
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ji Hyun Park | -- | 2155 | 2023-01-30 07:48:46 | | | |
| 2 | Lindsay Dong | Meta information modification | 2155 | 2023-01-31 08:51:34 | | |
Video Upload Options
Attention deficit hyperactivity disorder (ADHD) is a prevalent neurodevelopmental disorder that can diminish the quality of life of both children and adults in academic, occupational, and social contexts. The kynurenine pathway (KP) contains a set of enzymatic reactions involved in tryptophan (TRP) degradation. It is known to be associated with the risk of developing ADHD.
1. Introduction
2. The Kynurenine Pathway
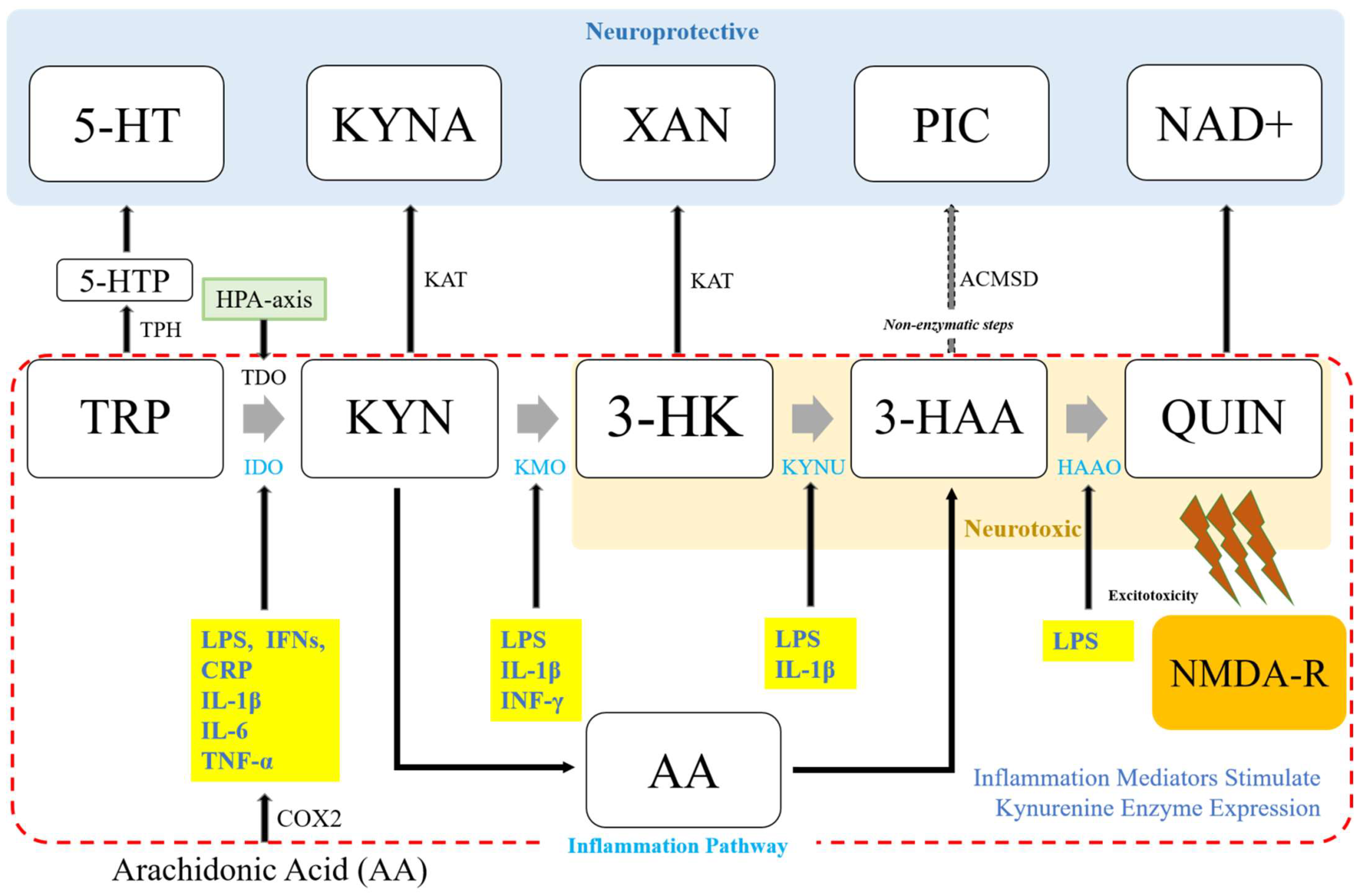
3. Inflammatory Cytokine-Mediated Regulation of Kynurenine Metabolism
3.1. Indoleamine 2,3-Dioxygenase (IDO) and Inflammation Mediators
3.2. Kynurenine-3-Monooxygenase (KMO) and Inflammation Mediators
3.3. Kynurenine Aminotransferases (KATs) and Inflammation Mediators
4. Genetic Links between Inflammation and Kynurenine Metabolism in ADHD
Genetic studies have supported that gene polymorphisms are linked to the inflammatory pathway in ADHD. In a total of 398 subjects, Smith et al. [30] evaluated a set of 164 single-nucleotide polymorphisms (SNPs) from 31 candidate genes and found that two SNPs in the ciliary neurotrophic factor receptor (CNTFR) were associated with the severity of ADHD inattentive symptom. Odell et al. [31] conducted a population-based association study with 546 ADHD patients vs. 546 controls and proposed an association between CNTFR and ADHD in both children and adults. They also reported an association between ADHD and major histocompatibility complex genes, demonstrating the role of inflammation and autoimmunity in this disorder.
