Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guido Gembillo | -- | 2293 | 2023-01-25 16:40:43 | | | |
| 2 | Sirius Huang | Meta information modification | 2293 | 2023-01-28 03:32:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gembillo, G.; Labbozzetta, V.; Giuffrida, A.E.; Peritore, L.; Calabrese, V.; Spinella, C.; Stancanelli, M.R.; Spallino, E.; Visconti, L.; Santoro, D. Role of Copper in Diabetic Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/40498 (accessed on 07 February 2026).
Gembillo G, Labbozzetta V, Giuffrida AE, Peritore L, Calabrese V, Spinella C, et al. Role of Copper in Diabetic Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/40498. Accessed February 07, 2026.
Gembillo, Guido, Vincenzo Labbozzetta, Alfio Edoardo Giuffrida, Luigi Peritore, Vincenzo Calabrese, Claudia Spinella, Maria Rita Stancanelli, Eugenia Spallino, Luca Visconti, Domenico Santoro. "Role of Copper in Diabetic Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/40498 (accessed February 07, 2026).
Gembillo, G., Labbozzetta, V., Giuffrida, A.E., Peritore, L., Calabrese, V., Spinella, C., Stancanelli, M.R., Spallino, E., Visconti, L., & Santoro, D. (2023, January 25). Role of Copper in Diabetic Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/40498
Gembillo, Guido, et al. "Role of Copper in Diabetic Kidney Disease." Encyclopedia. Web. 25 January, 2023.
Copy Citation
Copper is a fundamental element for the homeostasis of the body. It is the third most abundant essential transition metal in humans. Changes in the concentration of copper in the blood are responsible for numerous diseases affecting various organs, including the heart, brain, kidneys, and liver. One of the most interesting aspects of copper balance is its influence on diabetes and the progression of its complications, such as diabetic kidney disease (DKD).
Diabetic Kidney Disease
copper
diabetes
zinc
diabetic nephropathy
chronic kidney disease
gestational diabetes mellitus
1. Introduction
Copper plays an important role in the regulation of numerous enzymes and the synthesis of structural components and is involved in many physiological pathways and biological processes including angiogenesis, response to hypoxia, and neuromodulation [1].
A key role in preventing copper deficiency or toxicity is played by the P-type Wilson ATPase, which is responsible for transporting copper from the liver into the secretory pathway (about 50% of copper is excreted via bile, the rest via other gastrointestinal secretions) [2]. Mutations of this gene lead to a lack of copper transport from the liver into the bile and to a deficient incorporation of copper into ceruloplasmin. Other copper-containing enzymes are: Zinc-Cu superoxide dismutase, which plays a fundamental role in oxidative processes; dopamine mono-oxygenase, which is involved in the synthesis of neurotransmitters; lysyl oxidase, which is involved in bone formation; Leiden factor V, the deficiency of which leads to coagulation disorders; cytochrome C oxidase, the deficiency of which can manifest itself through several systemic symptoms [3].
Copper deficiency is characterized by hair and skin changes, muscle weakness, neurological disorders such as ataxia, neuropathy and cognitive impairment, edema, hepatosplenomegaly, and osteoporosis. It can also lead to anemia and neutropenia, the main hematologic features of copper deficiency.
In addition, copper is involved in processes that regulate oxidative stress (OS). Under physiological conditions, there is a balance between the products of metabolic processes that use oxygen (O2) as fuel for energy production, the so-called reactive oxygen species (ROS) and antioxidant agents. When this balance is disturbed, an increase in circulating ROS leads to the phenomenon of OS, which can cause damage to several cellular structures. If not adequately controlled, OS may be involved in the development of chronic and/or degenerative diseases such as cancer and cardiovascular disease [4]. In addition, minor copper deficiencies may contribute to the onset and progression of several pathologies, including diabetes. On the other hand, excessive copper concentration in the body can cause toxicity in many human organs, resulting in various diseases and, in rare cases, death.
Diabetes and Diabetic Kidney Disease (DKD) represent a real pandemic problem both for the public economy and for global health [5]. The development of novel therapies has helped to counteract this global phenomenon and ensure a more personalized approach, but dietary regulation and the adequate intake of essential elements are an indispensable aspect of treatment strategies [6][7].
