
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcin Zieliński | -- | 2840 | 2023-01-19 11:30:32 | | | |
| 2 | Peter Tang | Meta information modification | 2840 | 2023-01-20 02:17:41 | | |
Video Upload Options
Microbial fuel cells are increasingly an object of interest due to the possibility of simultaneous electrical energy production and wastewater treatment, and connecting them with other technologies. In addition, this technology is used to recover different resources such as nutrients, e.g., nitrogen and phosphorus or critical metals, and for desalination and biodetection. One of the essential aspects on which intensive works are in progress is the material used to build electrodes. There has been considerable development in materials for electrodes used in microbial fuel cells. More and more often, scientists reach for materials based on the most recent achievements of technology, and one of them is graphene. This material is increasingly used in industry and science, including microbial fuel cells. Graphene is connected with other materials and modified in numerous ways, and it seems to be a perfect alternative for standard carbon or stainless steel electrodes.
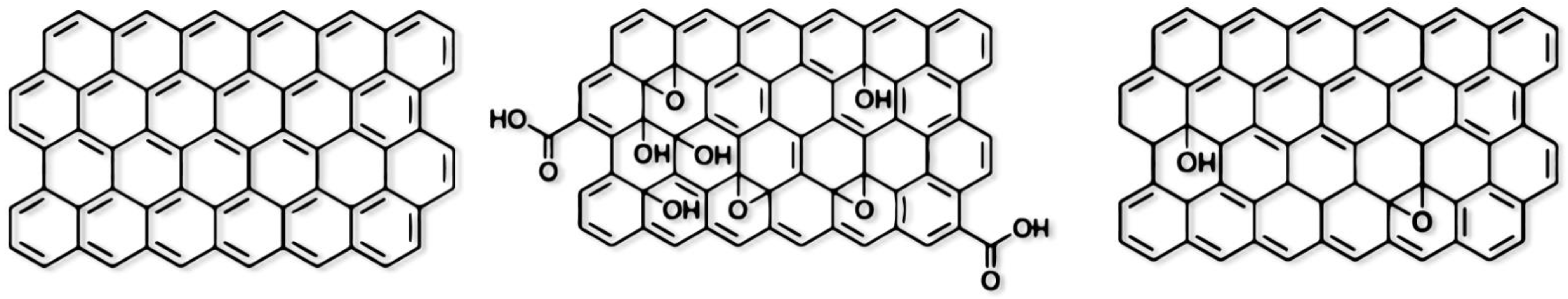
1. Graphene as Material
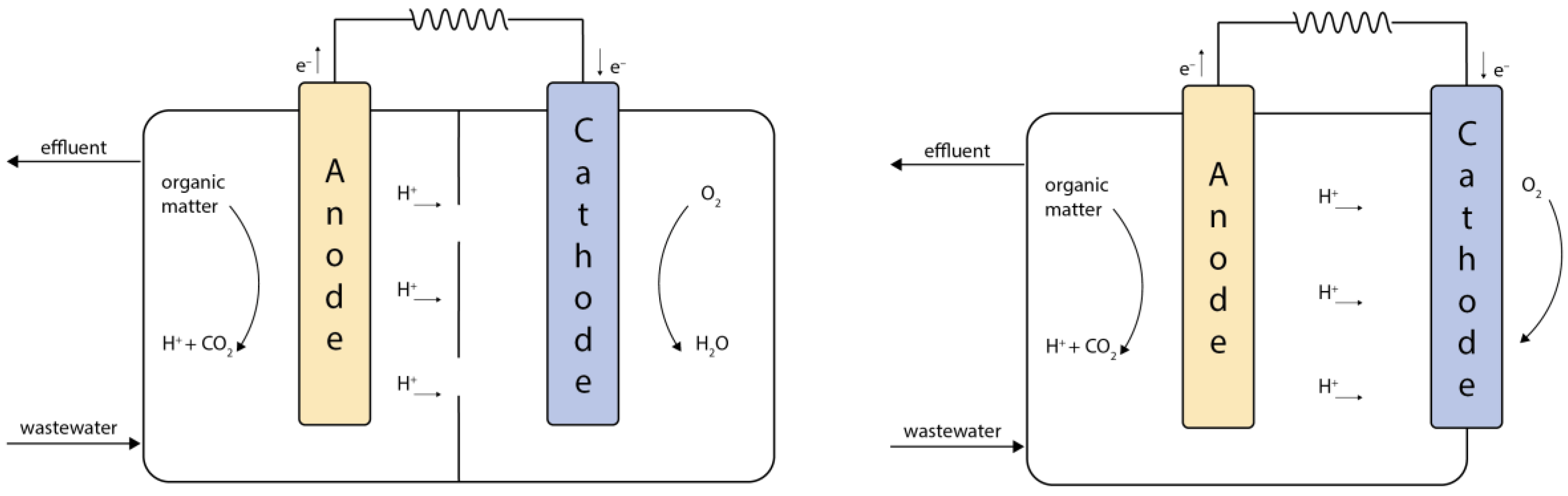
2. Microbial Fuel Cell Principles of Operation
2.1. Constructrion of a Microbial Fuel Cell
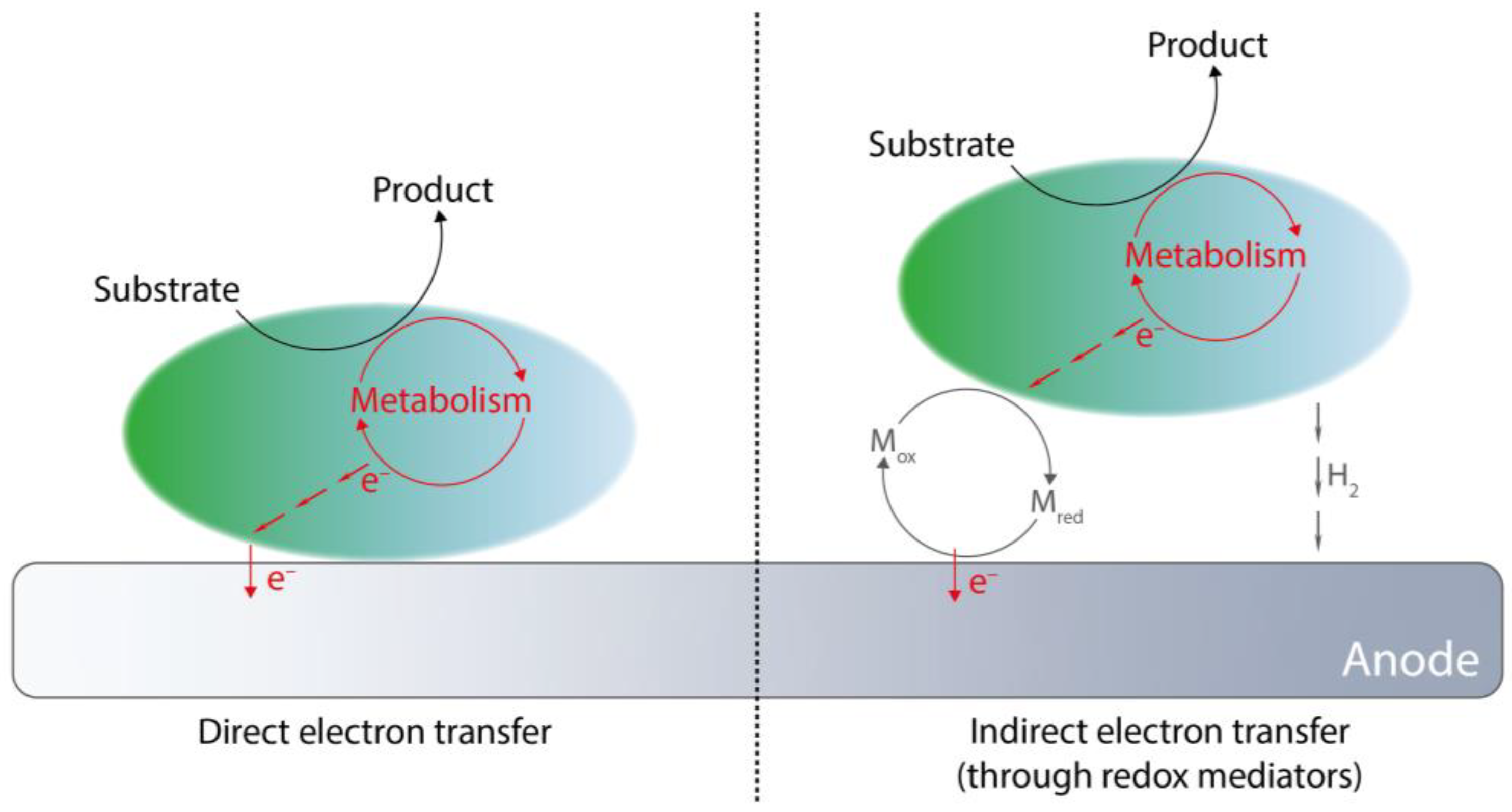
2.2. Extracellular Electron Transfer
3. Graphene Applications in Microbial Fuel Cells
3.1. Anodes Based on Graphene Used in MFC
3.2. Graphene Used in the Cathode Chamber of MFC
3.3. Graphene Advantages in Terms of Microbial Fuel Cells
References
- Heyrovska, R. Various Carbon to Carbon Bond Lengths Inter-related via the Golden Ratio, and their Linear Dependence on Bond Energies. Graphene 2008, 5, 35–38.
