Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Junjie Luo | -- | 1976 | 2023-01-30 08:09:19 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 1977 | 2023-01-31 08:59:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Y.; Huang, Y.; Xu, C.; An, P.; Luo, Y.; Jiao, L.; Luo, J.; Li, Y. Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/40552 (accessed on 07 February 2026).
Liu Y, Huang Y, Xu C, An P, Luo Y, Jiao L, et al. Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/40552. Accessed February 07, 2026.
Liu, Yu, Yuejia Huang, Chong Xu, Peng An, Yongting Luo, Lei Jiao, Junjie Luo, Yongzhi Li. "Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases" Encyclopedia, https://encyclopedia.pub/entry/40552 (accessed February 07, 2026).
Liu, Y., Huang, Y., Xu, C., An, P., Luo, Y., Jiao, L., Luo, J., & Li, Y. (2023, January 30). Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. In Encyclopedia. https://encyclopedia.pub/entry/40552
Liu, Yu, et al. "Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases." Encyclopedia. Web. 30 January, 2023.
Copy Citation
High mortality rates due to cardiovascular diseases (CVDs) have attracted worldwide attention. It has been reported that mitochondrial dysfunction is one of the most important mechanisms affecting the pathogenesis of CVDs. Mitochondrial DNA (mtDNA) mutations may result in impaired oxidative phosphorylation (OXPHOS), abnormal respiratory chains, and ATP production. In dysfunctional mitochondria, the electron transport chain (ETC) is uncoupled and the energy supply is reduced, while reactive oxygen species (ROS) production is increased.
cardiovascular disease
mitochondrial dysfunction
mitochondrial DNA mutation
1. Mitochondrial Dysfunctions and CVDs
The pathogenesis of cardiovascular diseases (CVDs) is constantly being explored. Although the pathogenesis of CVDs is not fully understood; a study from the perspective of mitochondrial dysfunction and the analysis of the relationship between mitochondrial dysfunction and CVDs will help to better understand and solve the problems related to CVDs. Here, Figure 1 summarizes the relationships between primary CVDs and mitochondrial dysfunction, and further analyzed the four important features of mitochondria in CVDs in detail.
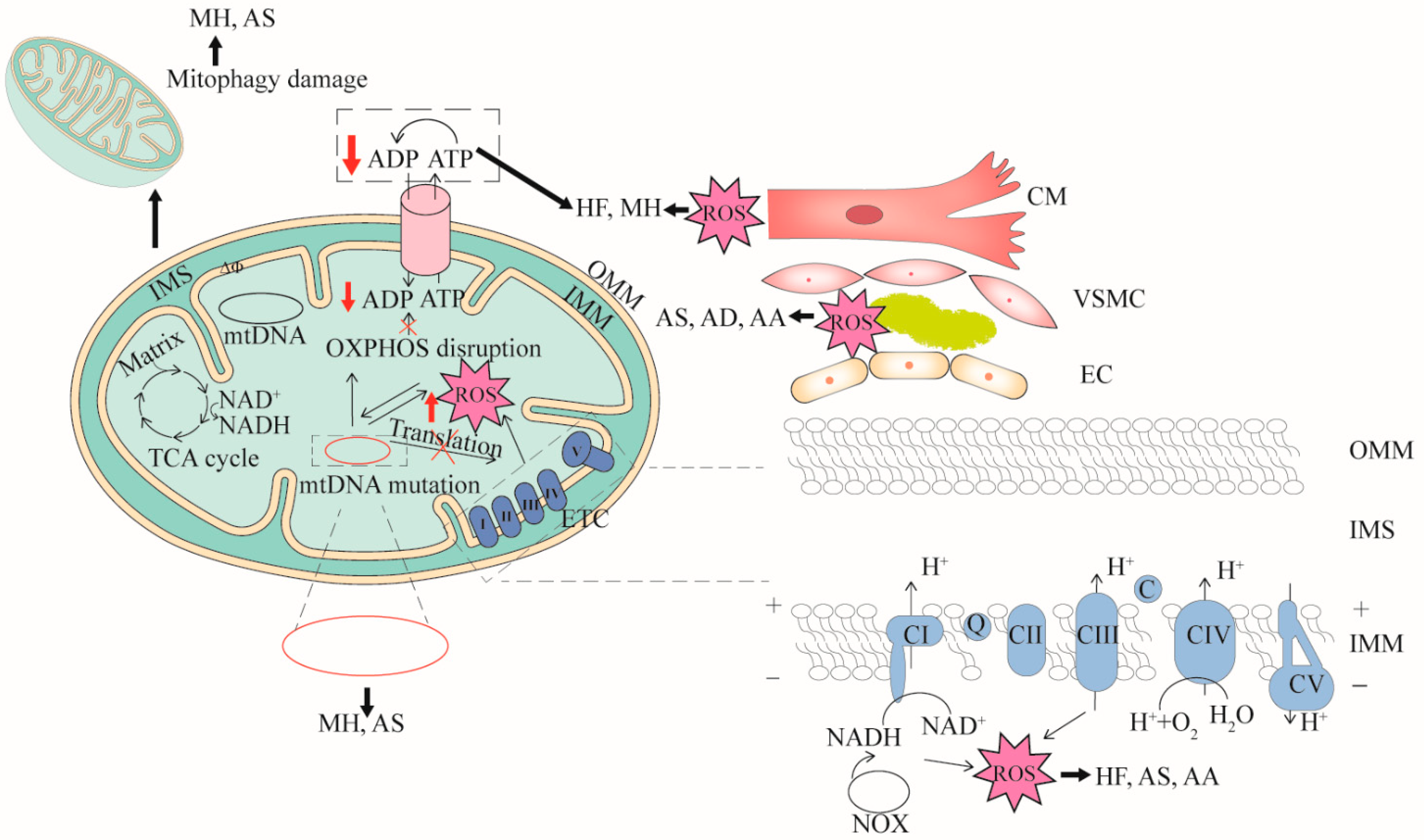
Figure 1. The relationships between mitochondrial dysfunction and CVDs. Four features (mtDNA mutation, mitophagy damage, decreased OXPHOS and increased ROS) associated with mitochondrial dysfunction are demonstrated. mtDNA mutation can cause dysfunction of mitochondrial respiratory chain complex or cytochrome transcription related to OXPHOS. When OXPHOS is impaired, ATP synthesis is reduced and excess ROS is generated. mtDNA mutation directly affects mitochondrial function or ROS production. In turn, high levels of ROS damage mitochondria. Cardiomyocyte cells (CM), vascular smooth muscle cells (VSMC), and endothelial cells (EC) that are impaired by mitochondrial dysfunction can cause CVDs. mtDNA mutation can cause myocardial hypertrophy (MH) and atherosclerosis (AS), mitophagy damage can cause MH and AS, decreased OXPHOS can cause heart failure (HF), aortic aneurysm (AA) and AS, and increased ROS can cause AS, aortic dissection (AD) and AA. OMM, outer mitochondrial membrane; IMS, inter membrane space; IMM, inner mitochondrial membrane.
