Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khetam Sounbuli | -- | 3196 | 2023-01-20 06:28:22 | | | |
| 2 | Rita Xu | -8 word(s) | 3188 | 2023-01-28 03:08:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sounbuli, K.; Mironova, N.; Alekseeva, L. Neutrophil Heterogeneity in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/40438 (accessed on 07 February 2026).
Sounbuli K, Mironova N, Alekseeva L. Neutrophil Heterogeneity in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/40438. Accessed February 07, 2026.
Sounbuli, Khetam, Nadezhda Mironova, Ludmila Alekseeva. "Neutrophil Heterogeneity in Cancer" Encyclopedia, https://encyclopedia.pub/entry/40438 (accessed February 07, 2026).
Sounbuli, K., Mironova, N., & Alekseeva, L. (2023, January 20). Neutrophil Heterogeneity in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/40438
Sounbuli, Khetam, et al. "Neutrophil Heterogeneity in Cancer." Encyclopedia. Web. 20 January, 2023.
Copy Citation
Neutrophils represent the most abundant cell type of leukocytes in the human blood and have been considered a vital player in the innate immune system and the first line of defense against invading pathogens. Neutrophils play an active role in the immune response during cancer development. They could exhibit both pro-oncogenic and anti-tumor activities under the influence of various mediators in the tumor microenvironment. Neutrophils can be divided into several subpopulations, thus contradicting the traditional concept of neutrophils as a homogeneous population with a specific function in the innate immunity and opening new horizons for cancer therapy.
neutrophil heterogeneity
tumor-associated neutrophils
cancer therapy
1. Introduction
Neutrophils represent the most abundant cell type of leukocytes in human blood and the second most in mice [1]. Neutrophils are named for their ability to be stained with a mixture of alkaline and acidic dyes [2]. Mature neutrophils are differentiated from hematopoietic stem cells in the bone marrow in a process called granulopoiesis and are produced in high quantities, up to 1011 per day in healthy individuals [3]. They are the first line of defense against pathogens, which explains the high susceptibility of people with neutropenia to infections [4]. Neutrophils were always considered a homogeneous population with specific functions in innate immunity, most likely due to their short life span, which limited the ability to investigate their diverse activities or even expect them. The recent observations of neutrophil heterogeneity in the steady state [5], in different tissues [6], and in pathology [7][8] have dramatically altered the old paradigm of neutrophil homogeneity. The recent reconsideration of neutrophil biology was achieved thanks to advances in biotechnology, which enabled researchers to investigate cells at a single-cell resolution [9]. In cancer, neutrophil actions are diverse and heterogeneous. Neutrophil blood levels increase during cancer progression [3]. Neutrophilia is associated with poor prognosis in many cancer types [10]. In addition to quantitative changes, qualitative changes in neutrophils upon cancer were observed. These changes include alterations in neutrophil morphology and function. The observation of tumor-associated neutrophils (TANs) producing neutrophil extracellular traps (NETs) was a hint of the possible role of neutrophils in the tumor microenvironment [11]. NETs, first observed by Brinkmann et al. in 2004, are web-like structures of neutrophilic genetic material decorated with the proteins of granules [12]. Later, NETs were shown to be involved in cancer metastasis [13]. In addition to NETs, neutrophils, after recruitment to the tumor microenvironment, could gain an anti-tumor (N1) or a pro-tumor (N2) phenotype [14]. Neutrophil polarization seems to be a complicated process affected by several tumor-derived factors. Besides this classification, a high percent of neutrophils in the circulation of cancer patients were shown to have a lower density (low-density neutrophils, LDNs) [15] and to exhibit some features of immaturity and immunosuppressive function (granulocytic-myeloid-derived suppressor cells (g-MDSCs) [16]. The recently described interactions between neutrophils and tumors prompted the scientific community to develop neutrophil-based cancer therapies. Achievements in this field are very promising and have reached the generation of chimeric antigen receptor neutrophils (CAR-neutrophils) [17].
2. Neutrophil Heterogeneity in Cancer: N1/N2, NDN/LDN, and g-MDSC
2.1. N1 vs. N2
The story of neutrophil heterogeneity in cancer started with Fridlender et al.’s study, suggesting for the first time the N1/N2 functional classification of TANs. The researchers introduced a new classification of neutrophils, analogous to the M1/M2 macrophage classification: N1—neutrophils with pro-inflammatory properties and anti-tumor functions, and N2—neutrophils with anti-inflammatory and pro-tumor functions [14]. Various factors influence the polarization of the neutrophil phenotype (Figure 1, Table 1).
In a pioneer study, using three mouse tumor models: mesothelioma AB12, hybridoma, and Kras-derived lung cancer, the ability of transforming growth factor beta (TGF-β) to play a role in neutrophil polarization was demonstrated [14]. TGF-β inhibition with the small TGF-β type 1 receptor kinase (ALK5) inhibitor SM16 increased the levels of neutrophil chemoattractants in the tumor microenvironment, resulting in neutrophil recruitment [14]. In all tumor models, the gene expression profiles of TANs from SM16-treated tumors revealed a significant decrease in arginase levels and a significant increase in tumor necrosis factor alpha (TNF-α) and intercellular adhesion molecule 1 (ICAM1) levels compared with TANs from SM16-untreated mice [14]. Arginase overexpression could lead to L-arginine depletion in the tumor microenvironment, which impairs T cell function and supports tumor immune escape [18]. Elevated levels of TNF-α and ICAM1 indicate the pro-inflammatory status of TANs from SM16-treated tumors. Functional analysis revealed enhanced cytotoxicity of TANs isolated from SM16-treated tumors against tumor cells, while TANs from untreated tumors were found to be noncytotoxic. In mesothelioma AB12 tumors of SM16-treated mice, in vivo depletion of CD8+ T cells by mAb injection canceled the reduction in tumor growth, indicating a dependence of TAN anti-tumor effects on CD8+ T cells. In SM16-untreated mice, in vivo TAN depletion with or without CD8+ T cell depletion led to a significant decrease in tumor size, indicating the pro-tumor activities of TANs [14]. The findings of this research provide a basic understanding of the morphological and functional differences between neutrophil N1 and N2 phenotypes, which are primarily regulated by TGF-β.