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29.
- Singh, S.B.; De, M. Effects of gaseous environments on physicochemical properties of thermally exfoliated graphene oxides for hydrogen storage: A comparative study. J. Porous Mater. 2021, 28, 875–888.
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene Oxide Synthesis, Properties and Characterization Techniques: A Comprehensive Review. ChemEngineering 2021, 5, 64.
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190.
- Sheikh, R.; Karmaker, S.; Solayman, M.; Mayna, J. Bioelectricity from Anaerobic Co-Digestion of Organic Solid Wastes and Sewage Sludge Using Microbial Fuel Cells (MFCs). J. Sustain. Bioenergy Syst. 2018, 08, 95–106.
- Palanisamy, G.; Jung, H.Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621.
- Al-Asheh, S.; Al-Assaf, Y.; Aidan, A. Single-chamber microbial fuel cells’ behavior at different operational scenarios. Energies 2020, 13, 5458.
- Tatinclaux, M.; Gregoire, K.; Leininger, A.; Biffinger, J.C.; Tender, L.; Ramirez, M.; Torrents, A.; Kjellerup, B.V. Electricity generation from wastewater using a floating air cathode microbial fuel cell. Water-Energy Nexus 2018, 1, 97–103.
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575.
- Mahmoud, R.H.; Samhan, F.A.; Ibrahim, M.K.; Ali, G.H.; Hassan, R.Y.A. Formation of electroactive biofilms derived by nanostructured anodes surfaces. Bioprocess Biosyst. Eng. 2021, 44, 759–768.
- Kalathil, S.; Patil, S.A.; Pant, D. Microbial fuel cells: Electrode materials. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 309–318. ISBN 9780128098943.
- Tiwari, S.K.; Mishra, R.K.; Ha, S.K.; Huczko, A. Evolution of Graphene Oxide and Graphene: From Imagination to Industrialization. ChemNanoMat 2018, 4, 598–620.
- Tsang, C.H.A.; Huang, H.; Xuan, J.; Wang, H.; Leung, D.Y.C. Graphene materials in green energy applications: Recent development and future perspective. Renew. Sustain. Energy Rev. 2020, 120, 109656.
- Chaturvedi, A.; Kundu, P.P. Nanostructured Graphene Utilization in Microbial Fuel Cells for Green Energy and Wastewater Treatment: Recent Developments and Future Perspectives. J. Hazard. Toxic Radioact. Waste 2022, 26, 03122002.
- Ono, T.; Min, Y.; Shapira, P.; Zavan, B.; Kim, S.O.; Lammel, T. Advances in Graphene; Scientific Research Publishing: Wuhan, China, 2017.
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078.
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Comparative effects of graphene and graphene oxide on copper toxicity to Daphnia magna: Role of surface oxygenic functional groups. Environ. Pollut. 2018, 236, 962–970.
- Chen, Y.; Zhao, Z.; Li, S.; Li, B.; Weng, Z.; Fang, Y.; Lei, W.; Jiang, H. Fabrication of 3D graphene anode for improving performance of miniaturized microbial fuel cells. 3 Biotech 2022, 12, 302.
- Sayed, E.T.; Alawadhi, H.; Olabi, A.G.; Jamal, A.; Almahdi, M.S.; Khalid, J.; Abdelkareem, M.A. Electrophoretic deposition of graphene oxide on carbon brush as bioanode for microbial fuel cell operated with real wastewater. Int. J. Hydrogen Energy 2021, 46, 5975–5983.