1.1. mtDNA Mutation in CVDs
Human mtDNA is a circular double stranded genome with a length of 16,569 bp and consists of 37 genes, which support aerobic respiration and the production of cellular energy through OXPHOS. Unlike nuclear DNA, mtDNA is not protected by histones and does not recombine, resulting in approximately 10–100-fold higher mutation rates [1]. mtDNA mutations include point mutations deletions, fragment deletion and large scale mtDNA rearrangements, which can directly impair OXPHOS [2][3]. A large number of mitochondrial diseases are rooted in mtDNA mutations [4]. Notably, many specific disease mutations in mtDNA have been observed to cause cardiomyopathy, suggesting that mtDNA encoded proteins play a vital role in mitochondrial function of the heart [5]. Heart failure, a complex clinical syndrome that represents the end result of CVDs with multiple etiologies, is a bioenergetic disease with severe mtDNA mutations and mitochondrial dysfunction [6][7]. The MRPL44-disorder causes problems with the translation of a partial protein participating in OXPHOS, and it is associated with the clinical manifestation of cardiomyopathy in infancy [8]. In a mouse model of myocardial infarction (MI), the knockdown of the mouse lncRNA-SNHG8 gene significantly suppressed cardiac tissue injury [9]. Myocardial hypertrophy is a common inherited CVDs, and cardiomyocytes are associated with abnormal mitochondrial structure and dysfunction of mitophagy clearance, which makes it impossible to maintain mtDNA and functional integrity [10]. Hypertrophic cardiomyopathy is the predominant pattern of cardiomyopathy in mtDNA diseases, observed in nearly 40% of patients [11]. The studies have evaluated an association between mtDNA mutations and maternally inherited essential hypertension (MIEH), and these mutations may be one of the pathological mechanisms causing MIEH [3][12].
1.2. Mitophagy Damage in CVDs
Autophagy plays a positive role in maintaining cellular homeostasis in most cardiovascular-derived cells (e.g., cardiomyocytes, VSMCs) [13], and mitophagy is a kind of selective autophagy [14]. Mitophagy is one of the mitochondrial quality control pathways, and it can control and remove damaged mitochondria in cells [15]. During mitophagy, the damaged mitochondria are sequestered by double membrane vesicles and eventually become hydrolyzed by lysosomes [16]. Therefore, if mitophagy is impaired, the accumulation of dysfunctional mitochondria increases, which may lead to abnormal cell function and CVDs. Reducing mitochondrial dysfunction and lipid accumulation by activating mitophagy can help prevent diabetic cardiomyopathy caused by high fat diet [17]. In contrast, in BMAL1 deficient hESC-derived cardiomyocytes, impaired mitophagy is a key cause for the development of dilated cardiomyopathy [18].