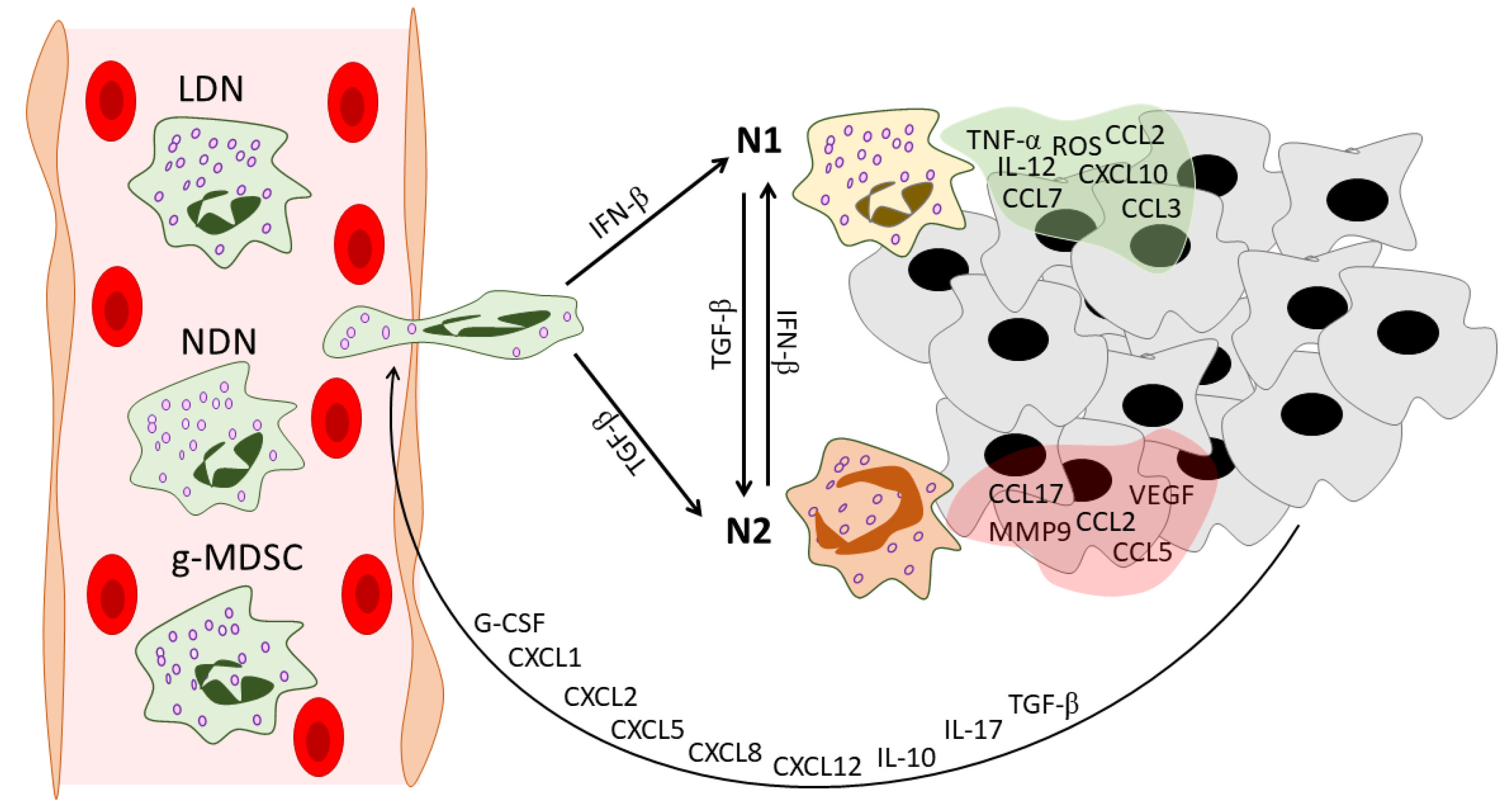
Figure 1. Neutrophil heterogeneity during tumor development. In the peripheral blood of cancer patients, three distinct populations of circulating neutrophils can be found: NDNs, LDNs, and g-MDSCs. Tumors recruit neutrophils via various mediators. These mediators include G-CSF [19], CXCL1 [20], CXCL2 [21], CXCL5 [22], CXCL8 [23], CXCL12 [24], IL-10 [19], IL-17 [25], and TGF-β [26]. After infiltration into the tumor microenvironment, neutrophils gain an N1 or N2 phenotype under the action of IFN-β [27] or TGF-β [14], respectively. Neutrophils in their turn reshape the tumor microenvironment: N1 TANs secrete pro-inflammatory anti-tumor mediators [14][28], while N2 TANs support tumor progression and angiogenesis and enhance the immunosuppressive tumor microenvironment [24][28]. NDNs—normal-density neutrophils, LDNs—low-density neutrophils, g-MDSCs—granulocytic-myeloid-derived suppressor cells, G-CSF—granulocyte colony-stimulating factor, CXCL—C-X-C motif chemokine ligand, CCL—C-C motif chemokine ligand, IL—interleukin, TGF-β—transforming growth factor beta, IFN-β—interferon beta, TNF-α—tumor necrosis factor alpha, ROS—reactive oxygen species, VEGF—vascular endothelial growth factor, MMP9—matrix metallopeptidase 9, TME—tumor microenvironment.