In the kidney, a correct balance of copper seems to be essential: an increased blood concentration of this ion in the kidney may condition its renal deposition, leading to nephrotoxicity associated with interstitial damage that can lead to progressive renal function impairment [8]. Copper excretion in the urine may be related to dissociation from the albumin–copper complex of the serum as it passes through the kidney. In diabetics with progressive renal dysfunction, urinary excretion of this element may be due to dissociations of both albumin–copper and ceruloplasmin–copper complexes filtering through the damaged glomerulus. Urinary copper overload of the altered renal tubules may play a role in the progression of renal dysfunction in patients with advanced CKD.
Previous studies have shown that hypercupremia is associated with the development of Chronic Kidney Disease (CKD) [9]. On the contrary, a reduction in the renal filtration rate leads to impaired renal excretion of copper and consequently to increased blood concentrations with corresponding potential complications [10].
The Mendelian randomization study by Ahmad et al. reports that genetically determined elevated circulating copper levels may be a causal risk factor for CKD and could possibly reduce estimated glomerular filtration rate (eGFR) and rapidly declining renal function [11]. A cross-sectional study of 3553 adults from Hunan, China, found that copper in urine is a risk factor for impaired kidney function [12]. Guo et al. demonstrated that whole blood copper levels were remarkably related to CKD risk and showed a positive dose–response relationship in the elderly Chinese population [13]. However, in another nested case-control study of 350 adolescents in northwestern Nicaragua, urinary Cu levels were found to have no significant association with loss of renal function in participants at risk for CKD of unclear etiology [14].
All of these findings show that the interaction between copper and kidney disease goes both ways, as imbalances in the homeostasis of circulating copper levels can also be associated with altered renal excretion and changes in protein metabolism in patients with CKD and with disease progression.
2. Role of Copper in Diabetic Kidney Disease
Renal failure and diabetes are also associated with disturbances in antioxidant homeostasis and chronic inflammation.
The study by Stancic A et al. [15] compared the activity of copper-zinc SOD in diabetic hypertensive patients with or without renal insufficiency and a control group. The results showed that SOD activity was significantly higher in diabetics with renal insufficiency, suggesting that disturbances in antioxidant homeostasis are associated with complications of diabetes such as hypertension and renal failure.