1.3. Mitochondrial OXPHOS Reduction in CVDs
Defects in the genes encoding the OXPHOS complex are responsible for triggering various diseases, especially those with high energy requirements [19]. Dysfunction of OXPHOS is considered as one of the main causes of CVDs [20]. In chronic HF patients, reduced succinyl-CoA levels in myocardial mitochondria cause decreased OXPHOS [21].
1.4. Mitochondrial-Derived ROS Increase in CVDs
Mitochondrial ROS production is closely related to the mitochondrial ETC and NADPH oxidase (NOX). In the process of mitochondrial electron transfer, complex I and complex III are the main sites of ROS generation [22][23]. NADPH acts as a substrate to generate ROS under the action of NOX. The NOX is rich in mitochondria, and under the combined action of ETC and NOX, ROS continuously accumulates [24]. As a toxic by-product, ROS can damage mitochondria and are involved in the pathomechanism of CVDs. In turn, damaged mitochondria induce a large amount of ROS to be released from adjacent mitochondria, which is known as ROS-induced ROS [25]. The increased mitochondrial ROS represents one of the pathogenic mechanisms for vascular diseases [26].
2. Strategies for Targeting Mitochondria to Treat CVDs
2.1. mtDNA Mutation and Treatment in CVDs
2.1.1. mtDNA Editing Therapy
Mitochondrial heterogeneity affects mtDNA stability through copy number alterations and point deletions [27]. Once mtDNA is cleaved and linearized, it is rapidly degraded [28]. By duplicating residual mtDNA, mtDNA can be repopulated to the original level. In general, mitochondrial gene editing may include four potential approaches: mitochondria targeted restriction endonuclease (RE) technology, zinc finger nuclease (ZFN) technology, transcription activator-like effector nuclease (TALEN) technology and CRISPR/Cas9 system. In fact, mtDNA editing is a promising therapeutic modality to treat heteroplasmic or mutant mtDNA diseases. Specific mtDNA was effectively eliminated in heart of mice by using a mitochondria targeted RE [29][30]. Mitochondrial-targeted ZFNs can selectively cleave and degrade pathogenic mtDNA bearing large scale deletions or point mutations [31].
2.1.2. Mitochondrial Replacement Therapy
mtDNA replacement therapy (MRT) is to use enucleated donor embryos as healthy mtDNA to replace undesired defective/mutated mtDNA to prevent mitochondria from being maternally inherited. MRT is a form of in vitro fertilization (IVF) that includes spindle transfer (ST), prokaryotic transfer (PNT) and polar body transfer (PBT) [32]. In fact, embryos from human nuclear transfer can contain low levels of mutated mtDNA, which may be suitable for treating degenerative diseases caused by mtDNA mutations [33]. This opens up the possibility of MRT for CVDs, a chronic noncommunicable degenerative disease.