Later, interferon beta (IFN-β) was identified as the orchestrator of neutrophil polarization toward the N1 phenotype in cancer patients and tumor-bearing mice [24][27][29]. In Ifnb1−/− mice after B16F10 melanoma implantation, enhanced tumor growth, angiogenesis, and metastasis were observed and accompanied by higher levels of TANs compared with tumors developed in Ifnb1+/+ mice. TANs isolated from Ifnb1−/− mice (Ifnb1−/−-TANs) highly expressed C-X-C motif chemokine receptor 4 (CXCR4) and its regulators c-Myc and signal transducer and activator of transcription 3 (STAT3), vascular endothelial growth factor (VEGF), and matrix metallopeptidase (MMP9) [24]. CXCR4 traffics neutrophils via a gradient of CXCL12, which was overexpressed in the tumors of Ifnb1−/− mice compared to controls [24][30]. MMP9 is a proteolytic enzyme that degrades the ECM and paves the way for new vessels [31]. VEGF plays a well-known key role in angiogenesis and is an important suppressor of anti-tumor immunity in the tumor microenvironment [32][33][34]. Altogether, high expression of CXCR4, VEGF, and MMP9 could serve as an ideal triad for successful neutrophil-induced angiogenesis. Interestingly, in vitro treatment of Ifnb1−/−-TANs with exogenous IFN-β decreased the expression of the abovementioned genes [24]. This study sheds light on the regulatory role of IFN-β in the acquisition of pro-angiogenic properties by neutrophils.
The absence of IFN-β was also associated with a prolonged life span of blood neutrophils and TANs [29]. Pro-angiogenic TANs from Ifnb1−/− mice were shown to have a prolonged life span in tumor-bearing mice, which could be explained by lower expression of FAS, active caspase 3 and 9, and an imbalance in the expression profiles of pro-apoptotic and anti-apoptotic genes [29]. Moreover, TANs from IFN-β–deficient mice showed a reduction in reactive oxygen species (ROS) production [29].
Table 1. Diverse neutrophil subpopulations in cancer in comparison with mature neutrophils in healthy individuals.
| Neutrophil Type | Markers | Origin | Maturity | Location/ Detection |
Life Span/ Turnover |
ROS Production |
Angiogenic Properties | NETosis | Interactions with Adaptive Immunity |
Other Features |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Murine | ||||||||||
| Mature neutrophils | CD11b+ CD16+ CD15+ CD14− [35][36] |
CD11b+ Ly6G+ Ly6C− [35][36] |
Hematopoietic stem cells in bone marrow [3] | In the final steps of granulopoiesis, neutrophils gain morphological and surface markers of maturity [3] | Bone marrow, peripheral blood, spleen, and tissues [37] | In blood, neutrophils have half-lives of 12.5 h for mice and 90 h for humans [38]; in tissues, neutrophils undergo apoptosis or reverse migration [35] | At the site of infection, neutrophils release large amounts of ROS as an antimicrobial mechanism [39] | Neutrophils in tissues may exhibit a non-immune angiogenic phenotype [6] | Undergo NETosis in response to various microorganisms and endogenous stimuli [40] | Are involved in complex interactions, including the activation and regulation of other immune cells [41] | N.D. |
| N1 TANs | Carry markers similar to mature neutrophils | Can come from both LDNs and, most likely, NDNs in the blood and tumor microenvironment [42] | Mature cells [14] | Intratumoral [14] | N.D. Polarization to N1 by IFNs could delay neutrophil apoptosis [43][44] |
Able to produce high levels of ROS [45] | IFN-β maintains the low levels of expression of angiogenic factors in N1 TANs [24] | Polarization to N1 by IFNs could ensure the capacity of N1 TANs to produce NETs [46] | Activate CD8+ T cells [14]; participate in antigen presentation [28] |
Hyper-segmented nucleus [14] | |
| N2 TANs | Carry markers similar to mature neutrophils | Can come from both NDNs and, most likely, LDNs [42] | Show morphological signs of immaturity [14][27] | Intratumoral [14] | N.D. Could have a prolonged life span [29] |
Reduced [29] | Produce high levels of CXCR4, VEGF, and MMP9 [24] | Reduced [27] | Could recruit Tregs [28]; produce high levels of arginase [14] | Circular nucleus [14][27] | |
| LDN | CD11b+ CD16+ CD15+ CD66+ Siglec8- CD36high CD61high CD41high Lox1high CD226high CD10 +/− [47] |
CD11b+ Ly6G+ [15] |
Could originate from NDNs under the action of tumor-derived factors [42] | Consist of both mature and immature populations [15] | Blood of cancer patients and tumor-bearing mice [15], could infiltrate tumors [42] | LDNs showed a lower rate of apoptosis in vitro in comparison to NDNs [15] | Increased [42] | N.D. | Immature LDNs in response to stimulation in vitro show increased ability to NETosis [48] | Express higher levels of PD-L1 in comparison to NDNs [49] | Lower phagocytic activity [42]; immature LDNs have greater bioenergetic capacity [48] |
| g-MDSC | CD11b+ CD15+ CD14− CD66b+ CD33+ HLA-DR- Lox1+ [19][50] |
CD11b+ Ly6G+ Ly6Clow [50] |
Granulocytic precursors [51] | Immature cells [35] | Bone marrow, blood, spleen, and tumors of tumor-bearing mice; blood and tumor environment of cancer patients [52] |
N.D. Their turnover could be regulated by the Fas-FasL pathway [53] |
Increased [54] | Could participate in tumor angiogenesis [55] | Could produce NETs under specific conditions [56] | Suppress T cells [57] | Lower density [58]; lower phagocytic activity [59] |
Andzinski et al. clearly showed the ability of IFN-β to polarize neutrophils in anti-tumor phenotype [27]. In tumor-bearing mice, upon IFN-β deficiency, neutrophil turnover and mobilization were faster and were combined with a higher percentage of immature neutrophils with ring-shaped nuclei in the blood [27]. In a co-culture with tumor cells, TANs from IFN-β-deficient mice showed significantly lower cytotoxicity and TNF-α expression in comparison with TANs from wild-type mice. However, the anti-tumor cytotoxicity of TANs was recovered after adding exogenous IFN-β to the co-culture [27]. Thus, the phenotypic switch of neutrophils could be regulated by TGF-β and type 1 IFN antagonistic signaling pathways [60][61].