Another genome-wide association study for 478 ADHD patients and 880 controls has suggested no significant SNPs [32]. However, a pathway analysis has revealed an association of ADHD with SNPs involved in gene expression regulation, cell adhesion, and inflammation [30]. One study has inspected the genomic overlap between ADHD and other psychiatric disorders in 318 individuals, including 93 who were diagnosed with ADHD, and found a similar inflammation-related genetic signature between ADHD and depression [33].
5. Dysregulation of the Kynurenine Pathway in ADHD
6. Potential Inflammatory Biomarkers in ADHD
| Biomarkers | Youth | References | Adult 1 | References |
|---|---|---|---|---|
| CRP | ↑ | [41][42] | ↑ | [43] |
| IL-1β | ↓ | [37] | ↔ | [43] |
| IL-6 | ↑ | [37][44][45] | ↔ | [46][47][48] |
| IL-10 | ↑ | [37][41][45] | ↔ | [46] |
| IL-13 | ↑ | [37][41] | ↔ | [46] |
| IL-16 | ↑ | [37][49] | ||
| TNF-α | ↓ | [37][46] | ↓ | [46] |
| Cortisol 2 | ↓ | [41][42][50][51] | ↓ | [52] |
References
- Riglin, L.; Collishaw, S.; Thapar, A.K.; Dalsgaard, S.; Langley, K.; Smith, G.D.; Stergiakouli, E.; Maughan, B.; O’Donovan, M.C.; Thapar, A. Association of genetic risk variants with attention-deficit/hyperactivity disorder trajectories in the general population. JAMA Psychiatry 2016, 73, 1285–1292.
- De Zeeuw, E.L.; van Beijsterveldt, C.E.M.; Ehli, E.A.; de Geus, E.J.C.; Boomsma, D.I. Attention deficit hyperactivity disorder symptoms and low educational achievement: Evidence supporting a causal hypothesis. Behav. Genet. 2017, 47, 278–289.
- Jangmo, A.; Stålhandske, A.; Chang, Z.; Chen, Q.; Almqvist, C.; Feldman, I.; Bulik, C.M.; Lichtenstein, P.; D’Onofrio, B.; Kuja-Halkola, R.; et al. Attention-deficit/hyperactivity disorder, school performance, and effect of medication. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 423–432.
- Evangelisti, M.; De Rossi, P.; Rabasco, J.; Donfrancesco, R.; Lionetto, L.; Capi, M.; Sani, G.; Simmaco, M.; Nicoletti, F.; Villa, M.P. Changes in serum levels of kynurenine metabolites in paediatric patients affected by ADHD. Eur. Child Adolesc. Psychiatry 2017, 26, 1433–1441.