The extent of copper excretion in urine was associated with the different stages of DKD. In studies by Ito S. et al. [16], 41 type 2 diabetic patients with different stages of nephropathy and 10 healthy controls were recruited and serum copper/albumin and copper/ceruloplasmin ratios were determined and tested whether they tended to dissociate in response to changes in urine pH. The results showed that urinary copper was significantly increased only in patients with macroalbuminuria. Urinary copper/ceruloplasmin and copper/albumin ratios were greater than in serum and equal between patients and healthy controls, except for the copper/albumin ratio in patients with macroalbuminuria. Reports in urine decreased when nephropathy worsened. Copper tends to dissociate from its carrier protein under acidic pH conditions. A damaged glomerulus due to nephropathy may cause greater dissociation of the copper/albumin and copper/ceruloplasmin complexes, and urinary copper overload may, in turn, play an important role in the progression of nephropathy (Figure 1).
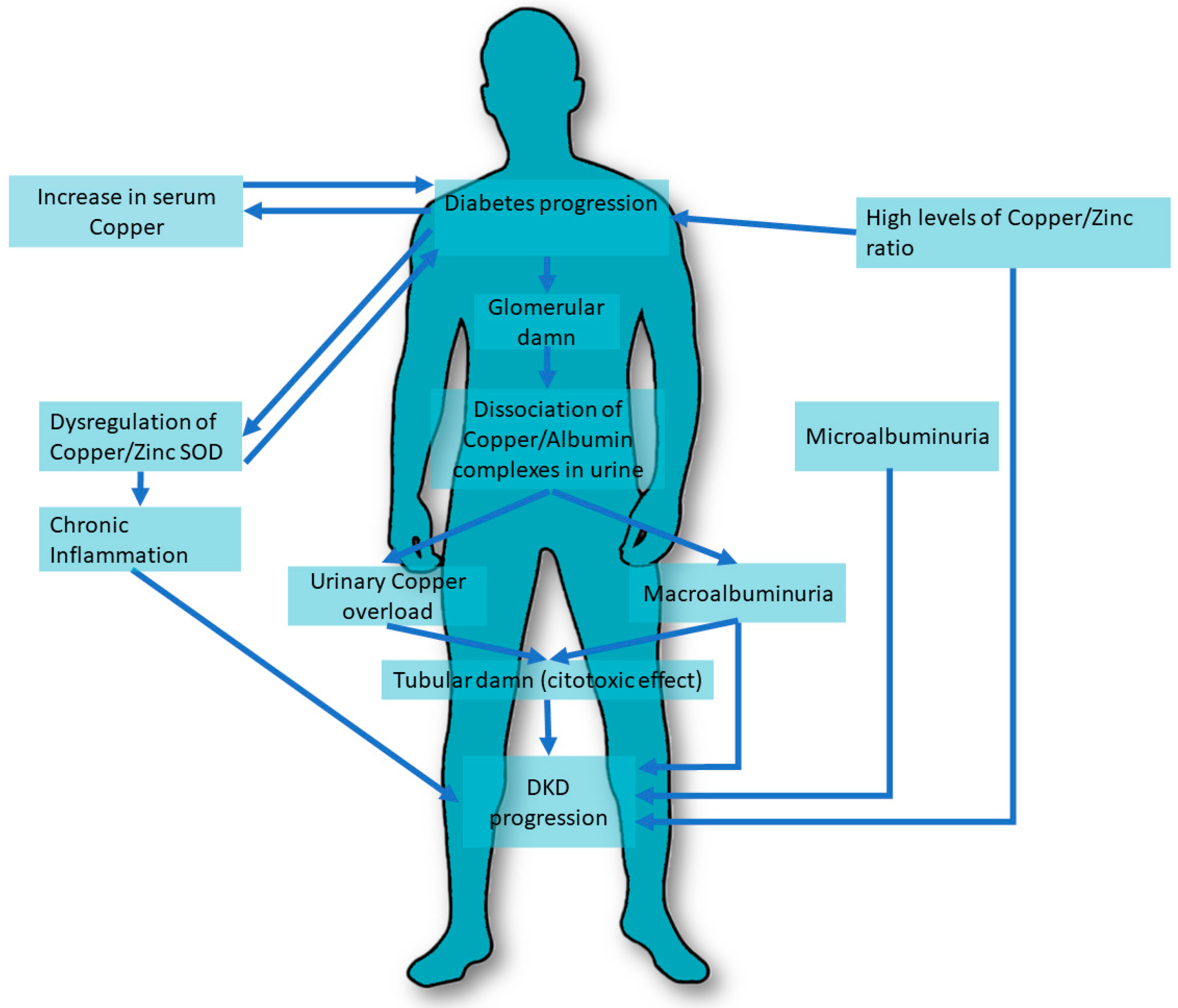
Figure 1. Copper pathways involved in DKD. DKD, Diabetic Kidney Disease. SOD, superoxide dismutase.
Copper is essential to ensure cardiovascular wellbeing. Several studies have shown that a deficiency of this ion may be a risk factor for the development of cardiovascular disease, especially in patients with T2DM with and without DKD. An example of this is the study by Al-Bayati et al. [17], which compared 55 patients with type 2 diabetes divided into two subgroups (the first group n = 31 with microalbuminuria between 30 and 299 μg/mg and a second group n = 29) with an albumin level below 30 μg/mg with 37 healthy subjects. The data showed an increase in urinary copper excretion in the group with microalbuminuria associated with a decrease in antioxidant enzymes compared to the control group, p < 0.05.
Talaei et al. [18] studied urinary copper levels in T2DM patients with microalbuminuria compared to patients without albuminuria by examining 42 patients with DKD and comparing them to a group of 40 healthy subjects. The 24h urinary copper levels were 36.14 μcg/L (14.54–57.74) and 14.77 μcg/L (10.17–19.37) in the case and control groups, respectively (p = 0.003). Diabetics with microalbuminuria appeared to have a greater urinary excretion in the 24 h, although a toxic effect of this high excretion in the progression of DKD cannot be excluded.
An important mechanism to evaluate the copper homeostasis is its association with zinc levels. Zinc is fundamental for the function of the antioxidant enzyme copper–zinc SOD, and an appropriate balance between these two micronutrients is of pivotal importance to controlling inflammation and reducing risk factors associated with DKD [19].
