Neutrophils represent the most abundant cell type of leukocytes in the human blood and have been considered a vital player in the innate immune system and the first line of defense against invading pathogens. Neutrophils play an active role in the immune response during cancer development. They could exhibit both pro-oncogenic and anti-tumor activities under the influence of various mediators in the tumor microenvironment. Neutrophils can be divided into several subpopulations, thus contradicting the traditional concept of neutrophils as a homogeneous population with a specific function in the innate immunity and opening new horizons for cancer therapy.
- neutrophil heterogeneity
- tumor-associated neutrophils
- cancer therapy
1. Introduction
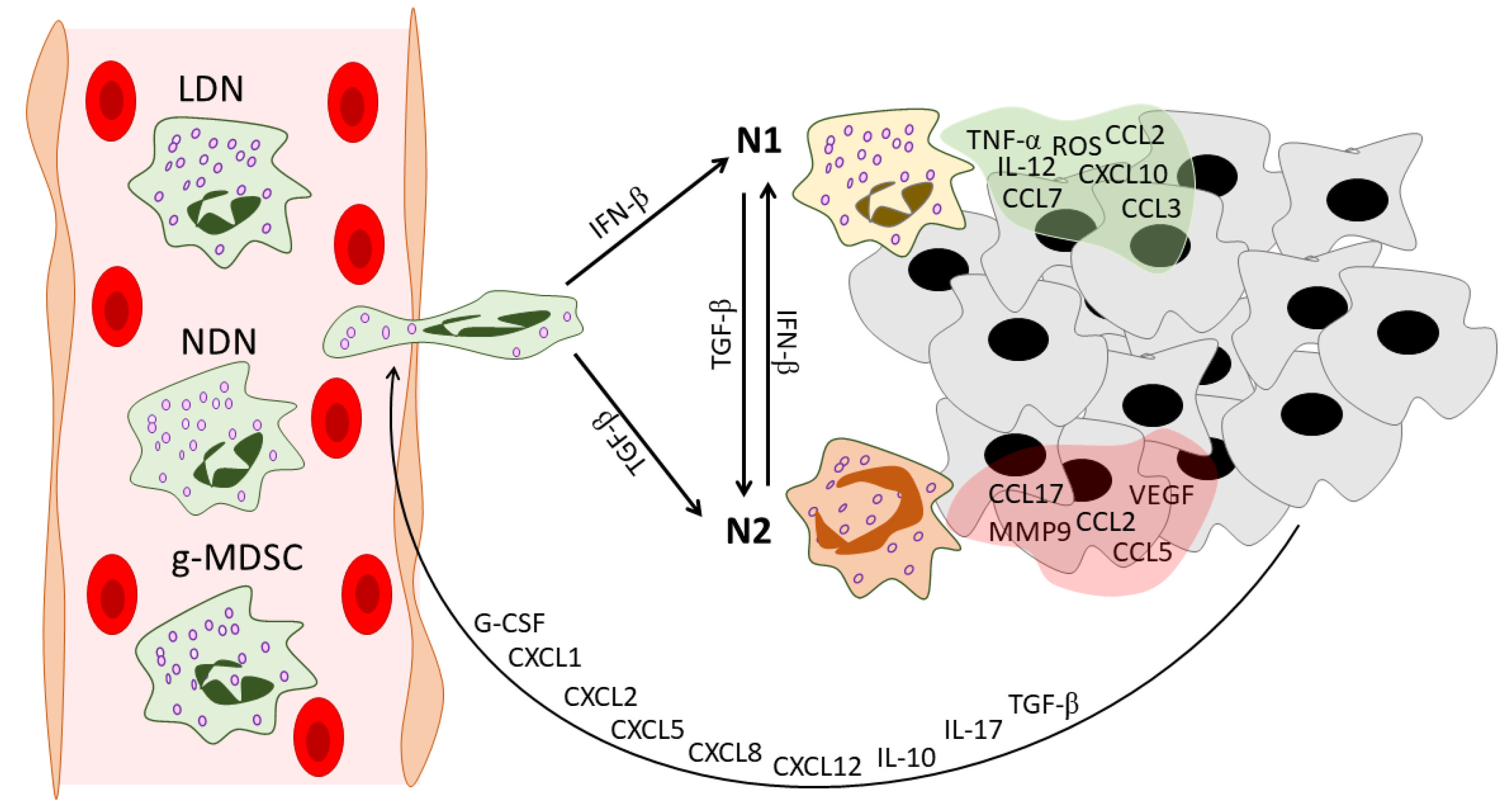
2. Neutrophil Heterogeneity in Cancer: N1/N2, NDN/LDN, and g-MDSC
2.1. N1 vs. N2
| Neutrophil Type | Markers | Origin | Maturity | Location/ Detection |
Life Span/ Turnover |
ROS Production |
Angiogenic Properties | NETosis | Interactions with Adaptive Immunity |
Other Features |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Human | Murine | ||||||||||
| Mature neutrophils | CD11b+ CD16+ CD15+ CD14− [35][36] |
CD11b+ Ly6G+ Ly6C− [35][36] |
Hematopoietic stem cells in bone marrow [3] | In the final steps of granulopoiesis, neutrophils gain morphological and surface markers of maturity [3] | Bone marrow, peripheral blood, spleen, and tissues [37] | In blood, neutrophils have half-lives of 12.5 h for mice and 90 h for humans [38]; in tissues, neutrophils undergo apoptosis or reverse migration [35] | At the site of infection, neutrophils release large amounts of ROS as an antimicrobial mechanism [39] | Neutrophils in tissues may exhibit a non-immune angiogenic phenotype [6] | Undergo NETosis in response to various microorganisms and endogenous stimuli [40] | Are involved in complex interactions, including the activation and regulation of other immune cells [41] | N.D. |
| N1 TANs | Carry markers similar to mature neutrophils | Can come from both LDNs and, most likely, NDNs in the blood and tumor microenvironment [42] | Mature cells [14] | Intratumoral [14] | N.D. Polarization to N1 by IFNs could delay neutrophil apoptosis [43][44] |