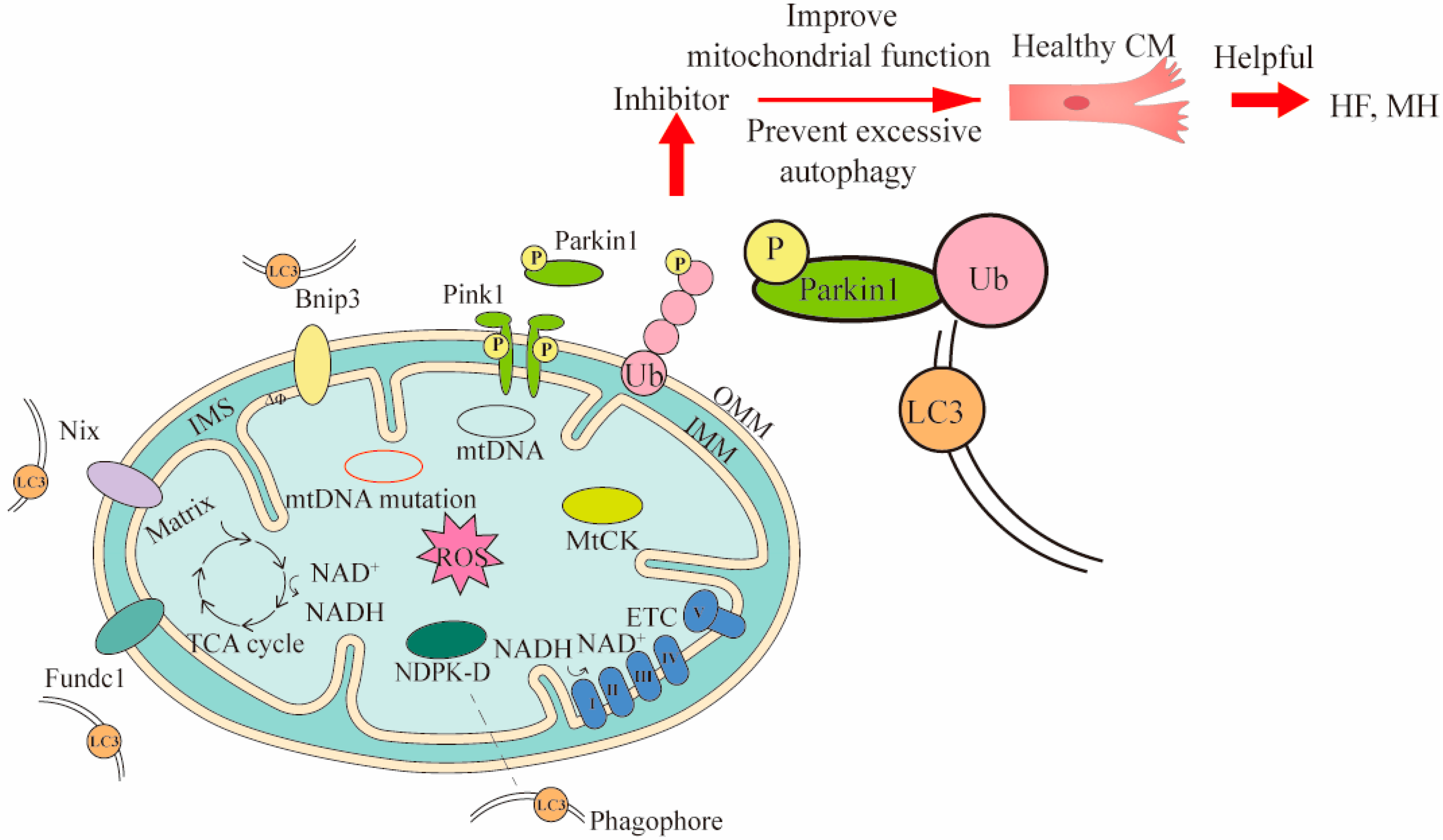
2.2. Mitophagy Therapy in CVDs
Mitophagy clears dysfunctional mitochondria under normal physiological conditions, and in response to pathological stress [34]. Currently, there are three mechanisms of mitophagy, including mitochondrial outer membrane receptor-mediated, Pink1/Parkin pathway, and lipid receptor-mediated mechanisms (Figure 2) [35]. Notably, the mechanism mediated by the Pink1/Parkin pathway is the most extensively studied. Under normal circumstances, the content of Pink1 is extremely low. When oxidative stress occurs and mitochondria damage is induced, Pink1 is activated and recruits Parkin to the mitochondrial outer membrane for phosphorylation. The phosphorylated Parkin ubiquitinates the substrate protein on the mitochondrial membrane [36]. These ubiquitinated proteins subsequently recruit specific autophagy-related receptors to interact with LC3-II to form autophagosomes [37].

Figure 2. Three mechanisms of mitophagy and the ways they intervene in treating CVDs. Three mechanisms of mitophagy include mitochondrial outer membrane receptor-mediated (such as Bnip3, Nix and Fundc1), Pink1/Parkin pathway, and lipid receptor-mediated mechanisms (such as MtCK, NDPK-D). The LC3 is located in the phagophore and binds to the corresponding receptor. The LC3 can bind to substances of different mitophagy mechanisms. To demonstrate the mitophagy occurring in CVDs, cardiomyocytes (CM) and Pink1/Parkin pathway were used to intervene in heart failure (HF) and myocardial hypertrophy (MH) by inhibitors. In Pink1/Parkin-dependent mitophagy, the Pink 1 accumulated on the damaged mitochondria is activated and recruits Parkin for phosphorylation. The phosphorylated Parkin binds to the ubiquitin attached to outer OMM, and finally binds to LC3 for mitophagy. The Bnip3 and Nix can directly bind LC3 and promote mitophagy. MtCK and NDPK-D as specific transporters can also directly bind LC3 for mitophagy to eliminate damaged mitochondria. The inhibitor acts on Pink1/Parkin pathway, and then prevents excessive mitophagy and improves mitochondrial function, thereby maintaining the healthy levels of cells associated with CVDs. OMM, outer mitochondrial membrane; IMS, inter membrane space; IMM, inner mitochondrial membrane.
