You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Annalisa D'Arco and Version 2 by Lindsay Dong.
The recent pandemic of SARS-CoV-2 virus has made evident critical issues relating to virus sensing and the need for deployable tools for adequate, rapid, effective viral recognition on a large-scale. Although many conventional molecular and immuno-based techniques are widely used for these purposes, they still have some drawbacks concerning sensitivity, safety, laboriousness, long-term collection and data analysis. Therefore, new rapidly emerging approaches have been introduced such as terahertz (THz)-based technologies. The emerging Terahertz (THz) technology is an ideal candidate for virus monitoring and detection purposes, offering various advantages which can be explored.
- THz radiation
- THz spectroscopy
- THz technology
- virus sensing
1. Introduction
Pandemic crises, caused by infections due to Zika, Ebola, and the recent severe acute respiratory syndrome coronaviruses such as SARS-CoV-2, are burdening the healthcare systems, seriously threating societal and economic stability [1]. Furthermore, the extent of the recent SARS-CoV-2 pandemic, its rapid large-scale spreading, the challenging management in many countries and the low reliability of early screening protocols have seriously impacted the healthcare systems. It is needless to say that effective virus diagnostic methods, based on rapid, reliable and accurate monitoring, can contribute toward controlling and preventing future pandemic events. Nowadays, the most widely used methods for viral diagnosis are mainly based on biomolecular techniques and immunoassays [2][3][4][5][6][7][8][2,3,4,5,6,7,8], namely, CRISPR–Cas12- [9] and CRISPR–Cas13-based SHERLOCK systems [10][11][10,11], real-time quantitative polymerase chain reaction (RT-PCR) [12][13][14][15][12,13,14,15], nucleic acid amplification tests (NAATs), immunofluorescence [16], enzymatic immunosorbent assays (ELISAs) [17][18][17,18] and side treatment point flow immunological assays (POCs) [19][20][19,20]. These molecular methods are considered the standard approaches for detecting the presence of viral components (genetic and/or protein material) in potentially infected individuals. However, in some cases, they may give rise to false negative results if the viral RNA charge at the time of detection is insufficient. For example, hemagglutination inhibition assays [21] exhibit low specificity under a certain agglutination level and when the samples contain non-specific hemagglutination factors [22]. Immunoassays may provide information on the status of ongoing viral infections and early exposure. Despite this, the main disadvantage of the aforementioned methods is the inability to identify the infection at a low viral charge, e.g., in the initial stage of the disease. This condition hardly depends on the immune response, which is detectable only several days after direct contact with the virus. In addition, most of the above-mentioned diagnosis processes have limitations, such as being time-consuming, labor-intensive and not reagent-free, as well as possessing poor sensitive and a slow detection process. These methods need sophisticated equipment and well-trained personnel to handle the tests. Therefore, complementary, reliable, fast, sensitive, easy-to-use and cost-effective point-of-care diagnostics methods are highly desirable. In such circumstances, various bio-sensor platforms, based on electrical, mechanical [23], optical [24] and plasmonic [24][25][26][27][28][24,25,26,27,28] approaches, have shown promising and appealing applications ranging from laboratory to clinical/medical investigations, with a high potential in miniaturized, real-time and label-free sensing [28][29][30][31][28,29,30,31]. All biosensor-based approaches have a common schematic layout, as reported in Figure 1. A specific bioreceptor surface selectively adsorbs/captures the analyte of interest and then generates a signal (in the form of light, voltage, current, charge or mass change, variation of refractive index, etc.) as a result of the interaction between the bioreceptor and the analyte. The bio-recognition event results in constituting highly sensitive detection and discrimination signals. These data are converted by a transducer into another form of energy, and then amplified and processed in order to record a direct, measurable and readable signal, generally proportional to the amount of interaction between the analyte–bioreceptor. Biosensor-based approaches exploit different intrinsic chemical, electrical or energetic properties of bio-macromolecules constituting virus structure. For example, concerning the electrical-based approach, viral biological molecules capacitance or impedance have been studied and considered as significant discriminative quantities. For instance, as Al Ahmad and co-workers showed, the electrical properties of viral suspensions depend upon protein, lipidic and envelope structures of the considered species; therefore, measurements of capacitance constitute a unique discrimination quantity. However, it has to be pointed out that only electrically polarizable virions can be detected and recognized with this method [32][33][32,33]. An innovative application of this electrical approach is reported in the study by MacCuspie et al. [34], who first exploited AC capacitance scanning probe microscopy to investigate biological samples, proving that different viruses have specific capacitance values. This is due to different capsid proteins and glycoproteins, which highly influence the dielectric properties defining the viral strain.
