Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Annalisa D'Arco | -- | 2007 | 2023-01-19 15:23:03 | | | |
| 2 | Lindsay Dong | -33 word(s) | 1974 | 2023-01-20 03:31:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mancini, T.; Marcelli, A.; Lupi, S.; D’arco, A. Terahertz Technologies for Virus Sensing. Encyclopedia. Available online: https://encyclopedia.pub/entry/40419 (accessed on 07 February 2026).
Mancini T, Marcelli A, Lupi S, D’arco A. Terahertz Technologies for Virus Sensing. Encyclopedia. Available at: https://encyclopedia.pub/entry/40419. Accessed February 07, 2026.
Mancini, Tiziana, Augusto Marcelli, Stefano Lupi, Annalisa D’arco. "Terahertz Technologies for Virus Sensing" Encyclopedia, https://encyclopedia.pub/entry/40419 (accessed February 07, 2026).
Mancini, T., Marcelli, A., Lupi, S., & D’arco, A. (2023, January 19). Terahertz Technologies for Virus Sensing. In Encyclopedia. https://encyclopedia.pub/entry/40419
Mancini, Tiziana, et al. "Terahertz Technologies for Virus Sensing." Encyclopedia. Web. 19 January, 2023.
Copy Citation
The recent pandemic of SARS-CoV-2 virus has made evident critical issues relating to virus sensing and the need for deployable tools for adequate, rapid, effective viral recognition on a large-scale. Although many conventional molecular and immuno-based techniques are widely used for these purposes, they still have some drawbacks concerning sensitivity, safety, laboriousness, long-term collection and data analysis. Therefore, new rapidly emerging approaches have been introduced such as terahertz (THz)-based technologies. The emerging Terahertz (THz) technology is an ideal candidate for virus monitoring and detection purposes, offering various advantages which can be explored.
THz radiation
THz spectroscopy
THz technology
virus sensing
1. Introduction
Pandemic crises, caused by infections due to Zika, Ebola, and the recent severe acute respiratory syndrome coronaviruses such as SARS-CoV-2, are burdening the healthcare systems, seriously threating societal and economic stability [1]. Furthermore, the extent of the recent SARS-CoV-2 pandemic, its rapid large-scale spreading, the challenging management in many countries and the low reliability of early screening protocols have seriously impacted the healthcare systems. It is needless to say that effective virus diagnostic methods, based on rapid, reliable and accurate monitoring, can contribute toward controlling and preventing future pandemic events. Nowadays, the most widely used methods for viral diagnosis are mainly based on biomolecular techniques and immunoassays [2][3][4][5][6][7][8], namely, CRISPR–Cas12- [9] and CRISPR–Cas13-based SHERLOCK systems [10][11], real-time quantitative polymerase chain reaction (RT-PCR) [12][13][14][15], nucleic acid amplification tests (NAATs), immunofluorescence [16], enzymatic immunosorbent assays (ELISAs) [17][18] and side treatment point flow immunological assays (POCs) [19][20]. These molecular methods are considered the standard approaches for detecting the presence of viral components (genetic and/or protein material) in potentially infected individuals. However, in some cases, they may give rise to false negative results if the viral RNA charge at the time of detection is insufficient. For example, hemagglutination inhibition assays [21] exhibit low specificity under a certain agglutination level and when the samples contain non-specific hemagglutination factors [22]. Immunoassays may provide information on the status of ongoing viral infections and early exposure. Despite this, the main disadvantage of the aforementioned methods is the inability to identify the infection at a low viral charge, e.g., in the initial stage of the disease. This condition hardly depends on the immune response, which is detectable only several days after direct contact with the virus. In addition, most of the above-mentioned diagnosis processes have limitations, such as being time-consuming, labor-intensive and not reagent-free, as well as possessing poor sensitive and a slow detection process. These methods need sophisticated equipment and well-trained personnel to handle the tests. Therefore, complementary, reliable, fast, sensitive, easy-to-use and cost-effective point-of-care diagnostics methods are highly desirable. In such circumstances, various bio-sensor platforms, based on electrical, mechanical [23], optical [24] and plasmonic [24][25][26][27][28] approaches, have shown promising and appealing applications ranging from laboratory to clinical/medical investigations, with a high potential in miniaturized, real-time and label-free sensing [28][29][30][31]. All biosensor-based approaches have a common schematic layout, as reported in Figure 1. A specific bioreceptor surface selectively adsorbs/captures the analyte of interest and then generates a signal (in the form of light, voltage, current, charge or mass change, variation of refractive index, etc.) as a result of the interaction between the bioreceptor and the analyte. The bio-recognition event results in constituting highly sensitive detection and discrimination signals. These data are converted by a transducer into another form of energy, and then amplified and processed in order to record a direct, measurable and readable signal, generally proportional to the amount of interaction between the analyte–bioreceptor. Biosensor-based approaches exploit different intrinsic chemical, electrical or energetic properties of bio-macromolecules constituting virus structure. For example, concerning the electrical-based approach, viral biological molecules capacitance or impedance have been studied and considered as significant discriminative quantities. For instance, as Al Ahmad and co-workers showed, the electrical properties of viral suspensions depend upon protein, lipidic and envelope structures of the considered species; therefore, measurements of capacitance constitute a unique discrimination quantity. However, it has to be pointed out that only electrically polarizable virions can be detected and recognized with this method [32][33]. An innovative application of this electrical approach is reported in the study by MacCuspie et al. [34], who first exploited AC capacitance scanning probe microscopy to investigate biological samples, proving that different viruses have specific capacitance values. This is due to different capsid proteins and glycoproteins, which highly influence the dielectric properties defining the viral strain.
