Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tudor Sorin Pop | -- | 1564 | 2023-01-23 06:53:28 | | | |
| 2 | Peter Tang | Meta information modification | 1564 | 2023-01-30 08:57:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Feier, A.M.; Pop, T.S.; Borodi, P.; Zuh, S.; Oprișan, A.; Russu, O.; Bațagă, T. Orthopedic Biocompatible Implant. Encyclopedia. Available online: https://encyclopedia.pub/entry/40479 (accessed on 07 February 2026).
Feier AM, Pop TS, Borodi P, Zuh S, Oprișan A, Russu O, et al. Orthopedic Biocompatible Implant. Encyclopedia. Available at: https://encyclopedia.pub/entry/40479. Accessed February 07, 2026.
Feier, Andrei Marian, Tudor Sorin Pop, Paul-Gabriel Borodi, Sándor-György Zuh, Andrei Oprișan, Octav Russu, Tiberiu Bațagă. "Orthopedic Biocompatible Implant" Encyclopedia, https://encyclopedia.pub/entry/40479 (accessed February 07, 2026).
Feier, A.M., Pop, T.S., Borodi, P., Zuh, S., Oprișan, A., Russu, O., & Bațagă, T. (2023, January 23). Orthopedic Biocompatible Implant. In Encyclopedia. https://encyclopedia.pub/entry/40479
Feier, Andrei Marian, et al. "Orthopedic Biocompatible Implant." Encyclopedia. Web. 23 January, 2023.
Copy Citation
A biomaterial is a nonviable material used in a medical device, intended to interact with biological systems. In the field of orthopedics, implantology challenges emerge at the border of local reactions to metallic implants with personalized implant surfaces and general inflammatory reactions as a result of host–implant response.
biocompatibility
implant
degenerative joint diseases
1. Introduction
An increasing number of diagnosed degenerative joint pathologies in the foreseeable near future is projected [1]. General increase in life expectancy and quality of life facilitates patients access to regular medical examinations and are expected to increase the prevalence of certain degenerative joint diseases [2]. Secondary to this, a growth in their incidence both nationally and globally is also expected, bringing a rising demand for innovative and durable implantable devices in the field of orthopedics [3]. Currently, the prevalence of degenerative joint disease on the Eastern European continent is rising (13.4% in 2020) which drags demand for large-scale implantable devices [4]. A highlight in increasing prevalence of degenerative joint diseases in younger people is also anticipated. In addition to socio-economic and functional impact, there is the issue of properties related to mechanical strength, osseointegration and durability of the implant. Enhancing various properties of a specific implant requires a multidisciplinary approach with aid from interconnection of different specialties such as: material engineering, cell biology, orthopedic surgery and not only [5].
Presently, the world is endorsing a growing need for materials and implants of various tissues and the permanent development of cell culture in vitro studies is an upright practical instrument for investigating the biocompatibility of future implantable materials. As a response to the well-known three “Rs” (“reduction”, “refinement” and “replacement”) brought to literature by English academics in the 1960s, in vitro biocompatibility research has been reduced and, in most cases, diminished to laboratory studies that no longer or drastically reduce animal sacrifice [6].
2. Biomaterial: Brief History and Definition
There are plenty of historical descriptions and reports of procedures for introducing different types of devices in the human body [1][2]. These ancient reports include procedures performed for replacing teeth, different bony structures or wound regeneration attempts [3]. However, biocompatible materials did not exist as we distinguish them today. Throughout history, the word “biomaterial” per se was not used in academic language and it was reported under distinctive names. The term was mostly synonymous with “implantable device”, “prosthesis”, “material augment”, etc. In the mid-18th century, as scientific communities became more robust and industrialized, the area of implantable materials gained additional popularity.
It was in the middle of 19th century when conferences and scientific gatherings around the world began methodically focusing on implantable devices and their usage as replacements in different anatomic parts. The first “almost-definition” of a biomaterial was made in 1967 by pioneer orthopedic surgeon Jonathan Cohen [4]. He defined all materials (metals, bone and derivatives used as bone grafts, plastics ceramics and composites) as “biomaterials” excluding drugs and fabrics used for sutures [5].