Several authors have associated the Zn/Cu ratio with glycemic status, renal function, and metabolic parameters in patients with and without T2DM. Hamasaki et al. [20] conducted a cross-sectional study of 149 diabetic and 206 non-diabetic patients measuring the levels of Zn and Cu, their ratio, the prevalence of type 2 diabetes, and the degree of renal function. A high Zn/Cu ratio was associated with improved renal function scores (β = 0.137, p = 0.014) and a reduced risk of poor glycemic control in patients with type 2 diabetes, which was assessed by multivariate logistic regression analysis (HbA1c ≥ 7%) (odds ratio = 0.382; 95% confidence interval, 0.165–0.884; p = 0.025).
In another cross-sectional study conducted by Takao et al. [21], the authors analyzed data from the Asahi Diabetes Complications Study to assess the role of the copper/zinc ratio in the DKD population. These data showed that a higher value of this ratio was associated with a higher prevalence of renal involvement during the course of T2DM.
While several studies have demonstrated a possible association between urinary copper excretion and DKD progression, the results related to serum copper levels are still controversial. Serum copper concentration appears to be altered in T1DM and T2DM patients compared to controls, with and without DKD [1][2][8][22]. These data are in contrast to the data of Prabodh et al. [23], who showed that there are no differences in serum copper levels in the patients with DKD compared to a group of healthy subjects. A group of 40 DKD patients and 40 control subjects were compared and fasting glucose, post-meal glucose, glycated hemoglobin, microalbuminuria, copper, and magnesium levels were determined. The results showed that the mean concentrations of fasting and postprandial glycemia, glycated hemoglobin, and microalbuminuria were significantly higher in the patients than in the control group. Mean copper levels in the DKD group, 165.42 ± 5.71 μg/dL, showed no significant differences compared with controls, 166.6 ± 5.48 μg/dL, (p> 0.05). These results suggest that hypomagnesemia may be related to the development of DKD, and copper levels do not seem to play a prominent role in the development of DKD.
The available literature confirms a possible association between urinary copper excretion and DKD, while the role of serum levels of this ion needs further investigation. The copper/zinc ratio may be a useful biomarker for both DM and the treatment of DKD: the correct balance of these two ions seems to counteract inflammatory processes and to be associated with adequate metabolic homeostasis in the DM population [24] (Table 1).
Table 1. Copper in Diabetic Kidney Disease. Cu, Copper. SOD, Superoxide Dismutase. T2DM, Type 2 Diabetes Mellitus. Zn, Zinc.
| DIABETIC KIDNEY DISEASE | Study, Year | Population | Control | Results |
| Ito et al. [16] 2001 Case-Control Study |
Group I: 15 diabetic patients with normoalbuminuria Age 60 ± 7 Group II: 14 diabetic patients with microalbuminuria Age 61 ± 9 Group III: 12 patients with macroalbuminuria Age 66 ± 8 |
10 healthy subjects Age 56 ± 10 |
Serum copper levels, serum ceruloplasmin levels, and serum copper/ceruloplasmin ratio were not different among the four groups Serum copper/albumin ratio increased in group III in comparison with group I (p < 0.05) Urinary copper concentration did not differ between groups I, II, and the control group, but its concentration was significantly higher in group III compared to other groups p < 0.001. Urinary cerulopasmin concentration significantly increased in group III in comparison with the other groups (p < 0.001), and it also increased in group II when compared with group I and the control group (p < 0.001). The copper/ceruloplasmin ratio in urine remarkably decreased in group III in comparison with the other groups (p < 0.001), and it also significantly decreased in group II when compared with group I and the control group (p < 0.001). The urinary copper/albumin ratio also decreased in group III compared to the other groups (p < 0.01) and decreased in group II in comparison with group I and control group (p < 0.001). Increase in urinary ceruloplasmin concentration correlated with urinary concentration of NAG (r = 0.846, p < 0.001)) and alfa-1-microglobulin (r = 0.608, p < 0.001) NAG and ·alfa-1-microglobulin concentrations in group II slightly increased in comparison with those in group I and control group (p < 0.05). NAG in group I was slightly higher than that in control group (p < 0.05). NAG and alfa- 1-microglobulin concentrations were clearly higher in group III than in the other groups (p < 0.001 and p < 0.01 |