In addition, when abnormal mitochondria undergo fission, they can trigger cardiovascular dysfunction [38][39]. Cytoplasmic GTPase dynamics related protein 1 (Drp1) regulates mitochondrial fission by interacting with proteins located at fission sites such as mitochondrial fission 1 (Fis1), mitochondrial fission factor (Mff), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49 and MiD51) [40].
2.3. Mitochondrial OXPHOS Reduction and Treatment in CVDs
2.3.1. Small Molecule Compounds Enhance Mitochondrial Function
SIRT3 is a mitochondrial protein deacetylase that regulates mitochondrial function and is considered as an emerging drug target for CVDs [41]. SIRT3 can make mitochondrial metabolic pathways and ROS detoxification activate, and increase ATP production [42]. Resveratrol improves mitochondrial OXPHOS in diabetic hearts and prevents the decline of SIRT3 activity in the heart by increasing ETC activity and mitochondrial function [43]. The polyphenolic compound polydatin can initiate SIRT3-regulated mitophagy to prevent MI [44].
When mtDNA is damaged at high levels, increased Poly(ADP-ribose) polymerase (PARP) activity leads to a decrease in NAD+ levels, resulting in impaired NAD+-dependent SIRT3 activation and ultimately cardiac mitochondrial dysfunction [45]. Therefore, targeted improvement of mitochondrial function through nutritional supplementation NAD+ or ketoesters may be useful in patients with heart failure [46]. NAD+ supplementation with nicotinamide riboside (NR) promotes mitophagy in a Pink1-dependent manner [47]. NR can reduce ROS production and maintain normal mitochondrial function in the presence of inflammatory triggers [48].
2.3.2. Nanomaterials Targeted Mitochondria to Improve Mitochondrial Function
Many drugs cannot precisely bind to damaged mitochondria, and they are even toxic to other tissues in the body. To solve these problems, precise targeted therapy has attracted much attention. Modification of nanoparticles with different components facilitates mitochondrial directed drug penetration [49]. A team has constructed a non invasive aerosol inhalation delivery system based on antioxidant nano drugs, which can target damaged mitochondria, clear ROS, and improve the targeting ability of nano-drugs to myocardium [50]. Artificial hybrid nanozymes created by protein reconstruction technology and nanotechnology can target mitochondria and scavenge ROS, thereby reducing mitochondrial oxidative damage [51].
2.4. Reduction or Elimination of Mitochondrial-Derived ROS in CVDs
ROS acts as highly active molecules in vivo, and antioxidants can effectively reduce or eliminate ROS. The in vitro hypoxia/reoxygenation model of H9c2 cells could simulate myocardial ischemia-reperfusion injury, and it found that the experimental group supplemented with vitamin D could inhibit the production of ROS in cardiomyocytes [52]. Melatonin is an indole heterocyclic compound produced by pineal cells in the pineal gland. It can effectively lower ROS production, thereby reducing oxidative stress and VSMC loss, preventing the deterioration of thoracic aortic aneurysm and dissection [53]. Fullerenol nanoparticles are introduced into an alginate hydrogel to form a fullerenol/alginate hydrogel with antioxidant activity. This injectable cell delivery vector can treat myocardial infarction by effectively reducing ROS levels [54]. Cardioprotection of tetrahedral DNA nanostructures can significantly decrease oxidative stress and play a positive role in protecting against myocardial ischemia-reperfusion injury [55].
Fortunately, the antioxidant CoQ10 has been used in the clinical treatment of CVDs and has good curative effect. Ubiquinone, the oxidized form of CoQ10, transports electrons in the mitochondrial ETC and plays a crucial role in mitochondrial energy production. CoQ10 can transport H+ to thermally dissipate chemosmotic gradients via uncoupling proteins (UCP-1, 2 and 3). After uncoupling, the reduction level of electron carriers is reduced, thereby reducing the production of ROS [56]. Moreover, the reduced form of CoQ10 is also an active agent involved in antioxidant function, which can scavenge ROS production due to mitochondrial dysfunction [57]. Meanwhile, CoQ10 helps recycle other antioxidants such as radical forms of vitamin C and vitamin E [58]. CoQ10 has been shown to increase ATP production in cardiomyocytes, enhance oxidative effects, and improve endothelial function and lipid profile [59]. Comparing CoQ10 with placebo, the therapeutic effect of CoQ10 was more significant in the long term [60].
References
- Luo, J.; Shen, S.; Xia, J.; Wang, J.; Gu, Z. Mitochondria as the Essence of Yang Qi in the Human Body. Phenomics 2022, 2, 336–348.
- Zhu, Z.; Wang, X. Significance of Mitochondria DNA Mutations in Diseases. In Mitochondrial DNA and Diseases; Advances in Experimental Medicine and Biology Book Series; Springer: Singapore, 2017; Volume 1038, pp. 219–230.