However, the fate of neutrophils to be friend or foe is probably decided by multiple factors, and not only in the tumor microenvironment but outside it. For example, Yan et al. showed that interleukin 6 (IL-6) along with granulocyte colony-stimulating factor (G-CSF) induces the neutrophil N2 phenotype in the bone marrow, a process most likely regulated by the immune suppressor cytokine IL-35 [62][63]. Moreover, it has also been suggested that neutrophils act differently depending on the stage of tumor development [64][65]. TANs isolated from early tumors produced higher levels of NO, H2O2, and TNF-α and demonstrated greater cytotoxicity against tumor cells in comparison with TANs isolated from late-stage tumors [64]. Interestingly, tumor growth was unaffected by neutrophil depletion during the early stages of tumor development. In contrast, after tumor establishment, neutrophil depletion led to a significant reduction in tumor growth, indicating a pro-tumorigenic effect of neutrophils at the late stage of tumor development [64]. Neutrophil migratory properties also vary in different stages of tumor development [65]. At early stages, neutrophils show enhanced migratory and metabolic potential with no immunosuppressive function. However, in later stages, neutrophils lose their elevated migratory and metabolic properties and gain an immunosuppressive phenotype [65].
Shaul et al. deeply analyzed the N1 and N2 phenotypes of neutrophils using microarray analysis and identified different transcriptomic signatures of N1 versus N2 neutrophils [28]. In the N1 profile, 136 genes were overexpressed and 2 genes were downregulated with a fold change of ≥10 [28]. N2 TANs showed a relative downregulation of genes associated with cytoskeletal organization and actin polymerization compared with bone marrow neutrophils and N1 TANs, suggesting that after neutrophil infiltration into the tumor, N2-polarized TANs lose the ability to organize the cytoskeleton and to leave the tumor microenvironment [28]. N1 TANs showed an upregulation of many genes associated with antigen presentation, especially major histocompatibility complex type 1 (MHC-I)-related loci. Moreover, many integrins and membrane receptors associated with neutrophil immune responses are overexpressed in N1 compared with N2 TANs. For example, IFN-γ receptor 1 is expressed in bone marrow naive neutrophils and N1 TANs but is significantly downregulated in N2 TANs, which may result in a loss of communication between neutrophils and IFN-γ-releasing cytotoxic T cells [28]. N1 TANs have pro-inflammatory properties with higher expression levels of the pro-inflammatory cytokines IL-12 and TNF-α as well as various chemokines that attract T cells and macrophages—C-X-C motif chemokine ligand 10 (CXCL10) and C-C motif chemokine ligands 2, 3, and 7 (CCL2, CCL3, and CCL7) [28]. CCL17, which recruits Tregs, is downregulated in N1 TANs compared to N2 TANs, another mechanism of the immunosuppressive function of N2 TANs [28].
Ohms et al. first polarized human neutrophils in vitro [45]. A cocktail containing lipopolysaccharide (LPS), IFN-γ, and IFN-β was used to polarize neutrophils toward an N1-like phenotype, while L-lactate, adenosine, TGF-β, IL10, prostaglandin E2 (PGE2), and G-CSF together were used to polarize neutrophils toward an N2-like phenotype [45]. Since neutrophils have a short life span and spontaneously undergo apoptosis, pan-caspase inhibitor was added during the polarization process [45]. The cytokine profile and functional features of in vitro-polarized neutrophils correspond to those of in vivo-polarized ones, allowing the investigation of deeply different phenotypes of neutrophils in vitro [45]. Lovászi et al. applied the protocol provided by Ohms et al. [45] to investigate the role of the neutrophilic A2A adenosine receptor (A2AAR) in neutrophil polarization [66]. A2AAR-specific agonist CGS21680 was added to the N1 polarization cocktail, and A2AAR-selective antagonist ZM241385 was added to neutrophils before adding the N2 polarization cocktail. The activation of A2AAR skewed N1 neutrophils to the N2 phenotype, while blocking A2AAR suppressed N2 polarization, which indicates the crucial role of the adenosine–A2AAR axis in N2 neutrophil polarization [66]. The discovery of the pro- and anti-inflammatory profiles of N1 and N2 neutrophils, respectively, has led to a wide investigation of these two phenotypes in several physiological and pathological conditions, including inflammatory diseases [67][68], bone regeneration [69], ischemia [70], myocardial infraction [71], and Alzheimer’s disease [72]. Of note, N1/N2 neutrophil classification in terms of infection could differ from N1/N2 TANs described in terms of tumor, which should be considered when moving from one research field to another. However, LPS-stimulated neutrophils showed a phenotype similar to that of anti-tumor N1 neutrophils, which may indicate a relationship between the pro-inflammatory and anti-tumor functions of neutrophils [73].
2.2. NDN vs. LDN
In differential density centrifugation, the main proportion of neutrophils is purified in a high-density layer and called high-density neutrophils (HDNs). However, a significant proportion of neutrophils were found to co-purify with the low-density mononuclear cell layer and are called low-density neutrophils (LDNs) [15] (Figure 1, Table 1). This heterogeneity in neutrophil density was described in 1983 [74]. To avoid confusion, since the term HDN does not refer to a specific neutrophil subpopulation except neutrophils with unaltered normal density, normal-density neutrophils (NDNs) seems to be a more suitable term [75]. It should be noted that TANs can come from both NDNs and LDNs [42], but because LDNs are more likely to have a pro-tumor phenotype [76], researchers hypothesized that N1 TANs come from the NDN fraction and N2 TANs come from the LDN fraction after entering the tumor microenvironment from the bloodstream.