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. In DSM-IIIR; American Psychiatric Press: Washington, DC, USA, 1987; pp. 3–24.
- Takahashi, N.; Ishizuka, K.; Inada, T. Peripheral biomarkers of attention-deficit hyperactivity disorder: Current status and future perspective. J. Psychiatr. Res. 2021, 137, 465–470.
- Paris, J.; Bhat, V.; Thombs, B. Is adult attention-deficit hyperactivity disorder being overdiagnosed? Can. J. Psychiatry 2015, 60, 324–328.
- Moynihan, R.; Doust, J.; Henry, D. Preventing overdiagnosis: How to stop harming the healthy. BMJ 2012, 344, e3502.
- Elia, J.; Glessner, J.T.; Wang, K.; Takahashi, N.; Shtir, C.J.; Hadley, D.; Sleiman, P.M.; Zhang, H.; Kim, C.E.; Robison, R. Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat. Genet. 2012, 44, 78–84.
- Moroni, F.; Russi, P.; Carlá, V.; Lombardi, G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci. Lett. 1988, 94, 145–150.
- Campbell, B.; Charych, E.; Lee, A.; Möller, T. Kynurenines in CNS disease: Regulation by inflammatory cytokines. Front. Neurosci. 2014, 8, 12.
- Heyes, M.P.; Chen, C.Y.; Major, E.O.; Saito, K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem. J. 1997, 326, 351–356.
- Amori, L.; Guidetti, P.; Pellicciari, R.; Kajii, Y.; Schwarcz, R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J. Neurochem. 2009, 109, 316–325.
- Ullah, I.; Awan, H.A.; Aamir, A.; Diwan, M.N.; de Filippis, R.; Awan, S.; Irfan, M.; Fornaro, M.; Ventriglio, A.; Vellante, F.; et al. Role and perspectives of inflammation and C-Reactive Protein (CRP) in psychosis: An economic and widespread tool for assessing the disease. Int. J. Mol. Sci. 2021, 22, 13032.
- Hirata, F.; Hayaishi, O. Possible participation of superoxide anion in the intestinal tryptophan 2,3-dioxygenase reaction. J. Biol. Chem. 1971, 246, 7825–7826.
- Verlaet, A.A.J.; Breynaert, A.; Ceulemans, B.; De Bruyne, T.; Fransen, E.; Pieters, L.; Savelkoul, H.F.J.; Hermans, N. Oxidative stress and immune aberrancies in attention-deficit/hyperactivity disorder (ADHD): A case–control comparison. Eur. Child Adolesc. Psychiatry 2019, 28, 719–729.
- Guidetti, P.; Amori, L.; Sapko, M.T.; Okuno, E.; Schwarcz, R. Mitochondrial aspartate aminotransferase: A third kynurenate-producing enzyme in the mammalian brain. J. Neurochem. 2007, 102, 103–111.
- Wang, M.; Ramos, B.P.; Paspalas, C.D.; Shu, Y.; Simen, A.; Duque, A.; Vijayraghavan, S.; Brennan, A.; Dudley, A.; Nou, E.; et al. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 2007, 129, 397–410.
- DiNatale, B.C.; Murray, I.A.; Schroeder, J.C.; Flaveny, C.A.; Lahoti, T.S.; Laurenzana, E.M.; Omiecinski, C.J.; Perdew, G.H. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol. Sci. 2010, 115, 89–97.
- Sathyasaikumar, K.V.; Tararina, M.; Wu, H.Q.; Neale, S.A.; Weisz, F.; Salt, T.E.; Schwarcz, R. Xanthurenic acid formation from 3-hydroxykynurenine in the mammalian brain: Neurochemical characterization and physiological effects. Neuroscience 2017, 367, 85–97.
- De Carvalho, L.P.; Bochet, P.; Rossier, J. The endogeneous agonist quinolinic acid and the non endogenous homoquinolinic acid discriminate between NMDAR2 receptor subunits. Neurochem. Int. 1996, 28, 445–452.