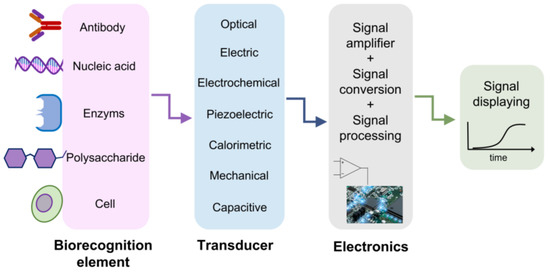
Figure 1. A schematic representation of biosensor structure. The biorecognition element is chosen to effectively catch the analyte of interest. The transducer reveals the presence of the analyte thanks to the variation of a physical quantity, depending on the approach exploited. The achieved signal is amplified, digitalized, processed and read on a display.
In this plethora of different techniques, another advantageous approach is represented by optical biosensors, which are essentially based on exploiting the different virus optical responses to an incident electromagnetic field. Different bio-macromolecular structures and compositions result in different refractive indexes and optical properties. Optical platforms have gained considerable attention for their potential in remote diagnosis schemes and their compatibility with physiological and serological solutions. Furthermore, within the optical-based sensor, a fundamental and essential role is played by the plasmonic approach, which instead is based on specific materials resonances (e.g., the surface plasmons), whose properties, and, in particular, their characteristic frequencies, are modified when a virus is present on their surfaces.
Figure 2 reports a graphical summary of the main detection approaches for virus sensing which are currently in use.
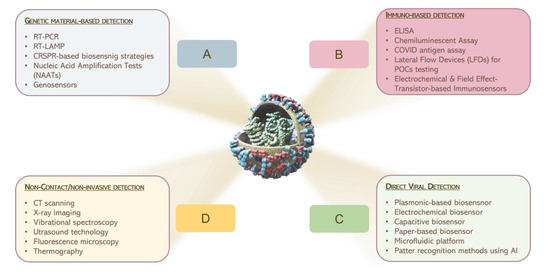
Figure 2.
Graphical illustration of the main consolidate processes for the detection of pathogens, currently in use.
In spite of the constraints listed above, several findings have been carried out on biological materials [69][79][80][69,79,80]. Concerning the low spatial resolution, THz radiation suffers from poor spatial resolution due to its large wavelength (λ = 300 µm @ 1 THz) [69][81][69,81]. The lateral dimensions of the typical viral pathogens range between 20–300 nm, thus their detection is very challenging because of their sub-wavelength dimensions [82]. The main obstacles for pathogenic monitoring are underlined by the work of Lee and co-workers [83].
They exploited THz spectroscopy to evaluate the optical parameters of H9N2 virus samples in the frequency range 0.2–2.0 THz. The scautholars did not show any identifiable spectral features between the absorbance of the freeze-dried virus pellet or the substrate. The weak sensitivity, low detectability and poor chemical selectivity due to the super-position of many biological vibration modes, essentially related to the protein content, prevented the use of THz for virus sensing. However, when a direct assessment fails, indirect virus detection is still possible. Indeed, the probing for antibody–antigen binding properties through THz spectroscopy is more sensitive compared to the standard ELISA [84][85][84,85].
Although direct detection is preferable, indirect methods may be favorably to overcome THz limitations and make THz technologies available for pathogenic sensing and biomolecular applications, e.g., in efficient graphene-based sensors, in micro-fluidic chips or in novel meta- and nano-materials [83]. Zhou et al. reported fascinating optoelectrical properties for graphene and predicted the possibility to raise the limit of detection (LOD) of biomolecules using graphene plasmons [82][85][82,85]. On the other hand, one of the challenges for biosensing is to perform measurements on minimal amounts. In this context, micro-fluidic chips are suitable for investigation in different physiological and serological environments [86][87]. They are able to select extremely small amounts of liquid and trap the molecules in the micro-fluidic channel, thus limiting the strong THz water absorption and providing very concentrated measurements. Devices based on meta- and nano-materials [85][87][88][89][90][91][92][93][85,88,89,90,91,92,93,94] have gained popularity as promising protein and DNA detection platforms [24][94][95][96][97][24,95,96,97,98] because of their operational simplicity, compactness and their attractive electromagnetic properties, such as the excitation of surface plasmon polaritons (SPPs) [98][99][100][101][99,100,101,102] and the localization and enhancement of the electric field associated with the incoming radiation.