The elevated levels of LDNs in the blood of cancer patients and tumor-bearing mice resulted in the study of their functions and the molecular pathways involved in their elevation during cancer development [15][47][77][78]. Interestingly, TGF-β was also involved in NDN to LDN switching [15]. Guglietta et al. showed that NETosis-induced blood clots could also switch NDN to LDN and suggested, based on gene expression profiling, that LDNs have an intermediate profile between an NDN and N2 [79]. In comparison to NDNs, LDNs from cancer patients overexpress CD66b, CD11b, and CD15 [15][80]. Shaul et al. performed cytometry by time-of-flight (CyTOF) analysis of NDNs and LDNs from healthy individuals and patients with lung cancer. Their data showed significant differences in the expression of CD10, CXCR4, CD94, and programmed death-ligand 1 (PD-L1) between NDNs and LDNs. In both healthy individuals and cancer patients, two populations of NDNs were identified: CD66bhigh/CD10high/CXCR4med/PDL1low and CD66bhigh/CD10med/CXCR4med/low/PDL1low neutrophils. Heterogeneous subsets in the LDN fraction from cancer patients were demonstrated and a unique subset defined by CD66high/CD10low/CXCR4high/PDL-1high/med was identified [78].
In patients with pancreatic ductal adenocarcinoma (PDAC), increased levels of circulating LDNs, which included cycling and non-cycling precursors, immature as well as mature neutrophils were observed [5]. The LDN fraction, isolated from the peripheral blood of stem cell donors receiving recombinant G-CSF, is composed of both immature (CD10−) and mature (CD10+) neutrophils [81]. Valadez-Cosmes et al. performed a high-dimensional screening of human cell surface markers and identified various markers that are overexpressed in LDNs which allowed them to discriminate between LDN and NDN subpopulations in cancer patients [47]. In the LDN subpopulation, the highest fold change was found in the CD36, CD41, CD61, and CD226 markers [47]. Functional analysis revealed impaired phagocytic activity, impaired ROS production, and the absence of anti-tumor activity in the LDN mature fraction, which corresponds to the results published by Marini et al. where mature (CD10+) LDNs inhibited T cell functions whereas immature (CD10−) LDNs enhanced them [15][81]. Furthermore, compared with NDNs, LDNs express higher levels of PD-L1 and can inhibit cytotoxic T cells and natural killer (NK) cells [49][82]. In a recent study, Arasanz et al. showed a possible role of circulating LDNs in the development of resistance to PD-1/PDL1 immunotherapy in non-small-cell lung cancer (NSCLC) patients [83]. In breast cancer patients, LDN levels were associated with a worse prognosis and were significantly higher in the case of metastatic cancer than in non-metastatic cases [77]. Similar results were observed in breast-cancer-bearing mice, where LDNs were involved in promoting liver metastasis [48]. In addition to studying the role of LDNs in cancer development, LDNs are actively investigated in inflammatory diseases [84], infections [85][86], and autoimmune diseases [87].
2.3. g-MDSCs
In the field of neutrophil heterogeneity, researchers should mention myeloid-derived suppressor cells (MDSCs) (Figure 1, Table 1). MDSCs are a population of immature myeloid cells derived from the granulocytic (g-MDSCs) or monocytic (m-MDSCs) lineages with a remarkable ability to suppress T cells [57]. MDSCs have been shown to accumulate in cancer patients and tumor-bearing mice and have also been observed under different conditions, including infection, chemotherapy, experimentally induced autoimmunity, and stress [88]. The similarity in the morphology and phenotype of g-MDSCs and mature neutrophils makes it difficult to distinguish between these populations [89]. Que et al. summarized the studies in which g-MDSCs were believed to be a neutrophil subset or a distinct population [90]. The researchers described a TAN as a “similar entity” to a g-MDSC, which is a suitable description in this context [90].
References
- Russo, M.; Nastasi, C. Targeting the Tumor Microenvironment: A Close Up of Tumor-Associated Macrophages and Neutrophils. Front. Oncol. 2022, 12, 871513.
- Borregaard, N.; Cowland, J.B. Granules of the Human Neutrophilic Polymorphonuclear Leukocyte. Blood 1997, 89, 3503–3521.
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113.
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil Kinetics in Health and Disease. Trends Immunol. 2010, 31, 318–324.
- Montaldo, E.; Lusito, E.; Bianchessi, V.; Caronni, N.; Scala, S.; Basso-Ricci, L.; Cantaffa, C.; Masserdotti, A.; Barilaro, M.; Barresi, S.; et al. Cellular and Transcriptional Dynamics of Human Neutrophils at Steady State and upon Stress. Nat. Immunol. 2022, 23, 1470–1483.
- Ballesteros, I.; Rubio-Ponce, A.; Genua, M.; Lusito, E.; Kwok, I.; Fernández-Calvo, G.; Khoyratty, T.E.; van Grinsven, E.; González-Hernández, S.; Nicolás-Ávila, J.Á.; et al. Co-Option of Neutrophil Fates by Tissue Environments. Cell 2020, 183, 1282–1297.e18.
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583.
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Lüscher, T.F.; Camici, G.G.; Liberale, L. Novel Findings in Neutrophil Biology and Their Impact on Cardiovascular Disease. Cardiovasc. Res. 2019, 115, 1266–1285.
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187.
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Neutrophils and Polymorphonuclear Myeloid-Derived Suppressor Cells: An Emerging Battleground in Cancer Therapy. Oncogenesis 2022, 11, 22.
- Berger-Achituv, S.; Brinkmann, V.; Abed, U.A.; Kühn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A Proposed Role for Neutrophil Extracellular Traps in Cancer Immunoediting. Front. Immunol. 2013, 4, 48.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science (80-) 2004, 303, 1532–1535.
- Kaltenmeier, C.; Simmons, R.L.; Tohme, S.; Yazdani, H.O. Neutrophil Extracellular Traps (NETs) in Cancer Metastasis. Cancers 2021, 13, 6131.