- Lestage, J.; Verrier, D.; Palin, K.; Dantzer, R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav. Immun. 2002, 16, 596–601.
- Connor, T.J.; Starr, N.; O’Sullivan, J.B.; Harkin, A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: A role for IFN-γ? Neurosci. Lett. 2008, 441, 29–34.
- Fujigaki, H.; Seishima, M.; Saito, K. Posttranslational modification of indoleamine 2,3-dioxygenase. Anal. Bioanal. Chem. 2012, 403, 1777–1782.
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Choudhury, S.; Musaelyan, K.; Myint, A.M.; Thuret, S.; Price, J.; Pariante, C.M. Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012, 37, 939–949.
- Jung, I.D.; Lee, C.-M.; Jeong, Y.-I.; Lee, J.S.; Park, W.S.; Han, J.; Park, Y.-M. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007, 581, 1449–1456.
- Tu, H.; Rady, P.L.; Juelich, T.; Smith, E.M.; Tyring, S.K.; Hughes, T.K. Cytokine regulation of tryptophan metabolism in the hypothalamic-pituitary-adrenal (HPA) axis: Implications for protective and toxic consequences in neuroendocrine regulation. Cell. Mol. Neurobiol. 2005, 25, 673–680.
- Yadav, M.C.; Burudi, E.; Alirezaei, M.; Flynn, C.C.; Watry, D.D.; Lanigan, C.M.; Fox, H.S. IFN-γ-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia 2007, 55, 1385–1396.
- Alberati-Giani, D.; Ricciardi-Castagnoli, P.; Köhler, C.; Cesura, A. Regulation of the kynurenine pathway by IFN-γ in murine cloned macrophages and microglial cells. In Recent Advances in Tryptophan Research; Springer: Boston, MA, USA, 1996; pp. 171–175.
- Smith, T.F.; Anastopoulos, A.D.; Garrett, M.E.; Arias-Vasquez, A.; Franke, B.; Oades, R.D.; Sonuga-Barke, E.; Asherson, P.; Gill, M.; Buitelaar, J.K. Angiogenic, neurotrophic, and inflammatory system SNPs moderate the association between birth weight and ADHD symptom severity. Am. J. Med. Genet. Part. B Neuropsychiatr. Genet. 2014, 165, 691–704.
- Odell, D.; Warren, R.P.; Warren, L.; Burger, R.A.; Maciulis, A. Association of genes within the major histocompatibility complex with attention deficit hyperactivity disorder. Neuropsychobiology 1997, 35, 181–186.
- Zayats, T.; Athanasiu, L.; Sonderby, I.; Djurovic, S.; Westlye, L.T.; Tamnes, C.K.; Fladby, T.; Aase, H.; Zeiner, P.; Reichborn-Kjennerud, T.; et al. Genome-wide analysis of attention deficit hyperactivity disorder in Norway. PLoS ONE 2015, 10, e0122501.
- De Jong, S.; Newhouse, S.J.; Patel, H.; Lee, S.; Dempster, D.; Curtis, C.; Paya-Cano, J.; Murphy, D.; Wilson, C.E.; Horder, J.; et al. Immune signatures and disorder-specific patterns in a cross-disorder gene expression analysis. Br. J. Psychiatry 2016, 209, 202–208.
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.P.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654.
- Oades, R.D.; Myint, A.-M.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: An exploration of associations of cytokines and kynurenine metabolites with symptoms and attention. Behav. Brain Funct. 2010, 6, 32.
- Irwin, M.; Belendiuk, K.; McCloskey, K.; Freedman, D.X. Tryptophan metabolism in children with attentional deficit disorder. Am. J. Psychiatry 1981, 138, 1082–1085.
- Oades, R.D.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J.; Myint, A.-M. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism-effects of medication. Behav. Brain Funct. 2010, 6, 29.
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233.
- Pillinger, T.; Osimo, E.F.; Brugger, S.; Mondelli, V.; McCutcheon, R.A.; Howes, O.D. A meta-analysis of immune parameters, variability, and assessment of modal distribution in psychosis and test of the immune subgroup hypothesis. Schizophr. Bull. 2019, 45, 1120–1133.