Only two years later, several symposiums were organized focusing predominantly on materials and their use for reconstructive surgery. Society For Biomaterials was founded by Dr. William Hall and his colleagues in 1974 with the aid of visionary bioengineers from Clemson University [6]. Therefore, a newly emerged organization was established and was set to accurately institute a new definition for the concept of biomaterials: “A biomaterial is a systematically, pharmacologically inert substance designed for implantation within or incorporation with a living system” [7].
Further on, a definition published by British professor David F. Williams gained wide criticism throughout previous decades [8][9]. He stated that a biomaterial is “a nonviable material used in a medical device, intended to interact with biological systems” [10]. It not only lacked explanations made on what precisely “nonviable” means, but the exclusion of biological tissues (bone grafts, tendons, ligaments, etc.) combined with various pharmacological products conveyed numerous controversies in the literature. As of today, half a century after the first attempts, the definition does not seem too far away but definitely multidisciplinary. The latest definition was proposed in United Kingdom in 1986 and approved afterwards in 1991 in a proceedings paper of Consensus Conference held by the European Society for Biomaterials: “Any substance or combination of substances, other than drugs, synthetic or natural in origin, which can be used for any period of time, which augments or replaces partially or totally any tissue, organ or function of the body, in order to maintain or improve the quality of life of the individual” [11].
The state-of-the-art definition agrees clearly and analytically on its previous troubling mentions and avoids almost every bias. It is a well-defined traceable result of several multidisciplinary meetings, mutual agreements and pooled opinions.
3. The Orthopedic Biomaterial
Up until a few decades ago, a new biomaterial introduced in the commercial lines of implantable devices manufacturers consisted of new bulk technologies such as: stents, wires, titanium cerclages, biodegradable screws and so on. Emerging concepts in present-day technology include extremely advanced biomaterials and include targeting nanocarriers with specially designed localized delivery systems [12]. In the field of orthopedics, implantology challenges emerge at the border of local reactions to metallic implants with personalized implant surfaces and general inflammatory reactions as a result of host–implant response [13]. While characteristics and occurrences of implantable device allergies seem to be left aside [14], an in-depth breakdown of adverse local reactions and methods of improving bone–implant interface osseointegration seem to arise [15].
Another breaking topic of significance remains around improving implants surface at a micro and more innovative, at nano scale level. This mainly consists of coating specific surfaced areas of implants [16][17] (e.g., trochanteric region of femoral stem, femoral condyle region of knee prosthesis). These procedures are generally advancing with numerous in vitro studies and subsequently implemented in clinical trials [18]. A detailed look at the steps involved in defining a good biocompatible implant is described in Figure 1.

Figure 1. Steps that are mandatory to bring potentially innovative biomaterial technology to an implantable level.
Orthopedic surgeons usually quantify the performance of an implant based on the rate of early aseptic revision of the surgical intervention (e.g., revision total hip arthroplasty). This is certainly biased due to implant malposition and technique errors. An example of quickly emerging basic science into clinical science are hip arthroplasty liners infused with Vitamin E [18]. Based on this and with a primordial aim of reducing revision rates and increasing commercial power, implant manufacturers bring innovative in vitro ideas to in vivo. This latest addition of Vitamin E to hip replacement polyethylene liners was found to reduce revision rates by 46% in a short-term follow-up meta-analysis [19].
Another novel, promising category of biomaterials is osteoinductive materials. There are several methods for imparting osteoinductive properties into scaffolds: surface modification, inclusion of growth factors (transforming growth factor, morphogenetic proteins, endothelial growth factors, etc.) and stem cells deposits [20]. Several authors reported methods for dual-delivery of growth factors or other constituents into wounded areas with the final aim of stimulating bone formation [21]. Incorporating nanoparticles into the scaffold used for bone tissue engineering is yet another method that may be used to deliver growth factors in applications related to orthopedics. The encapsulation of proteins into nanoparticles, which are subsequently transported via scaffolds, would allow for more precise control of their release and would provide the long-term sustained release patterns sought for particular growth factors [22].