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194.
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic Diversity and Plasticity in Circulating Neutrophil Subpopulations in Cancer. Cell Rep. 2015, 10, 562–573.
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res. 2007, 67, 425.
- Chang, Y.; Syahirah, R.; Wang, X.; Jin, G.; Torregrosa-Allen, S.; Elzey, B.D.; Hummel, S.N.; Wang, T.; Li, C.; Lian, X.; et al. Engineering Chimeric Antigen Receptor Neutrophils from Human Pluripotent Stem Cells for Targeted Cancer Immunotherapy. Cell Rep. 2022, 40, 111128.
- Niu, F.; Yu, Y.; Li, Z.; Ren, Y.; Li, Z.; Ye, Q.; Liu, P.; Ji, C.; Qian, L.; Xiong, Y. Arginase: An Emerging and Promising Therapeutic Target for Cancer Treatment. Biomed. Pharmacother. 2022, 149, 112840.
- Shaul, M.E.; Fridlender, Z.G. Cancer-Related Circulating and Tumor-Associated Neutrophils—Subtypes, Sources and Function. FEBS J. 2018, 285, 4316–4342.
- Li, L.; Xu, L.; Yan, J.; Zhen, Z.J.; Ji, Y.; Liu, C.Q.; Lau, W.Y.; Zheng, L.; Xu, J. CXCR2-CXCL1 Axis Is Correlated with Neutrophil Infiltration and Predicts a Poor Prognosis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 129.
- Zhang, H.; Ye, Y.L.; Li, M.X.; Ye, S.B.; Huang, W.R.; Cai, T.T.; He, J.; Peng, J.Y.; Duan, T.H.; Cui, J.; et al. CXCL2/MIF-CXCR2 Signaling Promotes the Recruitment of Myeloid-Derived Suppressor Cells and Is Correlated with Prognosis in Bladder Cancer. Oncogene 2017, 36, 2095–2104.
- Zhou, S.L.; Dai, Z.; Zhou, Z.J.; Chen, Q.; Wang, Z.; Xiao, Y.S.; Hu, Z.Q.; Huang, X.Y.; Yang, G.H.; Shi, Y.H.; et al. CXCL5 Contributes to Tumor Metastasis and Recurrence of Intrahepatic Cholangiocarcinoma by Recruiting Infiltrative Intratumoral Neutrophils. Carcinogenesis 2014, 35, 597–605.
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 Pathways in Cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71.
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils Responsive to Endogenous IFN-β Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J. Clin. Investig. 2010, 120, 1151–1164.
- Wu, L.; Awaji, M.; Saxena, S.; Varney, M.L.; Sharma, B.; Singh, R.K. IL-17–CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment. Am. J. Pathol. 2020, 190, 222–233.
- SenGupta, S.; Hein, L.E.; Xu, Y.; Zhang, J.; Konwerski, J.R.; Li, Y.; Johnson, C.; Cai, D.; Smith, J.L.; Parent, C.A. Triple-Negative Breast Cancer Cells Recruit Neutrophils by Secreting TGF-β and CXCR2 Ligands. Front. Immunol. 2021, 12, 973.
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; Von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int. J. Cancer 2016, 138, 1982–1993.
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-Associated Neutrophils Display a Distinct N1 Profile Following TGFβ Modulation: A Transcriptomics Analysis of pro- vs. Antitumor TANs. Oncoimmunology 2016, 5, e1232221.
- Andzinski, L.; Wu, C.F.; Lienenklaus, S.; Kröger, A.; Weiss, S.; Jablonska, J. Delayed Apoptosis of Tumor Associated Neutrophils in the Absence of Endogenous IFN-β. Int. J. Cancer 2015, 136, 572–583.
- De Filippo, K.; Rankin, S.M. CXCR4, the Master Regulator of Neutrophil Trafficking in Homeostasis and Disease. Eur. J. Clin. Investig. 2018, 48, e12949.
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370.
- Bu, M.T.; Chandrasekhar, P.; Ding, L.; Hugo, W. The Roles of TGF-β and VEGF Pathways in the Suppression of Antitumor Immunity in Melanoma and Other Solid Tumors. Pharmacol. Ther. 2022, 240, 108211.
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular Endothelial Growth Factor (VEGF)—Key Factor in Normal and Pathological Angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467.
- Zhang, Y.; Brekken, R.A. Direct and Indirect Regulation of the Tumor Immune Microenvironment by VEGF. J. Leukoc. Biol. 2022, 111, 1269–1286.
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune Suppression by Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells: Similarities and Differences. Cell. Mol. Life Sci. 2013, 70, 3813.
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of Neutrophil Surface Marker Changes in Health and Inflammation Using High-Throughput Screening Flow Cytometry. Exp. Cell Res. 2016, 342, 200–209.
- Christoffersson, G.; Phillipson, M. The Neutrophil: One Cell on Many Missions or Many Cells with Different Agendas? Cell Tissue Res. 2018, 371, 415–423.
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In Vivo Labeling with 2H2O Reveals a Human Neutrophil Lifespan of 5.4 Days. Blood 2010, 116, 625–627.
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373.
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2017, 18, 134–147.
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531.
- Arpinati, L.; Kaisar-iluz, N.; Shaul, M.E.; Groth, C.; Umansky, V.; Fridlender, Z.G. Tumor-Derived Factors Differentially Affect the Recruitment and Plasticity of Neutrophils. Cancers 2021, 13, 5082.
- Sakamoto, E.; Hato, F.; Kato, T.; Sakamoto, C.; Akahori, M.; Hino, M.; Kitagawa, S. Type I and Type II Interferons Delay Human Neutrophil Apoptosis via Activation of STAT3 and Up-Regulation of Cellular Inhibitor of Apoptosis 2. J. Leukoc. Biol. 2005, 78, 301–309.
