; Cunha, G.R.; Glickman, S.E.; Conley, A.J. Endocrine differentiation of fetal ovaries and testes of the spotted hyena (Crocuta crocuta): Timing of androgen-independent versus androgen-driven genital development. Reproduction 2006, 132, 649–659.
- Jiménez, R. Ovarian organogenesis in mammals: Mice cannot tell us everything. Sex. Dev. 2009, 3, 291–301.
- Carmona, F.D.; Lupiáñez, D.G.; Real, F.M.; Burgos, M.; Zurita, F.; Jiménez, R. SOX9 is not required for the cellular events of testicular organogenesis in XX mole ovotestes. J. Exp. Zool. B Mol. Dev. Evol. 2009, 312, 734–748.