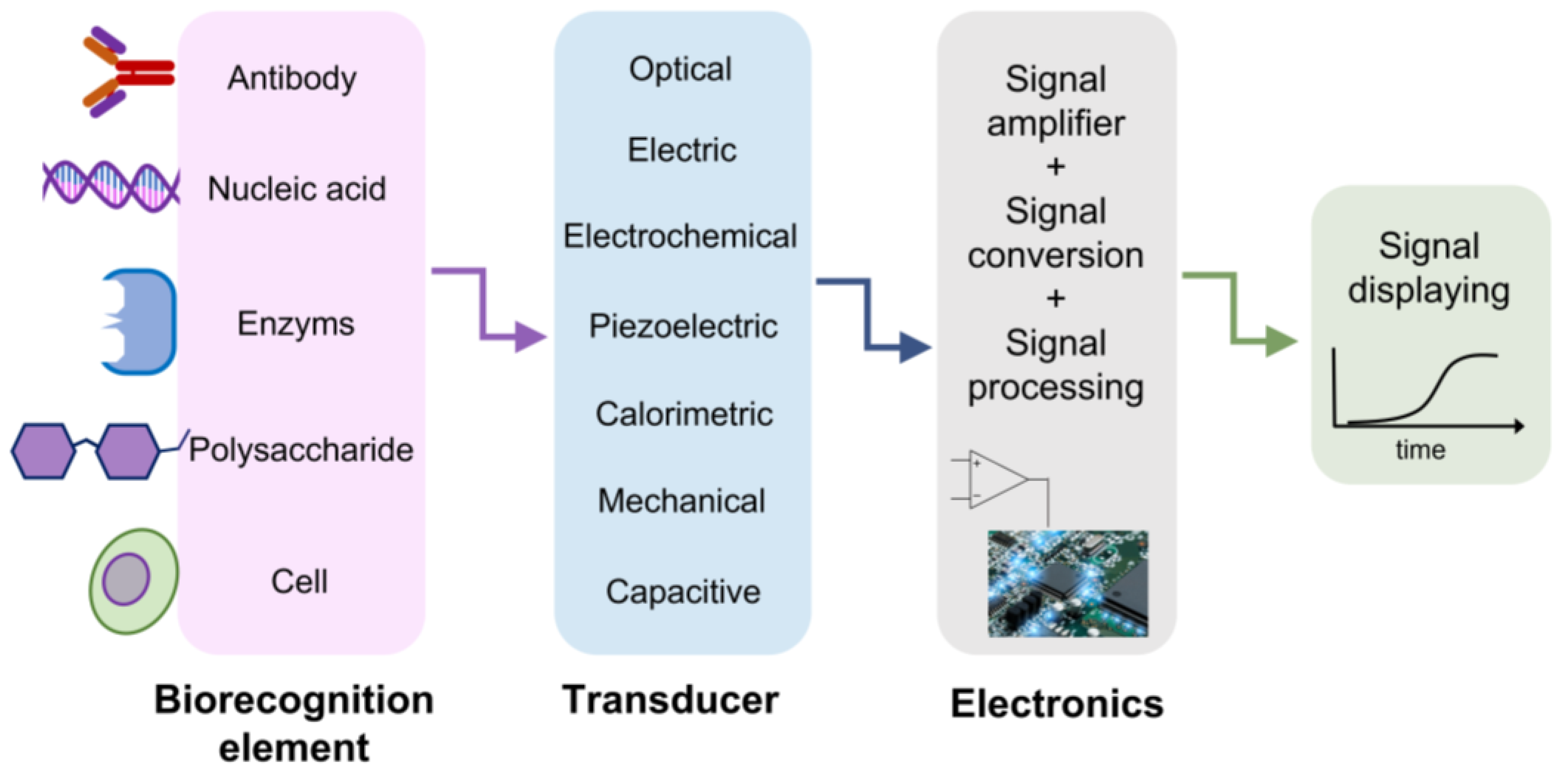
Figure 1. A schematic representation of biosensor structure. The biorecognition element is chosen to effectively catch the analyte of interest. The transducer reveals the presence of the analyte thanks to the variation of a physical quantity, depending on the approach exploited. The achieved signal is amplified, digitalized, processed and read on a display.
In this plethora of different techniques, another advantageous approach is represented by optical biosensors, which are essentially based on exploiting the different virus optical responses to an incident electromagnetic field. Different bio-macromolecular structures and compositions result in different refractive indexes and optical properties. Optical platforms have gained considerable attention for their potential in remote diagnosis schemes and their compatibility with physiological and serological solutions. Furthermore, within the optical-based sensor, a fundamental and essential role is played by the plasmonic approach, which instead is based on specific materials resonances (e.g., the surface plasmons), whose properties, and, in particular, their characteristic frequencies, are modified when a virus is present on their surfaces.
Figure 2 reports a graphical summary of the main detection approaches for virus sensing which are currently in use.