Able to produce high levels of ROS [45] | IFN-β maintains the low levels of expression of angiogenic factors in N1 TANs [24] | Polarization to N1 by IFNs could ensure the capacity of N1 TANs to produce NETs [46] | Activate CD8+ T cells [14]; participate in antigen presentation [28] |
Hyper-segmented nucleus [14] | |
| N2 TANs | Carry markers similar to mature neutrophils | Can come from both NDNs and, most likely, LDNs [42] | Show morphological signs of immaturity [14][27] | Intratumoral [14] | N.D. Could have a prolonged life span [29] |
Reduced [29] | Produce high levels of CXCR4, VEGF, and MMP9 [24] | Reduced [27] | Could recruit Tregs [28]; produce high levels of arginase [14] | Circular nucleus [14][27] | |
| LDN | CD11b+ CD16+ CD15+ CD66+ Siglec8- CD36high CD61high CD41high Lox1high CD226high CD10 +/− [47] |
CD11b+ Ly6G+ [15] |
Could originate from NDNs under the action of tumor-derived factors [42] | Consist of both mature and immature populations [15] | Blood of cancer patients and tumor-bearing mice [15], could infiltrate tumors [42] | LDNs showed a lower rate of apoptosis in vitro in comparison to NDNs [15] | Increased [42] | N.D. | Immature LDNs in response to stimulation in vitro show increased ability to NETosis [48] | Express higher levels of PD-L1 in comparison to NDNs [49] | Lower phagocytic activity [42]; immature LDNs have greater bioenergetic capacity [48] |
| g-MDSC | CD11b+ CD15+ CD14− CD66b+ CD33+ HLA-DR- Lox1+ [19][50] |
CD11b+ Ly6G+ Ly6Clow [50] |
Granulocytic precursors [51] | Immature cells [35] | Bone marrow, blood, spleen, and tumors of tumor-bearing mice; blood and tumor environment of cancer patients [52] |
N.D. Their turnover could be regulated by the Fas-FasL pathway [53] |
Increased [54] | Could participate in tumor angiogenesis [55] | Could produce NETs under specific conditions [56] | Suppress T cells [57] | Lower density [58]; lower phagocytic activity [59] |
2.2. NDN vs. LDN
2.3. g-MDSCs
This entry is adapted from the peer-reviewed paper 10.3390/ijms232415827
References
- Russo, M.; Nastasi, C. Targeting the Tumor Microenvironment: A Close Up of Tumor-Associated Macrophages and Neutrophils. Front. Oncol. 2022, 12, 871513.