|
| Talaei et al. [18] 2011 Cross-sectional study |
42 TD2M patients with microalbuminuria | 40 T2DM patients without microalbuminuria | Higher 24h urinary copper levels in microalbuminuria group compared to control group 36.14 μcg/L(14.54–57.74) vs. 14.77 μcg/L(10.17–19.37) p = 0.003 No significant difference between different subgroups based on HbA1C% levels |
|
| Prabodh et al. [23] 2011 Case-control Study |
40 patients with DKD Age 45–70 years |
40 healthy subjects Age matched |
No significant difference in serum copper levels between DKD group (165.42 ± 5.71 μg/dL) and control group (166.6 ± 5.48 μg/dL) (p> 0.05). No relation of Cu with microalbumin in DKD patients |
|
| Stancic et al. [15] 2012 Case-Control Study |
Hypertensive diabetic patients with or without renal insufficiency | Healthy subjects | Copper zinc superoxide dismutase activity was higher only in hypertensive diabetic with renal insufficiency | |
| Al Bayati et al. [17] 2015 Cross-sectional Study |
Group I: 31 T2DM patients with microalbuminuria between 30 and 299 μg/mg Age 49.5 ± 7.6 years Group II: 29 T2DM patients with microalbuminuria below 30 μg/mg Age 52.2 ± 8.2 years |
37 healthy subjects Age 48.9 ± 8.9 years |
Group I showed a significant increase in urinary Cu/creatinine ratio compared with controls: 53.3 ± 3.2 vs. 44.2 ± 5.3 p < 0.05 No significant difference between Group II and controls in urinary Cu/creatinine ratio SOD was significantly decreased in group I compared to control group 30.6 ± 3.3 vs. 45 ± 6 p < 0.05 No significant difference between Group II and controls in SOD |
|
| Hasamaki et al. [20] 2016 Cross-Sectional Study |
149 T2DM patients Age 61.1 ± 17.6 years |
206 non-diabetic patients | A high Zn/Cu ratio was associated with improved renal function levels (β = 0.137, p = 0.014) A high Zn/Cu ratio was associated with reduced risk of poor glycemic control in patients with type 2 diabetes, assessed by multivariate logistic regression analysis. (HbA1c ≥ 7%) (odds ratio = 0.382; 95% confidence interval, 0.165–0.884; p = 0.025) |
|
| Takao et al. [21] 2022 Cross-sectional Study |
651 patients with T2DM Age 65.1 ± 9.7 years |
No control group | Diabetic kidney disease was identified in 220 patients A Higher Cu/Zn ratios is correlated with more frequent renal involvement Higher Cu levels in DKD patients compared to non DKD patients (100.5–15.5 vs. 97.0–15.6) p = 0.007 Higher Cu/Zn ratio in DKD patients compared to non DKD patients (1.247–0.265 vs. 1.155–0.242) p < 0.0001 |
References
- Brewer, G.J. Copper in medicine. Curr. Opin. Chem. Biol. 2003, 7, 207–212.
- Prohaska, J.R. Biochemical functions of copper in animals. In Essential and Toxic Trace Elements in Human Health and Disease; Prasad, A.S., Ed.; Alan R Liss: New York, NY, USA, 1988.
- Danks, D.M. Copper deficiency in humans. Annu. Rev. Nutr. 1988, 8, 235–257.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Gembillo, G.; Ingrasciotta, Y.; Crisafulli, S.; Luxi, N.; Siligato, R.; Santoro, D.; Trifirò, G. Kidney Disease in Diabetic Patients: From Pathophysiology to Pharmacological Aspects with a Focus on Therapeutic Inertia. Int. J. Mol. Sci. 2021, 22, 4824.
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808.
- Amatruda, M.; Gembillo, G.; Giuffrida, A.E.; Santoro, D.; Conti, G. The Aggressive Diabetic Kidney Disease in Youth-Onset Type 2 Diabetes: Pathogenetic Mechanisms and Potential Therapies. Medicina 2021, 57, 868.
- Niu, Y.Y.; Zhang, Y.Y.; Zhu, Z.; Zhang, X.Q.; Liu, X.; Zhu, S.Y.; Song, Y.; Jin, X.; Lindholm, B.; Yu, C. Elevated intracellular copper contributes a unique role to kidney fibrosis by lysyl oxidase mediated matrix crosslinking. Cell Death Dis. 2020, 11, 211.
- Iyanda, A.A.; Anetor Adeniyi, F.A. Altered copper level and renal dysfunction in Nigerian women using skin-whitening agents. Biol. Trace Elem. Res. 2011, 143, 1264–1270.