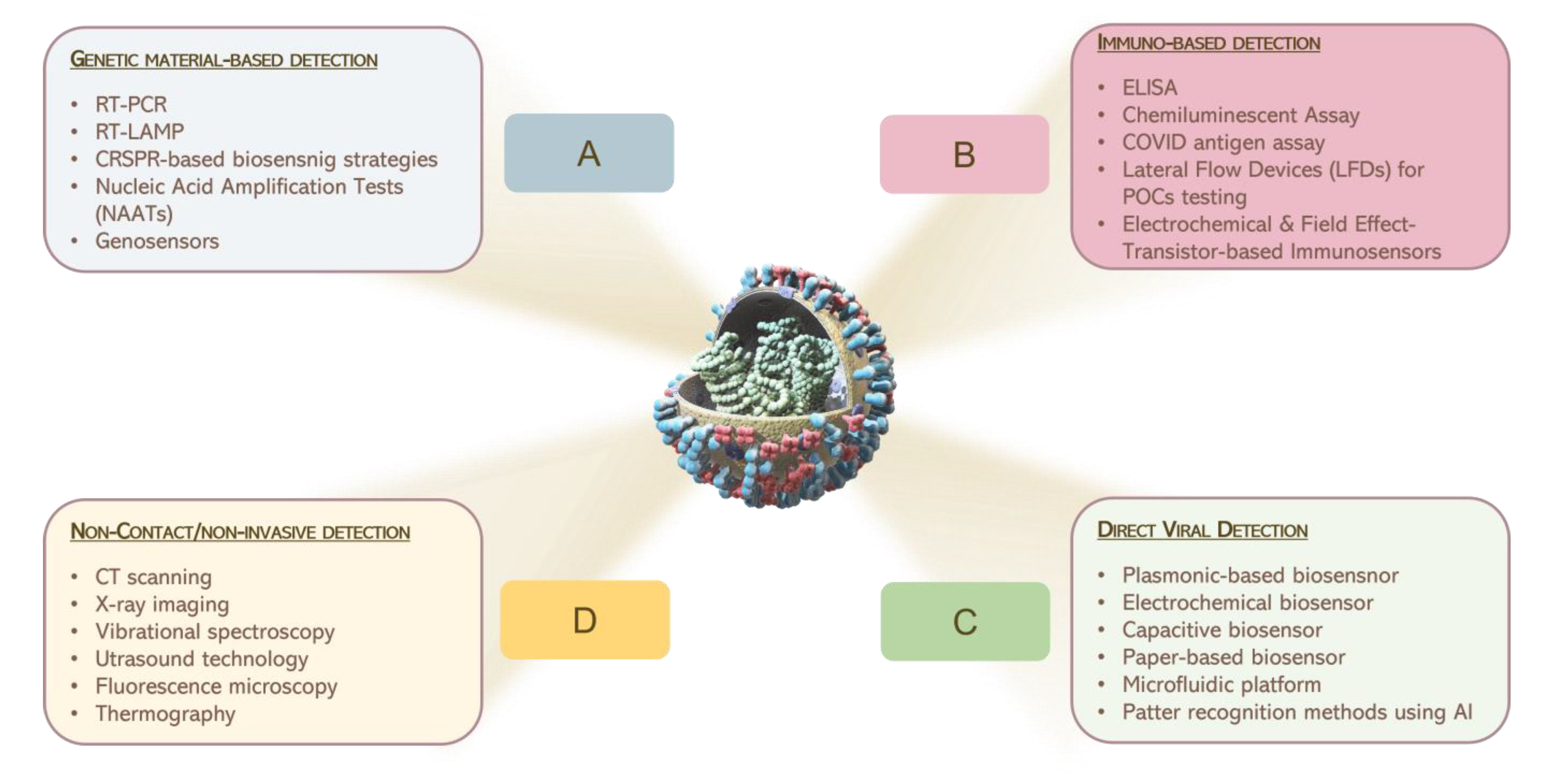
Figure 2. Graphical illustration of the main consolidate processes for the detection of pathogens, currently in use.
2. THz Technology for Virus Sensing
Despite the extensive attention given to microwave, infrared and visible regions, there is a small gap between microwave and infrared (0.1–10 THz, 3–330 cm−1), called the THz spectral range. This region of the electromagnetic spectrum has been often ignored because of the technological difficulties in THz generation and detection. In recent years, THz technology has grown, driven by improvements in sources, detector responses [35][36][37][38][39][40][41][42] and the availability of new materials with a strong THz response [43][44]. This has promoted the diffusion of THz research into various areas, e.g., air-quality and gas sensing [45][46][47][48][49][50], material sciences [42][44][51][52][53][54], microelectronics and security [55][56], agri-food quality [57], cultural heritage [58], in addition to biomedicine and bio-imaging [59][60][61][62][63]. For biomedical and biochemical issues, THz radiation is really appealing because of its low photon energy (around few meV, 4 meV @ 1 THz), and too low to heat materials and/or induce atom/molecule ionization; therefore, it enables non-destructive and non-ionizing sensing [64]. This is in contrast with other spectroscopic techniques, including ultraviolet or X-rays, where high-energy photons (>>eV) induce damage to the bio-sample [64]. In addition, THz radiation, characterized by low photon energy, is associated with energy levels matching low-frequency vibrational modes, including the collective vibrations of intermolecular and intramolecular interactions, such as hydrogen bonds [65], the phonon modes of crystalline molecular solids and vibrations of many macromolecules. In these circumstances, THz spectroscopy has been employed to investigate low-frequency vibrational modes of amino acids and proteins, due to its sensitivity to intermolecular interactions, such as hydrogen bonds, which in turn are dependent on molecular conformations and surrounding environments [66][67]. Therefore, THz radiation directly identifies a material’s spectral properties constituting its molecular fingerprint, offering, in this way, a chemical specificity to imaging and spectroscopy experiments, in a label-free, non-contact and non-invasive mode [59][68][69]. Moreover, it is worth to point out that non-polar materials (such as paper, cloths and plastic) are usually transparent in the THz range [55][70][71]. In contrast, the high sensitivity to polar molecules, such as water (absorption coefficient around 220 cm−1 for pure water @ 1 THz), and the low spatial resolution are the main drawbacks of THz radiation [72][73]. In fact, the extreme absorption, shown by THz radiation for polar molecules, specifically water, restricts the penetrability of THz waves from tens to hundreds of microns in hydrated samples. The diagnostic capability, especially in the case of biomedical applications in vivo or on fresh tissues, is then reduced. Nevertheless, the high sensitivity of water content can be used like an endogenous marker for the differentiation between fresh healthy and pathological tissues and preventing a wider range of applications in biology [69][74]. Referring to THz spectroscopy, many layouts and materials are used for THz signal collection [75][76][77], showing high performance in terms of the signal-to-noise ratio (SNR) and coherent detection mode. Because THz spectroscopy is insensitive to the thermal background, it has a high SNR, not requiring the use of cooled detectors [78]. Concerning the coherent detection mode, the temporal profile of THz electric field is directly recorded. Therefore, both amplitude and phase of the THz pulse electric field can be simultaneously measured, and the optical parameters, including the sample absorption coefficient and refractive index, can be estimated without using Kramers–Kronig relations [79]. In Table 1, major THz technology features are reported; the advantages and disadvantages are summarized and listed to have an overall view on its potential in terms of detection and discrimination.