- Borregaard, N.; Cowland, J.B. Granules of the Human Neutrophilic Polymorphonuclear Leukocyte. Blood 1997, 89, 3503–3521.
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113.
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil Kinetics in Health and Disease. Trends Immunol. 2010, 31, 318–324.
- Montaldo, E.; Lusito, E.; Bianchessi, V.; Caronni, N.; Scala, S.; Basso-Ricci, L.; Cantaffa, C.; Masserdotti, A.; Barilaro, M.; Barresi, S.; et al. Cellular and Transcriptional Dynamics of Human Neutrophils at Steady State and upon Stress. Nat. Immunol. 2022, 23, 1470–1483.
- Ballesteros, I.; Rubio-Ponce, A.; Genua, M.; Lusito, E.; Kwok, I.; Fernández-Calvo, G.; Khoyratty, T.E.; van Grinsven, E.; González-Hernández, S.; Nicolás-Ávila, J.Á.; et al. Co-Option of Neutrophil Fates by Tissue Environments. Cell 2020, 183, 1282–1297.e18.
- Silvestre-Roig, C.; Fridlender, Z.G.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583.
- Bonaventura, A.; Montecucco, F.; Dallegri, F.; Carbone, F.; Lüscher, T.F.; Camici, G.G.; Liberale, L. Novel Findings in Neutrophil Biology and Their Impact on Cardiovascular Disease. Cardiovasc. Res. 2019, 115, 1266–1285.
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187.
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Neutrophils and Polymorphonuclear Myeloid-Derived Suppressor Cells: An Emerging Battleground in Cancer Therapy. Oncogenesis 2022, 11, 22.
- Berger-Achituv, S.; Brinkmann, V.; Abed, U.A.; Kühn, L.I.; Ben-Ezra, J.; Elhasid, R.; Zychlinsky, A. A Proposed Role for Neutrophil Extracellular Traps in Cancer Immunoediting. Front. Immunol. 2013, 4, 48.
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science (80-) 2004, 303, 1532–1535.
- Kaltenmeier, C.; Simmons, R.L.; Tohme, S.; Yazdani, H.O. Neutrophil Extracellular Traps (NETs) in Cancer Metastasis. Cancers 2021, 13, 6131.
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194.
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic Diversity and Plasticity in Circulating Neutrophil Subpopulations in Cancer. Cell Rep. 2015, 10, 562–573.
- Gabrilovich, D.I.; Bronte, V.; Chen, S.H.; Colombo, M.P.; Ochoa, A.; Ostrand-Rosenberg, S.; Schreiber, H. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res. 2007, 67, 425.
- Chang, Y.; Syahirah, R.; Wang, X.; Jin, G.; Torregrosa-Allen, S.; Elzey, B.D.; Hummel, S.N.; Wang, T.; Li, C.; Lian, X.; et al. Engineering Chimeric Antigen Receptor Neutrophils from Human Pluripotent Stem Cells for Targeted Cancer Immunotherapy. Cell Rep. 2022, 40, 111128.
- Niu, F.; Yu, Y.; Li, Z.; Ren, Y.; Li, Z.; Ye, Q.; Liu, P.; Ji, C.; Qian, L.; Xiong, Y. Arginase: An Emerging and Promising Therapeutic Target for Cancer Treatment. Biomed. Pharmacother. 2022, 149, 112840.