- Shi, M.M.; Jiang, Y.G.; Shi, L. Electromicrobiology and biotechnological applications of the exoelectrogens Geobacter and Shewanella spp. Sci. China Technol. Sci. 2019, 62, 1670–1678.
- Cao, B.; Zhao, Z.; Peng, L.; Shiu, H.-Y.; Ding, M.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver Nanoparticles Boost Charge-Extraction Efficiency in Shewanella Microbial Fuel Cells. Science 2021, 373, 1336–1340.
- Yaqoob, A.A.; Ibrahim, M.N.M.; Yaakop, A.S.; Rafatullah, M. Utilization of biomass-derived electrodes: A journey toward the high performance of microbial fuel cells. Appl. Water Sci. 2022, 12, 99.
- Song, R.B.; Zhou, S.; Guo, D.; Li, P.; Jiang, L.P.; Zhang, J.R.; Wu, X.; Zhu, J.J. Core/Satellite Structured Fe3O4/Au Nanocomposites Incorporated with Three-Dimensional Macroporous Graphene Foam as a High-Performance Anode for Microbial Fuel Cells. ACS Sustain. Chem. Eng. 2020, 8, 1311–1318.
- Wang, R.; Yan, M.; Li, H.; Zhang, L.; Peng, B.; Sun, J.; Liu, D.; Liu, S.; Wang, R.W.; Yan, M.; et al. FeS2 Nanoparticles Decorated Graphene as Microbial-Fuel-Cell Anode Achieving High Power Density. Adv. Mater. 2018, 30, 1800618.
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K.; Bhawani, S.A.; Khan, A.; Asiri, A.M.; Khan, M.R.; Azam, M.; Alammari, A.M. Cellulose Derived Graphene/Polyaniline Nanocomposite Anode for Energy Generation and Bioremediation of Toxic Metals via Benthic Microbial Fuel Cells. Polymers 2020, 13, 135.
- Li, Z.L.; Yang, S.K.; Song, Y.; Xu, H.Y.; Wang, Z.Z.; Wang, W.K.; Dang, Z.; Zhao, Y.Q. In-situ modified titanium suboxides with polyaniline/graphene as anode to enhance biovoltage production of microbial fuel cell. Int. J. Hydrogen Energy 2019, 44, 6862–6870.
- Lin, X.Q.; Li, Z.L.; Liang, B.; Nan, J.; Wang, A.J. Identification of biofilm formation and exoelectrogenic population structure and function with graphene/polyanliline modified anode in microbial fuel cell. Chemosphere 2019, 219, 358–364.
- Li, Y.; Liu, J.; Chen, X.; Yuan, X.; Li, N.; He, W.; Feng, Y. Enhanced electricity generation and extracellular electron transfer by polydopamine–reduced graphene oxide (PDA–rGO) modification for high-performance anode in microbial fuel cell. Chem. Eng. J. 2020, 387, 123408.
- Noori, M.T.; Bhowmick, G.D.; Tiwari, B.R.; Ghangrekar, O.M.; Ghangrekar, M.M.; Mukherjee, C.K. Carbon Supported Cu-Sn Bimetallic Alloy as an Excellent Low-Cost Cathode Catalyst for Enhancing Oxygen Reduction Reaction in Microbial Fuel Cell. J. Electrochem. Soc. 2018, 165, F621–F628.
- Santoro, C.; Kodali, M.; Kabir, S.; Soavi, F.; Serov, A.; Atanassov, P. Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J. Power Sources 2017, 356, 371–380.
- Yang, W.; Chata, G.; Zhang, Y.; Peng, Y.; Lu, J.E.; Wang, N.; Mercado, R.; Li, J.; Chen, S. Graphene oxide-supported zinc cobalt oxides as effective cathode catalysts for microbial fuel cell: High catalytic activity and inhibition of biofilm formation. Nano Energy 2019, 57, 811–819.
- Xin, S.; Shen, J.; Liu, G.; Chen, Q.; Xiao, Z.; Zhang, G.; Xin, Y. Electricity generation and microbial community of single-chamber microbial fuel cells in response to Cu2O nanoparticles/reduced graphene oxide as cathode catalyst. Chem. Eng. J. 2020, 380, 122446.
- Li, J.C.; Hou, P.X.; Liu, C. Heteroatom-Doped Carbon Nanotube and Graphene-Based Electrocatalysts for Oxygen Reduction Reaction. Small 2017, 13, 1702002.