Table 1. Summary of THz spectroscopy. Advantages and disadvantages as a detection tool.
| Advantages | Disadvantages |
|---|---|
| Low-energy photon | Low spatial resolution (hundreds µm) |
| No-inflammable | Strong water absorption (220 cm−1 @ 1 THz) |
| No-ionizing radiation | Limited penetration inf fresh tissue |
| Sensitive to polar molecules | |
| Coherent detection | |
| No sample pre-treatment |
In spite of the constraints listed above, several findings have been carried out on biological materials [69][79][80]. Concerning the low spatial resolution, THz radiation suffers from poor spatial resolution due to its large wavelength (λ = 300 µm @ 1 THz) [69][81]. The lateral dimensions of the typical viral pathogens range between 20–300 nm, thus their detection is very challenging because of their sub-wavelength dimensions [82]. The main obstacles for pathogenic monitoring are underlined by the work of Lee and co-workers [83].
They exploited THz spectroscopy to evaluate the optical parameters of H9N2 virus samples in the frequency range 0.2–2.0 THz. The scholars did not show any identifiable spectral features between the absorbance of the freeze-dried virus pellet or the substrate. The weak sensitivity, low detectability and poor chemical selectivity due to the super-position of many biological vibration modes, essentially related to the protein content, prevented the use of THz for virus sensing. However, when a direct assessment fails, indirect virus detection is still possible. Indeed, the probing for antibody–antigen binding properties through THz spectroscopy is more sensitive compared to the standard ELISA [84][85].
Although direct detection is preferable, indirect methods may be favorably to overcome THz limitations and make THz technologies available for pathogenic sensing and biomolecular applications, e.g., in efficient graphene-based sensors, in micro-fluidic chips or in novel meta- and nano-materials [83]. Zhou et al. reported fascinating optoelectrical properties for graphene and predicted the possibility to raise the limit of detection (LOD) of biomolecules using graphene plasmons [82][85]. On the other hand, one of the challenges for biosensing is to perform measurements on minimal amounts. In this context, micro-fluidic chips are suitable for investigation in different physiological and serological environments [86]. They are able to select extremely small amounts of liquid and trap the molecules in the micro-fluidic channel, thus limiting the strong THz water absorption and providing very concentrated measurements. Devices based on meta- and nano-materials [85][87][88][89][90][91][92][93] have gained popularity as promising protein and DNA detection platforms [24][94][95][96][97] because of their operational simplicity, compactness and their attractive electromagnetic properties, such as the excitation of surface plasmon polaritons (SPPs) [98][99][100][101] and the localization and enhancement of the electric field associated with the incoming radiation.
Thus, the local dielectric changes generated by biological samples, such as viruses, may be successfully detected. In addition, since very thin water layers are required (a few tenths of a µm), these layouts easily overcome the limitation imposed by the strong THz water absorption [102]. The sensor specificity or biological selectivity may be increased with functionalization, e.g., anchoring the bio-analytes and/or bio-components of interest onto the meta- or nano-material platforms. Various approaches have been proposed: functionalization with alkanethiol molecules of well-ordered covalently bonded monolayers, the generation of hydroxyl groups by oxygen plasma or the surface chemical modifications using silane and silanol chemistries, the COx-H modification, the anchoring and/or the decoration with antigens [103][104][105][106].
References
- Campi, G.; Perali, A.; Marcelli, A.; Bianconi, A. Sars-CoV-2 world pandemic recurrent waves controlled by variants evolution and vaccination campaign. Sci. Rep. 2022, 12, 18108.
- Tsongalis, G. Branched DNA technology in molecular diagnostics. Am. J. Clin. Pathol. 2006, 126, 448–453.
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The disease and tools for detection. ACS Nano 2020, 14, 3822–3835.
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848.
- Yan, C.; Cui, J.; Huang, L.; Du, B.; Chen, L.; Xue, G.; Li, S.; Zhang, W.; Zhao, L.; Sun, Y.; et al. Rapid and visual detection of 2019 novel coronavirus (SARS-CoV-2) by a reverse transcription loop-mediated isothermal amplification assay. Clin. Microbiol. Infect. 2020, 26, 773–779.
- Carter, L.; Garner, L.; Smoot, J.; Li, Y.; Zhou, Q.; Saveson, C.J.-; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 2020, 6, 591–605.
- Sabalza, M.; Yasmin, R.; Barber, C.; Castro, T.; Malamud, D.; Kim, B.; Zhu, H.; Montagna, R.; Abrams, W. Detection of zika virus using reverse-transcription lamp coupled with reverse dot blot analysis in saliva. PLoS ONE 2018, 13, 019239.
- Bastos, M.L.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516.
- Broughton, J.; Deng, X.; Yu, G.; Fasching, C.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR–cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874.
- Kellner, M.; Koob, J.; Gootenberg, J.; Abudayyeh, O.; Zhang, F. Sherlock: Nucleic acid detection with CRISP nucleases. Nat. Prot. 2019, 14, 2986–3012.
- Patchsung, M.; Jantarug, K.; Pattama, A.; Aphicho, K.; Suraritdechachai, S.; Meesawat, P.; Sappakhaw, K.; Leelahakorn, N.; Ruenkam, T.; Wongsatit, T.; et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Engin 2020, 4, 1140–1149.
- Pan, Y.; Zhang, D.; Yang, P.; Poon, L.L.M.; Wang, Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020, 20, 411–412.