- Shaul, M.E.; Fridlender, Z.G. Cancer-Related Circulating and Tumor-Associated Neutrophils—Subtypes, Sources and Function. FEBS J. 2018, 285, 4316–4342.
- Li, L.; Xu, L.; Yan, J.; Zhen, Z.J.; Ji, Y.; Liu, C.Q.; Lau, W.Y.; Zheng, L.; Xu, J. CXCR2-CXCL1 Axis Is Correlated with Neutrophil Infiltration and Predicts a Poor Prognosis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 129.
- Zhang, H.; Ye, Y.L.; Li, M.X.; Ye, S.B.; Huang, W.R.; Cai, T.T.; He, J.; Peng, J.Y.; Duan, T.H.; Cui, J.; et al. CXCL2/MIF-CXCR2 Signaling Promotes the Recruitment of Myeloid-Derived Suppressor Cells and Is Correlated with Prognosis in Bladder Cancer. Oncogene 2017, 36, 2095–2104.
- Zhou, S.L.; Dai, Z.; Zhou, Z.J.; Chen, Q.; Wang, Z.; Xiao, Y.S.; Hu, Z.Q.; Huang, X.Y.; Yang, G.H.; Shi, Y.H.; et al. CXCL5 Contributes to Tumor Metastasis and Recurrence of Intrahepatic Cholangiocarcinoma by Recruiting Infiltrative Intratumoral Neutrophils. Carcinogenesis 2014, 35, 597–605.
- Liu, Q.; Li, A.; Tian, Y.; Wu, J.D.; Liu, Y.; Li, T.; Chen, Y.; Han, X.; Wu, K. The CXCL8-CXCR1/2 Pathways in Cancer. Cytokine Growth Factor Rev. 2016, 31, 61–71.
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils Responsive to Endogenous IFN-β Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J. Clin. Investig. 2010, 120, 1151–1164.
- Wu, L.; Awaji, M.; Saxena, S.; Varney, M.L.; Sharma, B.; Singh, R.K. IL-17–CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment. Am. J. Pathol. 2020, 190, 222–233.
- SenGupta, S.; Hein, L.E.; Xu, Y.; Zhang, J.; Konwerski, J.R.; Li, Y.; Johnson, C.; Cai, D.; Smith, J.L.; Parent, C.A. Triple-Negative Breast Cancer Cells Recruit Neutrophils by Secreting TGF-β and CXCR2 Ligands. Front. Immunol. 2021, 12, 973.
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; Von Köckritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int. J. Cancer 2016, 138, 1982–1993.
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-Associated Neutrophils Display a Distinct N1 Profile Following TGFβ Modulation: A Transcriptomics Analysis of pro- vs. Antitumor TANs. Oncoimmunology 2016, 5, e1232221.
- Andzinski, L.; Wu, C.F.; Lienenklaus, S.; Kröger, A.; Weiss, S.; Jablonska, J. Delayed Apoptosis of Tumor Associated Neutrophils in the Absence of Endogenous IFN-β. Int. J. Cancer 2015, 136, 572–583.
- De Filippo, K.; Rankin, S.M. CXCR4, the Master Regulator of Neutrophil Trafficking in Homeostasis and Disease. Eur. J. Clin. Investig. 2018, 48, e12949.
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370.
- Bu, M.T.; Chandrasekhar, P.; Ding, L.; Hugo, W. The Roles of TGF-β and VEGF Pathways in the Suppression of Antitumor Immunity in Melanoma and Other Solid Tumors. Pharmacol. Ther. 2022, 240, 108211.
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular Endothelial Growth Factor (VEGF)—Key Factor in Normal and Pathological Angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467.
- Zhang, Y.; Brekken, R.A. Direct and Indirect Regulation of the Tumor Immune Microenvironment by VEGF. J. Leukoc. Biol. 2022, 111, 1269–1286.