- Sondheimer, J.H.; Mahajan, S.K.; Rye, D.L.; Abu-Hamdan, D.K.; Migdal, S.D.; Prasad, A.S.; McDonald, F.D. Elevated plasma copper in chronic renal failure. Am. J. Clin. Nutr. 1988, 47, 896–899.
- Ahmad, S.; Ärnlöv, J.; Larsson, S.C. Genetically Predicted Circulating Copper and Risk of Chronic Kidney Disease: A Mendelian Randomization Study. Nutrients 2022, 14, 509.
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328.
- Guo, F.; Lin, Y.; Meng, L.; Peng, L.; Zhang, H.; Zhang, X.; Jin, M.; Wang, J.; Zhang, Y.; Tang, M.; et al. Association of copper exposure with prevalence of chronic kidney disease in older adults. Clin. Nutr. 2022, 41, 2720–2728.
- Smpokou, E.T.; González-Quiroz, M.; Martins, C.; Alvito, P.; Le Blond, J.; Glaser, J.; Aragón, A.; Wesseling, C.; Nitsch, D.; Pearce, N.; et al. Environmental exposures in young adults with declining kidney function in a population at risk of Mesoamerican nephropathy. Occup. Environ. Med. 2019, 76, 920–926, Erratum in Occup. Environ. Med. 2020, 77, 586.
- Stancic, A.; Rasic-Milutinovic, Z.; Perunicic-Pekovic, G.; Buzadzic, B.; Korac, A.; Otasevic, V.; Jankovic, A.; Vucetic, M.; Korac, B. Relation of CuZnSOD activity with renal insufficiency in hypertensive diabetic patients. Indian J. Biochem. Biophys. 2012, 49, 97–100.
- Ito, S.; Fujita, H.; Narita, T.; Yaginuma, T.; Kawarada, Y.; Kawagoe, M.; Sugiyama, T. Urinary copper excretion in type 2 diabetic patients with nephropathy. Nephron 2001, 88, 307–312.
- Al-Bayati, M.A.; Jamil, D.A.; Al-Aubaidy, H.A. Cardiovascular effects of copper deficiency on activity of superoxide dismutase in diabetic nephropathy. N. Am. J. Med. Sci. 2015, 7, 41–46.
- Talaei, A.; Jabari, S.; Bigdeli, M.H.; Farahani, H.; Siavash, M. Correlation between microalbuminuria and urinary copper in type two diabetic patients. Indian J. Endocrinol. Metab. 2011, 15, 316–319.
- Gembillo, G.; Visconti, L.; Giuffrida, A.E.; Labbozzetta, V.; Peritore, L.; Lipari, A.; Calabrese, V.; Piccoli, G.B.; Torreggiani, M.; Siligato, R.; et al. Role of Zinc in Diabetic Kidney Disease. Nutrients 2022, 14, 1353.
- Hamasaki, H.; Kawashima, Y.; Yanai, H. Serum Zn/Cu Ratio Is Associated with Renal Function, Glycemic Control, and Metabolic Parameters in Japanese Patients with and without Type 2 Diabetes: A Cross-sectional Study. Front. Endocrinol. 2016, 7, 147.
- Takao, T.; Yanagisawa, H.; Suka, M.; Yoshida, Y.; Onishi, Y.; Tahara, T.; Kikuchi, T.; Kushiyama, A.; Anai, M.; Takahashi, K.; et al. Synergistic association of the copper/zinc ratio under inflammatory conditions with diabetic kidney disease in patients with type 2 diabetes: The Asahi Diabetes Complications Study. J. Diabetes Investig. 2022, 13, 299–307.
- Isbir, T.; Tamer, L.; Taylor, A.; Isbir, M. Zinc, copper and magnesium status in insulin-dependent diabetes. Diabetes Res. 1994, 26, 41–45.
- Prabodh, S.; Prakash, D.S.; Sudhakar, G.; Chowdary, N.V.; Desai, V.; Shekhar, R. Status of copper and magnesium levels in diabetic nephropathy cases: A case-control study from South India. Biol. Trace Elem. Res. 2011, 142, 29–35.
- Laouali, N.; MacDonald, C.J.; Shah, S.; El Fatouhi, D.; Mancini, F.R.; Fagherazzi, G.; Boutron-Ruault, M.C. Dietary Copper/Zinc Ratio and Type 2 Diabetes Risk in Women: The E3N Cohort Study. Nutrients 2021, 13, 2502.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
28 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