- Kozłowska, E.; Brzezińska-Błaszczyk, E.; Agier, J.; Wysokiński, A.; Żelechowska, P. Alarmins (IL-33, sST2, HMGB1, and S100B) as potential biomarkers for schizophrenia. J. Psychiatr. Res. 2021, 138, 380–387.
- Chang, J.P.; Su, K.P.; Mondelli, V.; Pariante, C.M. Cortisol and inflammatory biomarker levels in youths with attention deficit hyperactivity disorder (ADHD): Evidence from a systematic review with meta-analysis. Transl. Psychiatry 2021, 11, 430.
- Chang, J.P.; Mondelli, V.; Satyanarayanan, S.K.; Chiang, Y.J.; Chen, H.T.; Su, K.P.; Pariante, C.M. Cortisol, inflammatory biomarkers and neurotrophins in children and adolescents with attention deficit hyperactivity disorder (ADHD) in Taiwan. Brain Behav. Immun. 2020, 88, 105–113.
- Yang, L.L.; Stiernborg, M.; Skott, E.; Söderström, Å.; Giacobini, M.; Lavebratt, C. Proinflammatory mediators and their associations with medication and comorbid traits in children and adults with ADHD. Eur. Neuropsychopharmacol. 2020, 41, 118–131.
- Elsadek, A.; Al-shokary, A.; Abdelghani, W.; Kamal, N.; Ibrahim, A.; El-shorbagy, H.; Suliman, H.; Barseem, N.; Abdel Maksoud, Y.; Azab, S.; et al. Serum levels of interleukin-6 and tumor necrosis factor alpha in children with attention-deficit hyperactivity disorder. J. Pediatr. Neurosci. 2020, 15, 402–408.
- Donfrancesco, R.; Nativio, P.; Borrelli, E.; Giua, E.; Andriola, E.; Villa, M.P. Serum cytokines in paediatric neuropsychiatric syndromes: Focus on Attention Deficit Hyperactivity Disorder. Minerva Pediatr. 2016, 73, 398–404.
- Misiak, B.; Wójta-Kempa, M.; Samochowiec, J.; Schiweck, C.; Aichholzer, M.; Reif, A.; Samochowiec, A.; Stańczykiewicz, B. Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 118, 110581.
- Corominas-Roso, M.; Armario, A.; Palomar, G.; Corrales, M.; Carrasco, J.; Richarte, V.; Ferrer, R.; Casas, M.; Ramos-Quiroga, J.A. IL-6 and TNF-α in unmedicated adults with ADHD: Relationship to cortisol awakening response. Psychoneuroendocrinology 2017, 79, 67–73.
- Leffa, D.T.; Caye, A.; Santos, I.; Matijasevich, A.; Menezes, A.; Wehrmeister, F.C.; Oliveira, I.; Vitola, E.; Bau, C.H.D.; Grevet, E.H.; et al. Attention-deficit/hyperactivity disorder has a state-dependent association with asthma: The role of systemic inflammation in a population-based birth cohort followed from childhood to adulthood. Brain Behav. Immun. 2021, 97, 239–249.
- Oades, R.D. An exploration of the associations of pregnancy and perinatal features with cytokines and tryptophan/kynurenine metabolism in children with attention-deficit hyperactivity disorder (ADHD). Atten. Deficit Hyperact. Disord. 2011, 3, 301–318.
- Llorens, M.; Barba, M.; Torralbas, J.; Nadal, R.; Armario, A.; Gagliano, H.; Betriu, M.; Urraca, L.; Pujol, S.; Montalvo, I.; et al. Stress-related biomarkers and cognitive functioning in adolescents with ADHD: Effect of childhood maltreatment. J. Psychiatr. Res. 2022, 149, 217–225.
- Isaksson, J.; Nilsson, K.W.; Lindblad, F. Early psychosocial adversity and cortisol levels in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2013, 22, 425–432.
- Scassellati, C.; Bonvicini, C.; Faraone, S.V.; Gennarelli, M. Biomarkers and attention-deficit/hyperactivity disorder: A systematic review and meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1003–1019.e1020.