- Pillay, J.; Tak, T.; Kamp, V.M.; Koenderman, L. Immune Suppression by Neutrophils and Granulocytic Myeloid-Derived Suppressor Cells: Similarities and Differences. Cell. Mol. Life Sci. 2013, 70, 3813.
- Lakschevitz, F.S.; Hassanpour, S.; Rubin, A.; Fine, N.; Sun, C.; Glogauer, M. Identification of Neutrophil Surface Marker Changes in Health and Inflammation Using High-Throughput Screening Flow Cytometry. Exp. Cell Res. 2016, 342, 200–209.
- Christoffersson, G.; Phillipson, M. The Neutrophil: One Cell on Many Missions or Many Cells with Different Agendas? Cell Tissue Res. 2018, 371, 415–423.
- Pillay, J.; Den Braber, I.; Vrisekoop, N.; Kwast, L.M.; De Boer, R.J.; Borghans, J.A.M.; Tesselaar, K.; Koenderman, L. In Vivo Labeling with 2H2O Reveals a Human Neutrophil Lifespan of 5.4 Days. Blood 2010, 116, 625–627.
- Nguyen, G.T.; Green, E.R.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front. Cell. Infect. Microbiol. 2017, 7, 373.
- Papayannopoulos, V. Neutrophil Extracellular Traps in Immunity and Disease. Nat. Rev. Immunol. 2017, 18, 134–147.
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the Activation and Regulation of Innate and Adaptive Immunity. Nat. Rev. Immunol. 2011, 11, 519–531.
- Arpinati, L.; Kaisar-iluz, N.; Shaul, M.E.; Groth, C.; Umansky, V.; Fridlender, Z.G. Tumor-Derived Factors Differentially Affect the Recruitment and Plasticity of Neutrophils. Cancers 2021, 13, 5082.
- Sakamoto, E.; Hato, F.; Kato, T.; Sakamoto, C.; Akahori, M.; Hino, M.; Kitagawa, S. Type I and Type II Interferons Delay Human Neutrophil Apoptosis via Activation of STAT3 and Up-Regulation of Cellular Inhibitor of Apoptosis 2. J. Leukoc. Biol. 2005, 78, 301–309.
- Aga, E.; Mukherjee, A.; Rane, D.; More, V.; Patil, T.; van Zandbergen, G.; Solbach, W.; Dandapat, J.; Tackenberg, H.; Ohms, M.; et al. Type-1 Interferons Prolong the Lifespan of Neutrophils by Interfering with Members of the Apoptotic Cascade. Cytokine 2018, 112, 21–26.
- Ohms, M.; Möller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in Vitro. Front. Immunol. 2020, 11, 532.
- Martinelli, S.; Urosevic, M.; Baryadel, A.; Oberholzer, P.A.; Baumann, C.; Fey, M.F.; Dummer, R.; Simon, H.U.; Yousefi, S. Induction of Genes Mediating Interferon-Dependent Extracellular Trap Formation during Neutrophil Differentiation. J. Biol. Chem. 2004, 279, 44123–44132.
- Valadez-Cosmes, P.; Maitz, K.; Kindler, O.; Raftopoulou, S.; Kienzl, M.; Santiso, A.; Mihalic, Z.N.; Brcic, L.; Lindenmann, J.; Fediuk, M.; et al. Identification of Novel Low-Density Neutrophil Markers Through Unbiased High-Dimensional Flow Cytometry Screening in Non-Small Cell Lung Cancer Patients. Front. Immunol. 2021, 12, 703846.
- Hsu, B.E.; Tabariès, S.; Johnson, R.M.; Andrzejewski, S.; Senecal, J.; Lehuédé, C.; Annis, M.G.; Ma, E.H.; Völs, S.; Ramsay, L.A.; et al. Immature Low-Density Neutrophils Exhibit Metabolic Flexibility That Facilitates Breast Cancer Liver Metastasis. Cell Rep. 2019, 27, 3902–3915.