- Jartti, T.; Soderlund-Venermo, M.; Hedman, K.; Ruuskanen, O.; Makela, M. New molecular virus detection methods and their clinical value in lower respiratory tract infections in children. Paediatr. Respir. Rev. 2013, 14, 38–45.
- Li, Y.; Yao, L.; Li, J.; Chen, L.; Song, Y.; Cai, Z.; Yang, C. Stability issues of RT-PCR testing of SAS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J. Med. Virol. 2020, 92, 903–908.
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features and future prospects. J. Microbiol. 2015, 53, 1–5.
- Vemula, S.V.; Zhao, J.; Liu, J.; Wang, X.; Biswas, S.; Hewlett, I. Current Approaches for Diagnosis of Influenza Virus Infections in Humans. Viruses 2016, 8, 96.
- Adams, E.; Ainsworth, M.; Anand, R.; Andersson, M.I.; Auckland, K.; Baillie, J.K.; Barnes, E.; Beer, S.; Bell, J.; Berry, T.; et al. Antibody testing for COVID-19: A report from the national covid scientific advisory panel. Wellcome Open Res. 2020, 5, 139.
- Gan, S.D.; Patel, K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Investig. Dermatol. 2013, 133, 12.
- Nguyen, T.; Bang, D.D.; Wolff, A. 2019 novel coronavirus disease (covid-19): Paving the road for rapid detection and point-of-care diagnostics. Micromachines 2020, 11, 306.
- Visseaux, B.; le Hingrat, Q.; Collin, G.; Bouzid, D.; Lebourgeois, S.; le Pluart, D.; Deconinck, L.; Lescure, F.X.; Lucet, J.C.; Bouadma, L.; et al. Evaluation of the qiastat-dx respiratory SARS-CoV-2 panel, the first rapid multiplex PCR commercial assay for SARS-CoV-2 detection. J. Clin. Microbiol. 2020, 58, e00630-20.
- Souf, S. Recent advances in diagnostic testing for viral infections. Biosci. Horiz. Int. J. Stud. Res. 2016, 9, hzw010.
- Soares, M.S.; Durigon, E.L.; Bersano, J.G.; Richtzenhain, L.J. Detection of porcine parvovirus DNA by the polymerase chain reaction assay using primers to the highly conserved nonstructural protein gene, NS-1. J. Virol. Methods 1999, 78, 191–198.
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors Based on Mechanical and Electrical Detection Techniques. Sensors 2020, 20, 5605.
- Xu, W.; Xie, L.; Ying, Y. Mechanisms and applications of terahertz metamaterial sensing: A review. Nanoscale 2017, 9, 13864–13878.
- Mauriz, E. Recent Progress in Plasmonic Biosensing Schemes for Virus Detection. Sensors 2020, 20, 4745.
- Yesilkoy, F.; Terborg, R.A.; Pello, J.; Belushkin, A.A.; Jahani, Y.; Pruneri, V.; Altug, H. Phase-sensitive plasmonic biosensor using a portable and large field-of-view interferometric microarray imager. Light Sci. Appl. 2018, 7, 17152.
- Ji, T.; Liu, Z.; Wang, G.; Guo, X.; Lai, C.; Chen, H.; Huang, S.; Xia, S.; Chen, B.; Jia, H.; et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens. Bioeletron. 2020, 166, 112455.
- Miller, B.S.; Bezinge, L.; Gliddon, H.D.; Huang, D.; Dold, G.; Gray, E.R.; Heaney, J.; Dobson, P.J.; Nastouli, E.; Morton, J.J.; et al. Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 2020, 587, 588–593.
- Moon, G.; Choi, J.r.; Lee, C.; Oh, Y.; Kim, K.; Kim, D. Machine learning-based design of meta-plasmonic biosensors with negative index metamaterials. Biosens. Bioelectron. 2020, 164, 112335.
- Maalouf, R.; Fournier-Wirth, C.; Coste, J.; Chebib, H.; Saïkali, Y.; Vittori, O.; Errachid, A.; Cloarec, J.P.; Martelet, C.; Jaffrezic-Renault, N. Label-free detection of bacteria by electrochemical impedance spectroscopy: Comparison to surface plasmon resonance. Anal. Chem. 2007, 79, 4879–4886.
- Guner, H.; Ozgur, E.; Kokturk, G.; Celik, M.; Esen, E.; Topal, E.A.; Ayas, S.; Uludag, Y.; Elbuken, C.; Dana, A. A smartphone-based surface plasmon resonance imaging (SPRI) platform for on-site biodetection. Sens. Actuators B Chem. 2017, 239, 571–577.
- Al Ahmad, M.; Mustafa, F.; Ali, L.M.; Karakkat, J.V.; Rizvi, T.A. Label-Free Capacitance-Based Identification of Viruses. Sci. Rep. 2015, 5, 9809.
- Cossettini, A.; Selmi, L. On the Response of Nanoelectrode Impedance Spectroscopy Measures to Plant, Animal, and Human Viruses. IEEE Trans. Nanobiosci. 2018, 17, 102–109.
- MacCuspie, R.I.; Nuraje, N.; Lee, S.-Y.; Runge, A.; Matsui, H. Comparison of Electrical Properties of Viruses Studied by AC Capacitance Scanning Probe Microscopy. J. Am. Chem. Soc. 2008, 130, 887–891.
- Siegel, P. Terahertz technology in biology and medicine. IEEE Trans. Microw. Theory 2004, 52, 2438–2447.
- Zhang, X. Terahertz wave imaging: Horizons and hurdles. Phys. Med. Biol. 2002, 47, 3667–3677.
- Wallace, V.P.; Taday, P.F.; Fitzgerald, A.J.; Woodward, R.M.; Cluff, J.; Pye, R.J.; Arnone, D.D. Terahertz pulsed imaging and spectroscopy for biomedical and pharmaceutical applications. Faraday Discuss. 2004, 126, 55–263.