Thus, the local dielectric changes generated by biological samples, such as viruses, may be successfully detected. In addition, since very thin water layers are required (a few tenths of a µm), these layouts easily overcome the limitation imposed by the strong THz water absorption [102][109]. The sensor specificity or biological selectivity may be increased with functionalization, e.g., anchoring the bio-analytes and/or bio-components of interest onto the meta- or nano-material platforms. Various approaches have been proposed: functionalization with alkanethiol molecules of well-ordered covalently bonded monolayers, the generation of hydroxyl groups by oxygen plasma or the surface chemical modifications using silane and silanol chemistries, the COx-H modification, the anchoring and/or the decoration with antigens [103][104][105][106][110,111,112,113].
2. THz Technology for Virus Sensing
Despite the extensive attention given to microwave, infrared and visible regions, there is a small gap between microwave and infrared (0.1–10 THz, 3–330 cm−1), called the THz spectral range. This region of the electromagnetic spectrum has been often ignored because of the technological difficulties in THz generation and detection. In recent years, THz technology has grown, driven by improvements in sources, detector responses [35][36][37][38][39][40][41][42][35,36,37,38,39,40,41,42] and the availability of new materials with a strong THz response [43][44][43,44]. This has promoted the diffusion of THz research into various areas, e.g., air-quality and gas sensing [45][46][47][48][49][50][45,46,47,48,49,50], material sciences [42][44][51][52][53][54][42,44,51,52,53,54], microelectronics and security [55][56][55,56], agri-food quality [57], cultural heritage [58], in addition to biomedicine and bio-imaging [59][60][61][62][63][59,60,61,62,63]. For biomedical and biochemical issues, THz radiation is really appealing because of its low photon energy (around few meV, 4 meV @ 1 THz), and too low to heat materials and/or induce atom/molecule ionization; therefore, it enables non-destructive and non-ionizing sensing [64]. This is in contrast with other spectroscopic techniques, including ultraviolet or X-rays, where high-energy photons (>>eV) induce damage to the bio-sample [64]. In addition, THz radiation, characterized by low photon energy, is associated with energy levels matching low-frequency vibrational modes, including the collective vibrations of intermolecular and intramolecular interactions, such as hydrogen bonds [65], the phonon modes of crystalline molecular solids and vibrations of many macromolecules. In these circumstances, THz spectroscopy has been employed to investigate low-frequency vibrational modes of amino acids and proteins, due to its sensitivity to intermolecular interactions, such as hydrogen bonds, which in turn are dependent on molecular conformations and surrounding environments [66][67][66,67]. Therefore, THz radiation directly identifies a material’s spectral properties constituting its molecular fingerprint, offering, in this way, a chemical specificity to imaging and spectroscopy experiments, in a label-free, non-contact and non-invasive mode [59][68][69][59,68,69]. Moreover, it is worth to point out that non-polar materials (such as paper, cloths and plastic) are usually transparent in the THz range [55][70][71][55,70,71]. In contrast, the high sensitivity to polar molecules, such as water (absorption coefficient around 220 cm−1 for pure water @ 1 THz), and the low spatial resolution are the main drawbacks of THz radiation [72][73][72,73]. In fact, the extreme absorption, shown by THz radiation for polar molecules, specifically water, restricts the penetrability of THz waves from tens to hundreds of microns in hydrated samples. The diagnostic capability, especially in the case of biomedical applications in vivo or on fresh tissues, is then reduced. Nevertheless, the high sensitivity of water content can be used like an endogenous marker for the differentiation between fresh healthy and pathological tissues and preventing a wider range of applications in biology [69][74][69,74]. Referring to THz spectroscopy, many layouts and materials are used for THz signal collection [75][76][77][75,76,77], showing high performance in terms of the signal-to-noise ratio (SNR) and coherent detection mode. Because THz spectroscopy is insensitive to the thermal background, it has a high SNR, not requiring the use of cooled detectors [78]. Concerning the coherent detection mode, the temporal profile of THz electric field is directly recorded. Therefore, both amplitude and phase of the THz pulse electric field can be simultaneously measured, and the optical parameters, including the sample absorption coefficient and refractive index, can be estimated without using Kramers–Kronig relations [79]. In Table 1, major THz technology features are reported; the advantages and disadvantages are summarized and listed to have an overall view on its potential in terms of detection and discrimination.
Table 1.
Summary of THz spectroscopy. Advantages and disadvantages as a detection tool.
| Advantages | Disadvantages | ||
|---|---|---|---|
| Low-energy photon | Low spatial resolution (hundreds µm) | ||
| No-inflammable | Strong water absorption (220 cm | −1 | @ 1 THz) |
| No-ionizing radiation | Limited penetration inf fresh tissue | ||
| Sensitive to polar molecules | |||
| Coherent detection | |||
| No sample pre-treatment |