- Yajuk, O.; Baron, M.; Toker, S.; Zelter, T.; Fainsod-Levi, T.; Granot, Z. The PD-L1/PD-1 Axis Blocks Neutrophil Cytotoxicity in Cancer. Cells 2021, 10, 1510.
- Zhou, J.; Nefedova, Y.; Lei, A.; Gabrilovich, D. Neutrophils and PMN-MDSCs: Their Biological Role and Interaction with Stromal Cells. Semin Immunol. 2018, 35, 19–28.
- Kusmartsev, S.; Nagaraj, S.; Gabrilovich, D.I. Tumor-Associated CD8+ T Cell Tolerance Induced by Bone Marrow-Derived Immature Myeloid Cells. J. Immunol. 2005, 175, 4583–4592.
- Vanhaver, C.; van der Bruggen, P.; Bruger, A.M. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872.
- Sinha, P.; Chornoguz, O.; Clements, V.K.; Artemenko, K.A.; Zubarev, R.A.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Express the Death Receptor Fas and Apoptose in Response to T Cell–Expressed FasL. Blood 2011, 117, 5381.
- Youn, J.-I.; Nagaraj, S.; Collazo, M.; Gabrilovich, D.I. Subsets of Myeloid-Derived Suppressor Cells in Tumor Bearing Mice. J. Immunol. 2008, 181, 5791.
- Yang, L.; DeBusk, L.M.; Fukuda, K.; Fingleton, B.; Green-Jarvis, B.; Shyr, Y.; Matrisian, L.M.; Carbone, D.P.; Lin, P.C. Expansion of Myeloid Immune Suppressor Gr+CD11b+ Cells in Tumor-Bearing Host Directly Promotes Tumor Angiogenesis. Cancer Cell 2004, 6, 409–421.
- Ortiz-Espinosa, S.; Morales, X.; Senent, Y.; Alignani, D.; Tavira, B.; Macaya, I.; Ruiz, B.; Moreno, H.; Remírez, A.; Sainz, C.; et al. Complement C5a Induces the Formation of Neutrophil Extracellular Traps by Myeloid-Derived Suppressor Cells to Promote Metastasis. Cancer Lett. 2022, 529, 70–84.
- Kramer, E.D.; Abrams, S.I. Granulocytic Myeloid-Derived Suppressor Cells as Negative Regulators of Anticancer Immunity. Front. Immunol. 2020, 11, 1963.
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498.
- Youn, J.-I.; Collazo, M.; Shalova, I.N.; Biswas, S.K.; Gabrilovich, D.I. Characterization of the Nature of Granulocytic Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. J. Leukoc. Biol. 2012, 91, 167.
- Pylaeva, E.; Lang, S.; Jablonska, J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front. Immunol. 2016, 7, 629.
- Giese, M.A.; Hind, L.E.; Huttenlocher, A. Neutrophil Plasticity in the Tumor Microenvironment. Blood 2019, 133, 2159–2167.
- Yan, B.; Wei, J.-J.; Yuan, Y.; Sun, R.; Li, D.; Luo, J.; Liao, S.-J.; Zhou, Y.-H.; Shu, Y.; Wang, Q.; et al. IL-6 Cooperates with G-CSF To Induce Protumor Function of Neutrophils in Bone Marrow by Enhancing STAT3 Activation. J. Immunol. 2013, 190, 5882–5893.
- Zou, J.-M.; Qin, J.; Li, Y.-C.; Wang, Y.; Li, D.; Shu, Y.; Luo, C.; Wang, S.-S.; Chi, G.; Guo, F.; et al. IL-35 Induces N2 Phenotype of Neutrophils to Promote Tumor Growth. Oncotarget 2017, 8, 33501–33514.
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-Associated Neutrophils (TAN) Develop pro-Tumorigenic Properties during Tumor Progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756.