- Curcio, A.; Petrarca, M. Diagnosing plasmas with wideband terahertz pulses. Opt. Lett. 2019, 44, 1011–1014.
- Cheon, H.; Yang, J.-H.; Son, H.J. Toward clinical cancer imaging using terahertz spectroscopy. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 8600109.
- Curcio, A.; Mou, S.; Palumbo, L.; Lupi, S.; Petrarca, M. Selection rules for the orbital angular momentum of optically produced THz radiation. Opt. Lett. 2021, 46, 1514–1517.
- Mou, S.; D’Arco, A.; Tomarchio, L.; Di Fabrizio, M.; Curcio, A.; Lupi, S.; Petrarca, M. Simultaneous elliptically and radially polarized THz from one-color laser-induced plasma filament. New J. Phys. 2021, 23, 063048.
- D’Arco, A.; Tomarchio, L.; Dolci, V.; Di Pietro, P.; Perucchi, A.; Mou, S.; Petrarca, M.; Lupi, S. Broadband anisotropic optical properties of the terahertz generator HMQ-TMS organic crystal. Condens. Matter 2020, 5, 47.
- Lupi, S.; Molle, A. Emerging Dirac materials for THz plasmonics. Appl. Mater. Today 2020, 20, 100732.
- Di Pietro, P.; Ortolani, M.; Limaj, O.; Di Gaspare, A.; Giliberti, V.; Giorgianni, F.; Brahlek, M.; Bansal, N.; Koirala, N.; Oh, S.; et al. Observation of dirac plasmons in a topological insulator. Nat. Nanotechnol. 2013, 8, 556–560.
- Galstyan, V.; D’Arco, A.; Di Fabrizio, M.; Poli, N.; Lupi, S.; Comini, E. Detection of volatile organic compounds: From chemical gas sensors to terahertz spectroscopy. Rev. Anal. Chem. 2021, 40, 33–57.
- D’Arco, A.; Di Fabrizio, M.; Dolci, V.; Marcelli, A.; Petrarca, M.; Della Ventura, G.; Lupi, S. Characterization of volatile organic compounds (VOCs) in their liquid-phase by terahertz time-domain spectroscopy. Biomed. Opt. Exp. 2019, 11, 1–7.
- Naftaly, M.; Vieweg, N.; Deninger, A. Industrial applications of terahertz sensing: State of play. Sensors 2019, 19, 4203.
- D’Arco, A.; Rocco, D.; Magboo, F.P.; Moffa, C.; Della Ventura, G.; Marcelli, A.; Palumbo, L.; Mattiello, L.; Lupi, S.; Petrarca, M. Terahertz continuous wave spectroscopy: A portable advanced method for atmospheric gas sensing. Opt. Exp. 2022, 30, 19005–19016.
- Smith, R.; Arnold, M. Selectivity of terahertz gas-phase spectroscopy. Anal. Chem. 2015, 87, 10679–10683.
- Rothbart, N.; Holz, O.; Koczulla, R.; Schmalz, K.; Hübers, H. Analysis of human breath by millimeter-wave/terahertz spectroscopy. Sensors 2019, 19, 12719.
- Macis, S.; Paolozzi, M.C.; D’Arco, A.; Tomarchio, L.; Di Gaspare, A.; Lupi, S. Terahertz Resonators Based on YBa2Cu3O7 High-Tc Superconductor. Appl. Sci. 2022, 12, 10242.
- Tomarchio, L.; Macis, S.; D’Arco, A.; Mou, S.; Grilli, A.; Romani, M.; Cestelli Guidi, M.; Hu, K.; Kukunuri, S.; Jeong, S.; et al. Disordered photonics behavior from terahertz to ultraviolet of a three-dimensional graphene network. NPG Asia Mater. 2021, 13, 73.
- Rau, J.V.; Fadeeva, I.V.; Forysenkova, A.A.; Davydova, G.A.; Fosca, M.; Filippov, Y.Y.; Antoniac, I.V.; Antoniac, A.; D’Arco, A.; Di Fabrizio, M.; et al. Strontium substituted tricalcium phosphate bone cement: Short and long-term time-resolved studies and in vitro properties. Adv. Mater. Interfaces 2022, 9, 2200803.
- Grazianetti, C.; Bonaventura, E.; Martella, C.; Molle, A.; Lupi, S. Optical Properties of Stanene-like Nanosheets on Al2O3: Implications for Xene Photonics. ACS Appl. Nano Mater. 2021, 4, 2351–2356.
- D’Arco, A.; Mussi, V.; Petrov, S.; Tofani, S.; Petrarca, M.; Beccherelli, R.; Dimitrov, D.; Marinova, V.; Lupi, S.; Zografopoulos, D. Fabrication and spectroscopic characterization of graphene transparent electrodes on flexible cyclo-olefin substrates for terahertz electro-optic applications. Nanotechnology 2020, 31, 364006.
- Federici, J.; Schulkin, B.; Huang, F.; Gary, D.; Barat, R.; Oliveira, F.; Zimdars, D. THz imaging and sensing for security applications—explosives, weapons and drugs. Semicond. Sci. Technol. 2005, 20, 266.
- Wang, K.; Sun, D.W.; Pu, H. Emerging non-destructive terahertz spectroscopic imaging technique: Principle and applications in the agri-food industry. Trend Food Sci. Technol. 2017, 67, 93–105.
- Cosentino, A. Terahertz and cultural heritage science: Examination of art and archeology. Technologies 2016, 4, 6.
- D’Arco, A.; Di Fabrizio, M.; Dolci, V.; Petrarca, M.; Lupi, S. THz pulsed imaging in biomedical applications. Condens. Matter 2020, 5, 25.