- Aga, E.; Mukherjee, A.; Rane, D.; More, V.; Patil, T.; van Zandbergen, G.; Solbach, W.; Dandapat, J.; Tackenberg, H.; Ohms, M.; et al. Type-1 Interferons Prolong the Lifespan of Neutrophils by Interfering with Members of the Apoptotic Cascade. Cytokine 2018, 112, 21–26.
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in Vitro. Front. Immunol. 2020, 11, 532.
- Martinelli, S.; Urosevic, M.; Baryadel, A.; Oberholzer, P.A.; Baumann, C.; Fey, M.F.; Dummer, R.; Simon, H.U.; Yousefi, S. Induction of Genes Mediating Interferon-Dependent Extracellular Trap Formation during Neutrophil Differentiation. J. Biol. Chem. 2004, 279, 44123–44132.
- Valadez-Cosmes, P.; Maitz, K.; Kindler, O.; Raftopoulou, S.; Kienzl, M.; Santiso, A.; Mihalic, Z.N.; Brcic, L.; Lindenmann, J.; Fediuk, M.; et al. Identification of Novel Low-Density Neutrophil Markers Through Unbiased High-Dimensional Flow Cytometry Screening in Non-Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 703846.
- Hsu, B.E.; Tabariès, S.; Johnson, R.M.; Andrzejewski, S.; Senecal, J.; Lehuédé, C.; Annis, M.G.; Ma, E.H.; Völs, S.; Ramsay, L.A.; et al. Immature Low-Density Neutrophils Exhibit Metabolic Flexibility That Facilitates Breast Cancer Liver Metastasis. Cell Rep. 2019, 27, 3902–3915.
- Yajuk, O.; Baron, M.; Toker, S.; Zelter, T.; Fainsod-Levi, T.; Granot, Z. The PD-L1/PD-1 Axis Blocks Neutrophil Cytotoxicity in Cancer. Cells 2021, 10, 1510.
- Zhou, J.; Nefedova, Y.; Lei, A.; Gabrilovich, D. Neutrophils and PMN-MDSCs: Their Biological Role and Interaction with Stromal Cells. Semin Immunol. 2018, 35, 19–28.
- Kusmartsev, S.; Nagaraj, S.; Gabrilovich, D.I. Tumor-Associated CD8+ T Cell Tolerance Induced by Bone Marrow-Derived Immature Myeloid Cells. J. Immunol. 2005, 175, 4583–4592.
- Vanhaver, C.; van der Bruggen, P.; Bruger, A.M. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872.
- Sinha, P.; Chornoguz, O.; Clements, V.K.; Artemenko, K.A.; Zubarev, R.A.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Express the Death Receptor Fas and Apoptose in Response to T Cell–Expressed FasL. Blood 2011, 117, 5381.
- Youn, J.-I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of Myeloid-Derived Suppressor Cells in Tumor Bearing Mice. J. Immunol. 2008, 181, 5791.
- Yang, L.; DeBusk, L.M.; Fukuda, K.; Fingleton, B.; Green-Jarvis, B.; Shyr, Y.; Matrisian, L.M.; Carbone, D.P.; Lin, P.C. Expansion of Myeloid Immune Suppressor Gr+CD11b+ Cells in Tumor-Bearing Host Directly Promotes Tumor Angiogenesis. Cancer Cell 2004, 6, 409–421.
- Ortiz-Espinosa, S.; Morales, X.; Senent, Y.; Alignani, D.; Tavira, B.; Macaya, I.; Ruiz, B.; Moreno, H.; Remírez, A.; Sainz, C.; et al. Complement C5a Induces the Formation of Neutrophil Extracellular Traps by Myeloid-Derived Suppressor Cells to Promote Metastasis. Cancer Lett. 2022, 529, 70–84.
- Kramer, E.D.; Abrams, S.I. Granulocytic Myeloid-Derived Suppressor Cells as Negative Regulators of Anticancer Immunity. Front. Immunol. 2020, 11, 1963.
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498.
- Youn, J.-I.; Collazo, M.; Shalova, I.N.; Biswas, S.K.; Gabrilovich, D.I. Characterization of the Nature of Granulocytic Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. J. Leukoc. Biol. 2012, 91, 167.
- Pylaeva, E.; Lang, S.; Jablonska, J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front. Immunol. 2016, 7, 629.
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil Plasticity in the Tumor Microenvironment. Blood 2019, 133, 2159–2167.
- Yan, B.; Wei, J.-J.; Yuan, Y.; Sun, R.; Li, D.; Luo, J.; Liao, S.-J.; Zhou, Y.-H.; Shu, Y.; Wang, Q.; et al. IL-6 Cooperates with G-CSF To Induce Protumor Function of Neutrophils in Bone Marrow by Enhancing STAT3 Activation. J. Immunol. 2013, 190, 5882–5893.
- Zou, J.-M.; Qin, J.; Li, Y.-C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.-S.; Chi, G.; Guo, F.; et al. IL-35 Induces N2 Phenotype of Neutrophils to Promote Tumor Growth. Oncotarget 2017, 8, 33501–33514.
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-Associated Neutrophils (TAN) Develop pro-Tumorigenic Properties during Tumor Progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756.
- Patel, S.; Fu, S.; Mastio, J.; Dominguez, G.A.; Purohit, A.; Kossenkov, A.; Lin, C.; Alicea-Torres, K.; Sehgal, M.; Nefedova, Y.; et al. Unique Pattern of Neutrophil Migration and Function during Tumor Progression. Nat. Immunol. 2018, 19, 1236–1247.
- Lovászi, M.; Németh, Z.H.; Pacher, P.; Gause, W.C.; Wagener, G.; Haskó, G. A2A Adenosine Receptor Activation Prevents Neutrophil Aging and Promotes Polarization from N1 towards N2 Phenotype. Purinergic Signal. 2022, 18, 345–358.