- Patel, S.; Fu, S.; Mastio, J.; Dominguez, G.A.; Purohit, A.; Kossenkov, A.; Lin, C.; Alicea-Torres, K.; Sehgal, M.; Nefedova, Y.; et al. Unique Pattern of Neutrophil Migration and Function during Tumor Progression. Nat. Immunol. 2018, 19, 1236–1247.
- Lovászi, M.; Németh, Z.H.; Pacher, P.; Gause, W.C.; Wagener, G.; Haskó, G. A2A Adenosine Receptor Activation Prevents Neutrophil Aging and Promotes Polarization from N1 towards N2 Phenotype. Purinergic Signal. 2022, 18, 345–358.
- Zhao, Z.; Liu, T.; Liang, Y.; Cui, W.; Li, D.; Zhang, G.; Deng, Z.; Chen, M.; Sha, K.; Xiao, W.; et al. N2-Polarized Neutrophils Reduce Inflammation in Rosacea by Regulating Vascular Factors and Proliferation of CD4+ T Cells. J. Investig. Dermatol. 2022, 142, 1835–1844.e2.
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 12, 3181.
- Cai, B.; Lin, D.; Li, Y.; Wang, L.; Xie, J.; Dai, T.; Liu, F.; Tang, M.; Tian, L.; Yuan, Y.; et al. N2-Polarized Neutrophils Guide Bone Mesenchymal Stem Cell Recruitment and Initiate Bone Regeneration: A Missing Piece of the Bone Regeneration Puzzle. Adv. Sci. 2021, 8, 2100584.
- Yao, J.; Zheng, J.; Cai, J.; Zeng, K.; Zhou, C.; Zhang, J.; Li, S.; Li, H.; Chen, L.; He, L.; et al. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Rat Hepatic Ischemia-Reperfusion Injury by Suppressing Oxidative Stress and Neutrophil Inflammatory Response. FASEB J. 2019, 33, 1695–1710.
- Nederlof, R.; Reidel, S.; Spychala, A.; Gödecke, S.; Heinen, A.; Lautwein, T.; Petzsch, P.; Köhrer, K.; Gödecke, A. Insulin-Like Growth Factor 1 Attenuates the Pro-Inflammatory Phenotype of Neutrophils in Myocardial Infarction. Front. Immunol. 2022, 13, 908023.
- Pakravan, N.; Hassan, Z.M.; Abbasi, A. Intra-Nasal Administration of Sperm Head Turns Neutrophil into Reparative Mode after PGE1- and/or Ang II Receptor-Mediated Phagocytosis Followed by Expression of Sperm Head’s Coding RNA. Int. Immunopharmacol. 2021, 98, 107696.
- Guimarães-Bastos, D.; Frony, A.C.; Barja-Fidalgo, C.; Moraes, J.A. Melanoma-Derived Extracellular Vesicles Skew Neutrophils into a pro-Tumor Phenotype. J. Leukoc. Biol. 2022, 111, 585–596.
- Pember, S.O.; Barnes, K.C.; Brandt, S.J.; Kinkade, J.M. Density Heterogeneity of Neutrophilic Polymorphonuclear Leukocytes: Gradient Fractionation and Relationship to Chemotactic Stimulation. Blood 1983, 61, 1105–1115.
- Cassatella, M.A.; Scapini, P. On the Improper Use of the Term High-Density Neutrophils. Trends Immunol. 2020, 41, 1059–1061.
- Kaisar-Iluz, N.; Arpinati, L.; Shaul, M.E.; Mahroum, S.; Qaisi, M.; Tidhar, E.; Fridlender, Z.G. The Bilateral Interplay between Cancer Immunotherapies and Neutrophils’ Phenotypes and Sub-Populations. Cells 2022, 11, 783.
- Saraiva, D.P.; Correia, B.F.; Salvador, R.; de Sousa, N.; Jacinto, A.; Braga, S.; Cabral, M.G.; Saraiva, D.P.; Correia, B.F.; Salvador, R.; et al. Circulating Low Density Neutrophils of Breast Cancer Patients Are Associated with Their Worse Prognosis Due to the Impairment of T Cell Responses. Oncotarget 2021, 12, 2388–2403.