In the field of musculoskeletal medicine and orthopedics, scientists are continuously pursuing an ideal substrate biomaterial to use as a delivery system for therapeutic stem cell use [21]. Almost any type of damaged tissue in the human body is susceptible to regeneration processes and subsequent immune regenerative reactions. Regenerative approaches using stem cells are the diamond of our medical generation and continue to rise with every new basic science breakthrough [20]. Stem cell encapsulation is a novel concept in which a population of cells are restrained and temporarily blocked inside a biocompatible matrix substrate [22]. The substrate allows for O2 and metabolites to pass through and provides several benefits [23]. It is currently used in minimally invasive cartilage procedures for delivering collagen and hyaluronic acid molecules to specific areas intra-articularly. Delivery includes a controlled and continuous supply of therapeutic agents and protection from host–immune cell reactions [24]. Bone specific clinical use includes bioactive constituents filled with encapsulated stem cells able to fill bone defects after trauma, tumor removals or revision arthroplasties [24].
It is undeniable that tissue engineering, combined with suitable, thoughtful basic science ideas and lab studies that are consequently applied clinically are the future of biomedicine.
4. From Laboratory to Clinical Practice: In Vitro Biocompatibility Testing
In the previous three decades, immense attention was ascribed to in vitro biocompatibility studies of novel biomaterials, consequently being detrimental to in vivo studies. An expectable outcome was a progress on cell-biomaterial interaction theories (cell adhesion, proliferation, viability, material roughness, surface adaptation, etc.) that resulted in a fast development of novel in vitro study models, products and their implementation in clinical practice [25].