- Zhao, Z.; Liu, T.; Liang, Y.; Cui, W.; Li, D.; Zhang, G.; Deng, Z.; Chen, M.; Sha, K.; Xiao, W.; et al. N2-Polarized Neutrophils Reduce Inflammation in Rosacea by Regulating Vascular Factors and Proliferation of CD4+ T Cells. J. Investig. Dermatol. 2022, 142, 1835–1844.e2.
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 12, 3181.
- Cai, B.; Lin, D.; Li, Y.; Wang, L.; Xie, J.; Dai, T.; Liu, F.; Tang, M.; Tian, L.; Yuan, Y.; et al. N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle. Adv. Sci. 2021, 8, 2100584.
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Rat Hepatic Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Neutrophil Inflammatory Response. FASEB J. 2019, 33, 1695–1710.
- Nederlof, R.; Reidel, S.; Spychala, A.; Gödecke, S.; Heinen, A.; Lautwein, T.; Petzsch, P.; Köhrer, K.; Gödecke, A. Insulin-Like Growth Factor 1 Attenuates the Pro-Inflammatory Phenotype of Neutrophils in Myocardial Infarction. Front. Immunol. 2022, 13, 908023.
- Pakravan, N.; Hassan, Z.M.; Abbasi, A. Intra-Nasal Administration of Sperm Head Turns Neutrophil into Reparative Mode after PGE1- and/or Ang II Receptor-Mediated Phagocytosis Followed by Expression of Sperm Head’s Coding RNA. Int. Immunopharmacol. 2021, 98, 107696.
- Guimarães-Bastos, D.; Frony, A.C.; Barja-Fidalgo, C.; Moraes, J.A. Melanoma-Derived Extracellular Vesicles Skew Neutrophils into a pro-Tumor Phenotype. J. Leukoc. Biol. 2022, 111, 585–596.
- Pember, S.O.; Barnes, K.C.; Brandt, S.J.; Kinkade, J.M. Density Heterogeneity of Neutrophilic Polymorphonuclear Leukocytes: Gradient Fractionation and Relationship to Chemotactic Stimulation. Blood 1983, 61, 1105–1115.
- Cassatella, M.A.; Scapini, P. On the Improper Use of the Term High-Density Neutrophils. Trends Immunol. 2020, 41, 1059–1061.
- Kaisar-Iluz, N.; Arpinati, L.; Shaul, M.E.; Mahroum, S.; Qaisi, M.; Tidhar, E.; Fridlender, Z.G. The Bilateral Interplay between Cancer Immunotherapies and Neutrophils’ Phenotypes and Sub-Populations. Cells 2022, 11, 783.
- Saraiva, D.P.; Correia, B.F.; Salvador, R.; de Sousa, N.; Jacinto, A.; Braga, S.; Cabral, M.G.; Saraiva, D.P.; Correia, B.F.; Salvador, R.; et al. Circulating Low Density Neutrophils of Breast Cancer Patients Are Associated with Their Worse Prognosis Due to the Impairment of T Cell Responses. Oncotarget 2021, 12, 2388–2403.
- Shaul, M.E.; Eyal, O.; Guglietta, S.; Aloni, P.; Zlotnik, A.; Forkosh, E.; Levy, L.; Weber, L.M.; Levin, Y.; Pomerantz, A.; et al. Circulating Neutrophil Subsets in Advanced Lung Cancer Patients Exhibit Unique Immune Signature and Relate to Prognosis. FASEB J. 2020, 34, 4204–4218.
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation Induced by C3aR-Dependent NETosis Drives Protumorigenic Neutrophils during Small Intestinal Tumorigenesis. Nat. Commun. 2016, 7, 11037.
- Liu, Y.; Hu, Y.; Gu, F.; Liang, J.; Zeng, Y.; Hong, X.; Zhang, K.; Liu, L. Phenotypic and Clinical Characterization of Low Density Neutrophils in Patients with Advanced Lung Adenocarcinoma. Oncotarget 2017, 8, 90969–90978.
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T.; et al. Mature CD10+ and Immature CD10− Neutrophils Present in G-CSF–Treated Donors Display Opposite Effects on T Cells. Blood 2017, 129, 1343–1356.
- Sun, R.; Xiong, Y.; Liu, H.; Gao, C.; Su, L.; Weng, J.; Yuan, X.; Zhang, D.; Feng, J. Tumor-Associated Neutrophils Suppress Antitumor Immunity of NK Cells through the PD-L1/PD-1 Axis. Transl. Oncol. 2020, 13, 100825.
- Arasanz, H.; Bocanegra, A.I.; Morilla, I.; Fernández-Irigoyen, J.; Martínez-Aguillo, M.; Teijeira, L.; Garnica, M.; Blanco, E.; Chocarro, L.; Ausin, K.; et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3846.
- Ning, X.; Wang, W.M.; Jin, H.Z.; Fang, W. Low-Density Granulocytes in Immune-Mediated Inflammatory Diseases. J. Immunol. Res. 2022, 2022, 1622160.
- Zhang, Y.; Wang, Q.; Mackay, C.R.; Ng, L.G.; Kwok, I. Neutrophil Subsets and Their Differential Roles in Viral Respiratory Diseases. J. Leukoc. Biol. 2022, 111, 1159–1173.
- Cabrera, L.E.; Pekkarinen, P.T.; Alander, M.; Nowlan, K.H.A.; Nguyen, N.A.; Jokiranta, S.; Kuivanen, S.; Patjas, A.; Mero, S.; Pakkanen, S.H.; et al. Characterization of Low-Density Granulocytes in COVID-19. PLoS Pathog. 2021, 17, e1009721.
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693.
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499.
- Aarts, C.E.M.; Kuijpers, T.W. Neutrophils as Myeloid-Derived Suppressor Cells. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12989.
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-Associated Neutrophils and Neutrophil-Targeted Cancer Therapies. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188762.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
763
Revisions:
2 times
(View History)
Update Date:
28 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