- Zhu, Y.; You, J.; Xu, C.; Gu, X. Associations of mitochondrial DNA 3777–4679 region mutations with maternally inherited essential hypertensive subjects in China. BMC Med. Genet. 2020, 21, 1–9.
- Siva, M.A.; Mahalakshmi, R.; Bhakta-Guha, D.; Guha, G. Gene therapy for the mitochondrial genome: Purging mutations, pacifying ailments. Mitochondrion 2018, 46, 195–208.
- DiMauro, S.; Schon, E.A. Mitochondrial Respiratory-Chain Diseases. N. Engl. J. Med. 2003, 348, 2656–2668.
- Wu, C.; Zhang, Z.; Zhang, W.; Liu, X. Mitochondrial dysfunction and mitochondrial therapies in heart failure. Pharmacol. Res. 2021, 175, 106038.
- Bray, A.W.; Ballinger, S.W. Mitochondrial DNA mutations and cardiovascular disease. Curr. Opin. Cardiol. 2017, 32, 267–274.
- Friederich, M.W.; Geddes, G.C.; Wortmann, S.B.; Punnoose, A.; Wartchow, E.; Knight, K.M.; Prokisch, H.; Creadon-Swindell, G.; Mayr, J.A.; Van Hove, J.L. Pathogenic variants in MRPL44 cause infantile cardiomyopathy due to a mitochondrial translation defect. Mol. Genet. Metab. 2019, 133, 362–371.
- Tang, J.; Tang, Q.-X.; Liu, S. METTL3-modified lncRNA-SNHG8 binds to PTBP1 to regulate ALAS2 expression to increase oxidative stress and promote myocardial infarction. Mol. Cell. Biochem. 2022.
- Ranjbarvaziri, S.; Kooiker, K.B.; Ellenberger, M.; Fajardo, G.; Zhao, M.; Roest, A.S.V.; Woldeyes, R.A.; Koyano, T.T.; Fong, R.; Ma, N.; et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 1714–1731.
- Lioncino, M.; Monda, E.; Caiazza, M.; Fusco, A.; Cirillo, A.; Dongiglio, F.; Simonelli, V.; Sampaolo, S.; Ruggiero, L.; Scarano, G. Cardiovascular involvement in mtDNA disease: Diagnosis, management, and therapeutic options. Heart Fail. Clin. 2022, 18, 51–60.
- Zhu, Y.; Gu, X.; Xu, C. Mitochondrial DNA 7908–8816 region mutations in maternally inherited essential hypertensive subjects in China. BMC Med. Genom. 2018, 11, 1–11.
- Bravo-San Pedro, J.M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 120, 1812–1824.
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-Arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in Human Diseases. Int. J. Mol. Sci. 2021, 22, 3903.
- Poznyak, A.V.; Nikiforov, N.G.; Wu, W.-K.; Kirichenko, T.V.; Orekhov, A.N. Autophagy and Mitophagy as Essential Components of Atherosclerosis. Cells 2021, 10, 443.
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42.
- Tong, M.; Saito, T.; Zhai, P.; Oka, S.-I.; Mizushima, W.; Nakamura, M.; Ikeda, S.; Shirakabe, A.; Sadoshima, J. Mitophagy Is Essential for Maintaining Cardiac Function During High Fat Diet-Induced Diabetic Cardiomyopathy. Circ. Res. 2019, 124, 1360–1371.
- Li, E.; Li, X.; Huang, J.; Xu, C.; Liang, Q.; Ren, K.; Bai, A.; Lu, C.; Qian, R.; Sun, N. BMAL1 regulates mitochondrial fission and mitophagy through mitochondrial protein BNIP3 and is critical in the development of dilated cardiomyopathy. Protein Cell 2020, 11, 661–679.
- Ghezzi, D.; Zeviani, M. Human diseases associated with defects in assembly of OXPHOS complexes. Essays Biochem. 2018, 62, 271–286.
- Stamerra, C.A.; Di Giosia, P.; Giorgini, P.; Ferri, C.; Sukhorukov, V.N.; Sahebkar, A. Mitochondrial Dysfunction and Cardiovascular Disease: Pathophysiology and Emerging Therapies. Oxidative Med. Cell. Longev. 2022, 2022, 1–16.
- Takada, S.; Maekawa, S.; Furihata, T.; Kakutani, N.; Setoyama, D.; Ueda, K.; Nambu, H.; Hagiwara, H.; Handa, H.; Fumoto, Y. Succinyl-CoA-based energy metabolism dysfunction in chronic heart failure. Proc. Natl. Acad. Sci. USA 2022, 119, e2203628119.
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031.
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31.