- Shaul, M.E.; Eyal, O.; Guglietta, S.; Aloni, P.; Zlotnik, A.; Forkosh, E.; Levy, L.; Weber, L.M.; Levin, Y.; Pomerantz, A.; et al. Circulating Neutrophil Subsets in Advanced Lung Cancer Patients Exhibit Unique Immune Signature and Relate to Prognosis. FASEB J. 2020, 34, 4204–4218.
- Guglietta, S.; Chiavelli, A.; Zagato, E.; Krieg, C.; Gandini, S.; Ravenda, P.S.; Bazolli, B.; Lu, B.; Penna, G.; Rescigno, M. Coagulation Induced by C3aR-Dependent NETosis Drives Protumorigenic Neutrophils during Small Intestinal Tumorigenesis. Nat. Commun. 2016, 7, 11037.
- Liu, Y.; Hu, Y.; Gu, F.; Liang, J.; Zeng, Y.; Hong, X.; Zhang, K.; Liu, L. Phenotypic and Clinical Characterization of Low Density Neutrophils in Patients with Advanced Lung Adenocarcinoma. Oncotarget 2017, 8, 90969–90978.
- Marini, O.; Costa, S.; Bevilacqua, D.; Calzetti, F.; Tamassia, N.; Spina, C.; De Sabata, D.; Tinazzi, E.; Lunardi, C.; Scupoli, M.T.; et al. Mature CD10+ and Immature CD10− Neutrophils Present in G-CSF–Treated Donors Display Opposite Effects on T Cells. Blood 2017, 129, 1343–1356.
- Sun, R.; Xiong, Y.; Liu, H.; Gao, C.; Su, L.; Weng, J.; Yuan, X.; Zhang, D.; Feng, J. Tumor-Associated Neutrophils Suppress Antitumor Immunity of NK Cells through the PD-L1/PD-1 Axis. Transl. Oncol. 2020, 13, 100825.
- Arasanz, H.; Bocanegra, A.I.; Morilla, I.; Fernández-Irigoyen, J.; Martínez-Aguillo, M.; Teijeira, L.; Garnica, M.; Blanco, E.; Chocarro, L.; Ausin, K.; et al. Circulating Low Density Neutrophils Are Associated with Resistance to First Line Anti-PD1/PDL1 Immunotherapy in Non-Small Cell Lung Cancer. Cancers 2022, 14, 3846.
- Ning, X.; Wang, W.M.; Jin, H.Z.; Fang, W. Low-Density Granulocytes in Immune-Mediated Inflammatory Diseases. J. Immunol. Res. 2022, 2022, 1622160.
- Zhang, Y.; Wang, Q.; Mackay, C.R.; Ng, L.G.; Kwok, I. Neutrophil Subsets and Their Differential Roles in Viral Respiratory Diseases. J. Leukoc. Biol. 2022, 111, 1159–1173.
- Cabrera, L.E.; Pekkarinen, P.T.; Alander, M.; Nowlan, K.H.A.; Nguyen, N.A.; Jokiranta, S.; Kuivanen, S.; Patjas, A.; Mero, S.; Pakkanen, S.H.; et al. Characterization of Low-Density Granulocytes in COVID-19. PLoS Pathog. 2021, 17, e1009721.
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the Pathogenesis of Rheumatoid Arthritis and Systemic Lupus Erythematosus: Same Foe Different M.O. Front. Immunol. 2021, 12, 649693.
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499.
- Aarts, C.E.M.; Kuijpers, T.W. Neutrophils as Myeloid-Derived Suppressor Cells. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12989.
- Que, H.; Fu, Q.; Lan, T.; Tian, X.; Wei, X. Tumor-Associated Neutrophils and Neutrophil-Targeted Cancer Therapies. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188762.