- Di Fabrizio, M.; D’Arco, A.; Mou, S.; Palumbo, L.; Petrarca, M.; Lupi, S. Performance evaluation of a THz pulsed imaging system: Point spread function, broadband THz beam visualization and image reconstruction. Appl. Sci. 2021, 11, 562.
- Mancini, T.; Mosetti, R.; Marcelli, A.; Petrarca, M.; Lupi, S.; D’Arco, A. Terahertz spectroscopic analysis in protein dynamics: Current status. Radiation 2022, 2, 100–123.
- Di Fabrizio, M.; Lupi, S.; D’Arco, A. Virus recognition with terahertz radiation: Drawbacks and potentialities. J. Phys. Photonics 2021, 3, 032001.
- Fardelli, E.; D’Arco, A.; Lupi, S.; Billi, D.; Moeller, R.; Cestelli Guidi, M. Spectroscopic evidence of the radioresistance of Chroococcidiopsis biosignatures: A combined Raman, FT-IR and THz-TDs spectroscopy study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 288, 122148.
- Manti, L.; D’Arco, A. Cooperative biological effects between ionizing radiation and other physical and chemical agents. Mutat. Res. Rev. Mutat. Res. 2010, 704, 115–122.
- Parrott, E.P.J.; Zeitler, J.A. Terahertz Time-Domain and Low-Frequency Raman Spectroscopy of Organic Materials. Appl. Spectrosc. 2015, 69, 1–25.
- Walther, M.; Plochocka, P.; Fischer, B.; Helm, H.; Jepsen, P. Collective vibrational modes in biological molecules investigated by terahertz time-domain spectroscopy. Biopolymers 2002, 67, 310–313.
- Lee, D.; Cheon, H.; Jeong, S.-Y.; Son, J.-H. Transformation of terahertz vibrational modes of cytosine under hydration. Sci Rep 2020, 10, 10271.
- Ghann, W.; Uddin, L. Terahertz (THz) spectroscopy: A cutting-edge technology terahertz spectroscopy—A cutting edge. In Terahertz (THz) Spectroscopy: A Cutting Edge Technology Terahertz Spectroscopy—A Cutting Edge; Uddin, J., Ed.; Chapman and Hall, IntechOpen Limited: London, UK, 2017.
- Yang, X.; Zhao, X.; Yang, K.; Liu, Y.; Liu, Y.; Fu, W.; Luo, Y. Biomedical applications of terahertz spectroscopy and imaging. Trends Biotechnol. 2016, 34, 810–824.
- Rezvani, S.J.; Di Gioacchino, D.; Tofani, S.; D’Arco, A.; Ligi, C.; Lupi, S.; Gatti, C.; Cestelli Guidi, M.; Marcelli, A. A cryogenic magneto-optical device for long wavelength radiation. Rev. Sci. Instrum. 2020, 91, 075103.
- Fedulova, E.; Nazarov, M.; Angeluts, A.; Kitai, M.; Sokolov, V.; Shkurinov, A. Studying of dielectric properties of polymers in the terahertz frequency range. In Optical Technologies in Biophysics and Medicine XIII, Proceedings of the Saratov Fall Meeting 2011, Saratov, Russia, 27–30 September 2011; SPIE: Bellingham, WA, USA, 2011.
- Marcelli, A.; Irizawa, A.; Lupi, S. THz: Research frontiers for new sources, imaging and other advanced technologies. Condens. Matter 2019, 6, 23.
- Lin, H.; Withayachumnankul, W.; Fischer, B.; Mickan, S.; Abbott, D. Gas recognition with terahertz time-domain spectroscopy and spectral catalog: A preliminary study. Terahertz Photonics 2008, 6840, 68400X.
- Zhang, X.-C.; Jingzhou, X. Introduction to THz Wave Photonics, 1st ed.; Springer: Boston, MA, USA, 2010.
- Burford, N.; El-Shenawee, M.O. Review of terahertz photoconductive antenna technology. Opt. Eng. 2017, 56, 010901.
- Dexheimer, S.L. Terahertz Spectroscopy: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2017.
- Naftaly, M. Terahertz Metrology; Artech House: London, UK, 2015.
- Seo, M.; Park, H.-R. Terahertz Biochemical Molecules-specific sensors. Adv. Opt. Mater. 2019, 8, 1900662.
- Ajito, K.; Ueno, Y. THz chemical imaging for biological applications. IEEE Trans. Terahertz Sci. Technol. 2011, 1, 293–300.
- Cooksey, C.C.; Greer, B.J.; Heilweil, E.J. Terahertz Spectroscopy of L-Proline in Reverse Aqueous Micelles. Chem. Phys. Lett. 2009, 467, 424–429.
- Abbe, E. Beitr¨age zur theorie des mikroskops und der mikroskopischen wahrnehmung. Archiv. Mikrosk. Anat. 1873, 9, 413–468.
- Park, S.J.; Cha, S.H.; Shin, G.A.; Ahn, Y.H. Sensing viruses using terahertz nano-gap metamaterials. Biomed. Opt. Express 2017, 8, 3551–3558.
- Lee, D.-K.; Kang, J.-H.; Know, J.; Lee, J.-S.; Lee, S.; Woo, D.; Kim, J.; Song, C.-S.; Park, Q.; Seo, M. Nano metamaterials for ultrasensitive terahertz biosensing. Sci. Rep. 2017, 7, 8146.
- Sun, Y.; Zhang, Y.; Pickwell-MacPherson, E. Investigating antibody interactions with a polar liquid using terahertz pulsed spectroscopy. Biophys. J. 2011, 100, 225–231.
- Zhou, R.; Wang, C.; Wendao, X.; Xie, L. Biological applications of terahertz technology based on nanomaterials and nanostructures. Nanoscale 2019, 11, 3445–3457.
- Zhou, R.; Wang, C.; Huang, Y.; Huang, K.; Wang, Y.; Xu, W.; Xie, L.; Ying, Y. Label-free Terahertz Microfluidic Biosensor for Sensitive DNA Detection Using Graphene-Metasurface Hybrid Structures. Biosens. Bioelectron. 2021, 188, 113336.