- Moghadam, Z.M.; Henneke, P.; Kolter, J. From Flies to Men: ROS and the NADPH Oxidase in Phagocytes. Front. Cell Dev. Biol. 2021, 9, 628991.
- Madamanchi, N.; Runge, M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007, 100, 460–473.
- López-Acosta, O.; Fortis-Barrera, M.D.L.A.; Barrios-Maya, M.A.; Ramírez, A.R.; Aguilar, F.J.A.; El-Hafidi, M. Reactive Oxygen Species from NADPH Oxidase and Mitochondria Participate in the Proliferation of Aortic Smooth Muscle Cells from a Model of Metabolic Syndrome. Oxidative Med. Cell. Longev. 2018, 2018, 1–10.
- Wang, W.; Wang, X. New potentials of mitochondrial DNA editing. Cell Biol. Toxicol. 2020, 36, 391–393.
- Peeva, V.; Blei, D.; Trombly, G.; Corsi, S.; Szukszto, M.J.; Rebelo-Guiomar, P.; Gammage, P.A.; Kudin, A.P.; Becker, C.; Altmüller, J.; et al. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat. Commun. 2018, 9, 1–11.
- Bacman, S.R.; Williams, S.L.; Garcia, S.; Moraes, C.T. Organ-specific shifts in mtDNA heteroplasmy following systemic delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2010, 17, 713–720.
- Bacman, S.R.; Williams, S.L.; Duan, N.; Moraes, C.T. Manipulation of mtDNA heteroplasmy in all striated muscles of newborn mice by AAV9-mediated delivery of a mitochondria-targeted restriction endonuclease. Gene Ther. 2012, 19, 1101–1106.
- Gammage, P.A.; Rorbach, J.; Vincent, A.I.; Rebar, E.J.; Minczuk, M. Mitochondrially targeted ZFN s for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol. Med. 2014, 6, 458–466.
- Sharma, H.; Singh, D.; Mahant, A.; Sohal, S.K.; Kesavan, A.K. Samiksha Development of mitochondrial replacement therapy: A review. Heliyon 2020, 6, e04643.
- Greggains, G.D.; Lister, L.M.; Tuppen, H.A.L.; Zhang, Q.; Needham, L.H.; Prathalingam, N.; Hyslop, L.A.; Craven, L.; Polanski, Z.; Murdoch, A.P.; et al. Therapeutic potential of somatic cell nuclear transfer for degenerative disease caused by mitochondrial DNA mutations. Sci. Rep. 2014, 4, 3844.
- Wang, B.; Nie, J.; Wu, L.; Hu, Y.; Wen, Z.; Dong, L.; Zou, M.-H.; Chen, C.; Wang, D.W. AMPKα2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018, 122, 712–729.
- Qiu, Z.; Wei, Y.; Song, Q.; Du, B.; Wang, H.; Chu, Y.; Hu, Y. The Role of Myocardial Mitochondrial Quality Control in Heart Failure. Front. Pharmacol. 2019, 10, 1404.
- McWilliams, T.G.; Muqit, M.M. PINK1 and Parkin: Emerging themes in mitochondrial homeostasis. Curr. Opin. Cell Biol. 2017, 45, 83–91.
- Bansal, M.; Moharir, S.C.; Swarup, G. Autophagy receptor optineurin promotes autophagosome formation by potentiating LC3-II production and phagophore maturation. Commun. Integr. Biol. 2018, 11, 1–4.
- Quiles, J.M.; Gustafsson, B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, 19, 723–736.
- Jin, J.-Y.; Wei, X.-X.; Zhi, X.-L.; Wang, X.-H.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2021, 42, 655–664.
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 2013, 24, 659–667.
- Sun, W.; Liu, C.; Chen, Q.; Liu, N.; Yan, Y.; Liu, B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2018, 2018, 1–11.
- Zhang, J.; Xiang, H.; Liu, J.; Chen, Y.; He, R.-R.; Liu, B. Mitochondrial Sirtuin 3: New emerging biological function and therapeutic target. Theranostics 2020, 10, 8315–8342.
- Bagul, P.K.; Katare, P.B.; Bugga, P.; Dinda, A.K.; Banerjee, S.K. SIRT-3 Modulation by Resveratrol Improves Mitochondrial Oxidative Phosphorylation in Diabetic Heart through Deacetylation of TFAM. Cells 2018, 7, 235.
- Zhang, M.; Zhao, Z.; Shen, M.; Zhang, Y.; Duan, J.; Guo, Y.; Zhang, D.; Hu, J.; Lin, J.; Man, W.; et al. Polydatin protects cardiomyocytes against myocardial infarction injury by activating Sirt3. Biochim. Et Biophys. Acta Mol. Basis Dis. 2017, 1863, 1962–1972.