After identifying the need for an improvement of a biomaterial, surface or feature, a clear description of novel mechanical properties is established. Cytotoxic testing commonly begins simultaneously with cell culture analysis in vitro and according to several authors they are followed by fluorescent staining of different types [26]. Culture testing implies an in-depth analysis of protein interactions and synthesis, cell viability, adhesion and proliferation processes [27]. Several particularities of each step involved are described in detail in Figure 2.
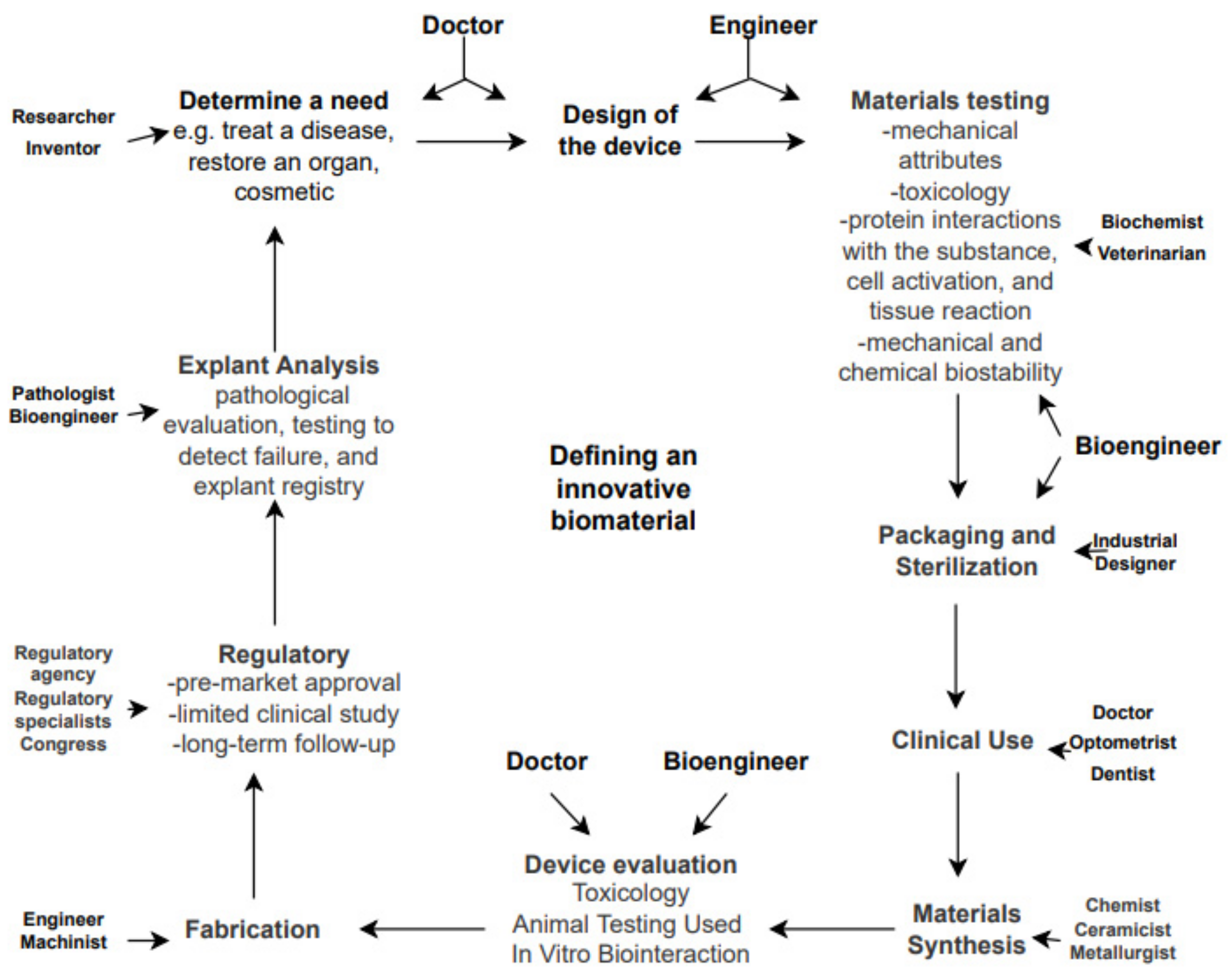
Figure 2. Disciplines involved in biomaterials science and the path from a need to a manufactured medical device.
Three main types of cells used are: tumor-derived osteoblastic cells, primary cells and commercial lines.
References
- Ko, W.H. Early History and Challenges of Implantable Electronics. ACM J. Emerg. Technol. Comput. Syst. 2012, 8, 8.
- Madry, H.; Grässel, S.; Nöth, U.; Relja, B.; Bernstein, A.; Docheva, D.; Kauther, M.D.; Katthagen, J.C.; Bader, R.; van Griensven, M.; et al. The future of basic science in orthopaedics and traumatology: Cassandra or Prometheus? Eur. J. Med. Res. 2021, 26, 56.
- Shah, J.B. The history of wound care. J. Am. Coll. Certif. Wound Spec. 2011, 3, 65–66.
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633.
- Cohen, J. Biomaterials in orthopedic surgery. Am. J. Surg. 1967, 1, 31–41.
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J. Biomaterials Science: A Multidisciplinary Endeavor; Elsevier: Amsterdam, The Netherlands, 2013.
- Park, J.B. Introduction. In Biomaterials Science and Engineering; Springer: Boston, MA, USA, 1984; ISBN 978-1-4612-9710-9.
- Todros, S.; Todesco, M.; Bagno, A. Biomaterials and Their Biomedical Applications: From Replacement to Regeneration. Processes 2021, 9, 1949.
- Zhang, K.; Ma, B.; Hu, K.; Yuan, B.; Sun, X.; Song, X.; Tang, Z.; Lin, H.; Zhu, X.; Zheng, Y.; et al. Evidence-based biomaterials research. Bioact Mater. 2022, 25, 495–503.
- Williams, D.F.; David, F. European Society for Biomaterials Definitions in biomaterials. In Proceedings of the Consensus Conference of the European Society for Biomaterials, Chester, UK, 3–5 March 1986; Elsevier: Amsterdam, The Netherlands; Chester, UK; Volume 4.