- Tang, Q.; Liang, M.; Yi, L.; Wong, P.K.; Wilmink, G.J.; Zhang, D.D.; Xin, H. Microfluidic devices for terahertz spectroscopy of live cells toward lab-on-a-chip applications. Sensors 2016, 16, 476.
- Chen, X.; Fan, W. Ultrasensitive terahertz metamaterial sensor based on spoof surface plasmon. Sci. Rep. 2017, 7, 2092.
- Samy Saadeldin, A.; Hameed, M.F.O.; Elkaramany, E.M.A.; Obayya, S.S.A. Highly sensitive terahertz metamaterial sensor. IEEE Sens. J. 2019, 19, 7993–7999.
- Nickpay, M.R.; Danaie, M.; Shahzadi, A. Highly Sensitive THz Refractive Index Sensor Based on Folded Split-Ring Metamaterial Graphene Resonators. Plasmonics 2022, 17, 237–248.
- Karthikeyan, M.; Jayabala, P.; Ramachandran, S.; Dhanabalan, S.S.; Sivanesan, T.; Ponnusamy, M. Tunable Optimal Dual Band Metamaterial Absorber for High Sensitivity THz Refractive Index Sensing. Nanomaterials 2022, 12, 2693.
- Cui, Z.; Wang, Y.; Shi, Y.; Zhu, Y.; Zhang, D.; Hong, Z.; Feng, X. Significant sensing performance of an all-silicon terahertz metasurface chip for Bacillus thuringiensis Cry1Ac protein. Photonics Res. 2022, 10, 740–746.
- Nickpay, M.R.; Danaie, M.; Shahzadi, A. Graphene-based metamaterial absorber for refractive index sensing applications in terahertz band. Diam. Relat. Mater. 2022, 130, 109539.
- Geng, Z.; Zhang, X.; Fan, Z.; Lv, X.; Chen, H. A Route to Terahertz Metamaterial Biosensor Integrated with Microfluidics for Liver Cancer Biomarker Testing in Early Stage. Sci. Rep. 2017, 7, 16378–16411.
- Bhardwaj, S.K.; Bhardwaj, N.; Kumar, V.; Bhatt, D.; Azzouz, A.; Bhaumik, J.; Kim, K.-H.; Deep, A. Recent progress in nanomaterial-based sensing of airborne viral and bacterial pathogens. Environ. Int. 2021, 146, 106183.
- Yang, Y.; Xu, D.; Zhang, W. High-sensitivity and Label-free Identification of a Transgenic Genome Using a Terahertz Meta-Biosensor. Opt. Express 2018, 26, 31589–31598.
- Zhou, J.; Zhao, X.; Huang, G.; Yang, X.; Zhang, Y.; Zhan, X.; Tian, H.; Xiong, Y.; Wang, Y.; Fu, W. Molecule- Specific Terahertz Biosensors Based on an Aptamer Hydrogel-Functionalized Metamaterial for Sensitive Assays in Aqueous Environments. ACS Sens. 2021, 6, 1884–1890.
- Zhang, Y.; Han, Z. Spoof surface plasmon based planar antennas for the realization of terahertz hotspots. Sci. Rep. 2015, 5, 18606.
- Cheng, D.; Xia, H.; Huang, X.; Zhang, B.; Liu, G.; Shu, G.; Fang, C.; Wang, J.; Luo, Y. Terahertz biosensing metamaterial absorber for virus detection based on spoof surface plasmon polaritons. Int. J. RF Microw. Computer-Aided Eng. 2018, 28, e21448.
- Chen, X.; Park, H.-R.; Pelton, M.; Piao, X.; Lindquist, N.C.; Im, H.; Kim, Y.J.; Ahn, J.S.; Ahn, K.J.; Park, N.; et al. Atomic layer lithography of wafer-scale nanogap arrays for extreme confinement of electromagnetic waves. Nat. Commun. 2013, 4, 2361.
- Tang, W.X.; Zhang, H.C.; Ma, H.F.; Jiang, W.X.; Cui, T.J. Concept, theory, design and applications of spoof surface plasmon polaritons at microwave frequencies. Adv. Opt. Mater. 2019, 7, 1800421.
- Hong, J.T.; Jun, S.W.; Cha, S.H.; Park, J.Y.; Lee, S.; Shin, G.A.; Ahn, Y.H. Enhanced sensitivity in THz plasmonic sensors with silver nanowires. Sci. Rep. 2018, 8, 15536.
- Terracciano, M.; Rea, I.; Politi, J.; De Stefano, L. Optical characterization of aminosilane-modified silicon dioxide surface for biosensing. J. Eur. Opt. Soc.-Rap. 2013, 8, 13075.
- De Stefano, L.; Oliviero, G.; Amato, J.; Borbone, N.; Piccialli, G.; Mayol, L.; Rendina, I.; Terracciano, M.; Rea, I. Aminosilane functionalizations of mesoporous oxidized silicon for oligonucleotide synthesis and detection. J. R. Soc. Interface 2013, 10, 20130160.
- Terracciano, M.; Galstyan, V.; Rea, I.; Casalino, M.; De Stefano, L.; Sberveglieri, G. Chemical modification of TiO2 nanotube arrays for label-free optical biosensing applications. Appl. Surf. Sci. 2017, 419, 235–240.
- Xiaojun, W.; Quan, B.; Pan, X.; Xinlong, X.; Xinchao, L.; Changzhi, G.; Wang, L. Alkanethiol-functionalized terahertz metamaterial as label-free, highly-sensitive and specific biosensor. Biosens. Bioelectron. 2013, 42, 626–631.
More
Information
Subjects:
Physics, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
808
Revisions:
2 times
(View History)
Update Date:
20 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