- Lauritzen, K.H.; Olsen, M.B.; Ahmed, M.S.; Yang, K.; Rinholm, J.E.; Bergersen, L.H.; Esbensen, Q.Y.; Sverkeli, L.J.; Ziegler, M.; Attramadal, H.; et al. Instability in NAD+ metabolism leads to impaired cardiac mitochondrial function and communication. eLife 2021, 10, e59828.
- O’Brien, K.D.; Tian, R. Boosting mitochondrial metabolism with dietary supplements in heart failure. Nat. Rev. Cardiol. 2021, 18, 685–686.
- Yang, B.; Dan, X.; Hou, Y.; Lee, J.; Wechter, N.; Krishnamurthy, S.; Kimura, R.; Babbar, M.; Demarest, T.; McDevitt, R.; et al. NAD + supplementation prevents STING-induced senescence in ataxia telangiectasia by improving mitophagy. Aging Cell 2021, 20, e13329.
- Zhou, B.; Wang, D.D.-H.; Qiu, Y.; Airhart, S.; Liu, Y.; Stempien-Otero, A.; O’Brien, K.D.; Tian, R. Boosting NAD level suppresses inflammatory activation of PBMCs in heart failure. J. Clin. Investig. 2020, 130, 6054–6063.
- Charles, C.; Cohen-Erez, I.; Kazaoka, B.; Melnikov, O.; Stein, D.E.; Sensenig, R.; Rapaport, H.; Orynbayeva, Z. Mitochondrial responses to organelle-specific drug delivering nanoparticles composed of polypeptide and peptide complexes. Nanomedicine 2020, 15, 2917–2932.
- Liu, C.; Chen, L.; Ma, Y.; Hu, K.; Wu, P.; Pan, L.; Chen, H.; Li, L.; Hu, H.; Zhang, J. Pulmonary circulation-mediated heart targeting for the prevention of heart failure by inhalation of intrinsically bioactive nanoparticles. Theranostics 2021, 11, 8550–8569.
- Zhang, Y.; Khalique, A.; Du, X.; Gao, Z.; Wu, J.; Zhang, X.; Zhang, R.; Sun, Z.; Liu, Q.; Xu, Z.; et al. Biomimetic Design of Mitochondria-Targeted Hybrid Nanozymes as Superoxide Scavengers. Adv. Mater. 2021, 33, e2006570.
- Lee, T.-L.; Lee, M.-H.; Chen, Y.-C.; Lee, Y.-C.; Lai, T.-C.; Lin, H.Y.-H.; Hsu, L.-F.; Sung, H.-C.; Lee, C.-W.; Chen, Y.-L. Vitamin D attenuates ischemia/reperfusion-induced cardiac injury by reducing mitochondrial fission and mitophagy. Front. Pharmacol. 2020, 11, 604700.
- Xia, L.; Sun, C.; Zhu, H.; Zhai, M.; Zhang, L.; Jiang, L.; Hou, P.; Li, J.; Li, K.; Liu, Z.; et al. Melatonin protects against thoracic aortic aneurysm and dissection through SIRT1-dependent regulation of oxidative stress and vascular smooth muscle cell loss. J. Pineal Res. 2020, 69, e12661.
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488.
- Zhang, M.; Zhu, J.; Qin, X.; Zhou, M.; Zhang, X.; Gao, Y.; Zhang, T.; Xiao, D.; Cui, W.; Cai, X. Cardioprotection of Tetrahedral DNA Nanostructures in Myocardial Ischemia-Reperfusion Injury. ACS Appl. Mater. Interfaces 2019, 11, 30631–30639.
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824.
- Gutierrez-Mariscal, F.M.; Larriva, A.P.A.-D.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 Supplementation for the Reduction of Oxidative Stress: Clinical Implications in the Treatment of Chronic Diseases. Int. J. Mol. Sci. 2020, 21, 7870.
- Dhanasekaran, M.; Ren, J. The Emerging Role of Coenzyme Q-10 in Aging, Neurodegeneration, Cardiovascular Disease, Cancer and Diabetes Mellitus. Curr. Neurovascular Res. 2005, 2, 447–459.
- Szczepańska, E.; Białek-Dratwa, A.; Janota, B.; Kowalski, O. Dietary Therapy in Prevention of Cardiovascular Disease (CVD)—Tradition or Modernity? A Review of the Latest Approaches to Nutrition in CVD. Nutrients 2022, 14, 2649.
- Mortensen, A.L.; Rosenfeldt, F.; Filipiak, K.J. Effect of coenzyme Q10 in Europeans with chronic heart failure: A sub-group analysis of the Q-SYMBIO randomized double-blind trial. Cardiol. J. 2019, 26, 147–156.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
562
Revisions:
2 times
(View History)
Update Date:
31 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