- Doherty, P.J. Biomaterial-Tissue Interfaces. In Proceedings of the Ninth European Conference on Biomaterials, Chester, UK, 9–11 September 1991; Doherty, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 1992.
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309.
- Sheng, X.; Wang, A.; Wang, Z.; Liu, H.; Wang, J.; Li, C. Advanced Surface Modification for 3D-Printed Titanium Alloy Implant Interface Functionalization. Front. Bioeng. Biotechnol. 2022, 10, 850110.
- Comino-Garayoa, R.; Cortés-Bretón Brinkmann, J.; Peláez, J.; López-Suárez, C.; Martínez-González, J.M.; Suárez, M.J. Allergies to Titanium Dental Implants: What Do We Really Know about Them? A Scoping Review. Biology 2020, 9, 404.
- Shin, Y.C.; Bae, J.-H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.-B.; Han, D.-W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 11.
- Hoskins, W.T.; Bingham, R.J.; Lorimer, M.; de Steiger, R.N. The Effect of Size for a Hydroxyapatite-Coated Cementless Implant on Component Revision in Total Hip Arthroplasty: An Analysis of 41,265 Stems. J. Arthroplast. 2020, 35, 1074–1078.
- Pap, K.; Vasarhelyi, G.; Gal, T.; Nemeth, G.; Abonyi, B.; Hangody, L.R.; Hangody, G.M.; Hangody, L. Evaluation of clinical outcomes of cemented vs uncemented knee prostheses covered with titanium plasma spray and hydroxyapatite: A minimum two years follow-up. Jt. Dis. Relat. Surg. 2018, 29, 65–70.
- Oral, E.; Wannomae, K.K.; Rowell, S.L.; Muratoglu, O.K. Diffusion of vitamin E in ultra-high molecular weight polyethylene. Biomaterials 2007, 28, 5225–5237.
- Cheng, Q.Y.; Zhang, B.F.; Wen, P.F.; Wang, J.; Hao, L.J.; Wang, T.; Cheng, H.G.; Wang, Y.K.; Guo, J.B.; Zhang, Y.M. Vitamin E-Enhanced Liners in Primary Total Hip Arthroplasty: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2021, 6, 3236679.
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen Biomater. 2018, 5, 197–211.
- Subbiah, R.; Hwang, M.P.; Van, S.Y.; Do, S.H.; Park, H.; Lee, K.; Kim, S.H.; Yun, K.; Park, K. Osteogenic/Angiogenic Dual Growth Factor Delivery Microcapsules for Regeneration of Vascularized Bone Tissue. Adv. Healthc. Mater. 2015, 4, 1982–1992.
- Wang, Z.; Wang, K.; Lu, X.; Li, M.; Liu, H.; Xie, C.; Meng, F.; Jiang, O.; Li, C.; Zhi, W. BMP-2 encapsulated polysaccharide nanoparticle modified biphasic calcium phosphate scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2015, 103A, 1520–1532.
- Shah, K. Encapsulated stem cells for cancer therapy. Biomatter 2013, 3, e24278.
- Hashemi, M.; Fatemeh, K. Application of encapsulation technology in stem cell therapy. Life Sci. 2015, 143, 139–146.
- Kulkarni, V.; Uttamani, J.R.; Asar, N.V.; Nares, S.; Tözüm, T.F. Evidence-Based Clinical Outcomes of Immediate and Early Loading of Short Endosseous Dental Implants: A Meta-analysis. Int. J. Oral Maxillofac. Implants. 2021, 36, 59–67.
- Czekanska, E.M.; Stoddart, M.J.; Ralphs, J.R.; Richards, R.G.; Hayes, J.S. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. A 2014, 102, 2636–2643.
- Tomoaia, G.; Mocanu, A.; Vida-Simiti, I.; Jumate, N.; Bobos, L.D.; Soritau, O.; Tomoaia-Cotisel, M. Silicon effect on the composition and structure of nanocalcium phosphates: In vitro biocompatibility to human osteoblasts. Mater. Sci. Eng. C 2014, 37, 37–47.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
649
Revisions:
2 times
(View History)
Update Date:
30 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




