Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gianfranco Picone | -- | 2025 | 2023-01-18 16:24:41 | | | |
| 2 | Sirius Huang | Meta information modification | 2025 | 2023-01-19 02:43:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ciampa, A.; Danesi, F.; Picone, G. NMR-Based Metabolomics in Food Quality Assessment. Encyclopedia. Available online: https://encyclopedia.pub/entry/40363 (accessed on 28 February 2026).
Ciampa A, Danesi F, Picone G. NMR-Based Metabolomics in Food Quality Assessment. Encyclopedia. Available at: https://encyclopedia.pub/entry/40363. Accessed February 28, 2026.
Ciampa, Alessandra, Francesca Danesi, Gianfranco Picone. "NMR-Based Metabolomics in Food Quality Assessment" Encyclopedia, https://encyclopedia.pub/entry/40363 (accessed February 28, 2026).
Ciampa, A., Danesi, F., & Picone, G. (2023, January 18). NMR-Based Metabolomics in Food Quality Assessment. In Encyclopedia. https://encyclopedia.pub/entry/40363
Ciampa, Alessandra, et al. "NMR-Based Metabolomics in Food Quality Assessment." Encyclopedia. Web. 18 January, 2023.
Copy Citation
The ability of nuclear magnetic resonance spectroscopy (NMR) to extract chemical information from a complex mixture is invaluable and widely described in literature. Many applications of this technique in the foodomics field have highlighted how NMR could characterize food matrices, and it can be used all along its “life chain”: from farm to fork and from fork to the digestion process.
nuclear magnetic resonance
bioactive compounds
food analysis
food quality
foodomics
1. Introduction
Food quality, food authenticity, and food safety are the most valuable targets to ensure and guarantee the needs of a world population that is increasing higher and higher. Consumers ask for much more security and, at the same time, for better information on food chemical and nutritional composition, origin, authenticity, and the effect of technological transformation on both the food’s molecular profile and human health [1]. Furthermore, the bioavailability and bioaccessibility of the nutrients and/or bioactive compounds (BC), and the prediction of nutrition efficiency are additional key concepts in which consumers begin to be interested in [2]. They also become more sensitive to the environmental aspect, considering the deep global warming alarm in 2022, one of the warmest years since 2005 [3]. Nowadays, the attention on “what we eat” reflects not only the food’s nutritional-health aspects but also “avoiding derivatization and favoring substances based on renewable sources” [4] by respecting the environment. In light of these global warming events, academics together with industry must work to ensure consumers’ requests and to improve their human life. These pathways go hand-hand with sustainable development inevitably.
Thus, how can academics answer to the consumers’ needs while being at the same time environmentally friendly? Secondly, how can NMR-based metabolomics play a key role in this scenario?
Before answering these two questions it is important to keep in mind the meaning of the word sustainable when we take into account academic research and, thus, analytical techniques. Płotka-Wasylka, et al. [5] defined sustainable as those analytical techniques that take into consideration three fundamental pillars: (1) the environment, (2) the economy, and (3) the society (Figure 1).
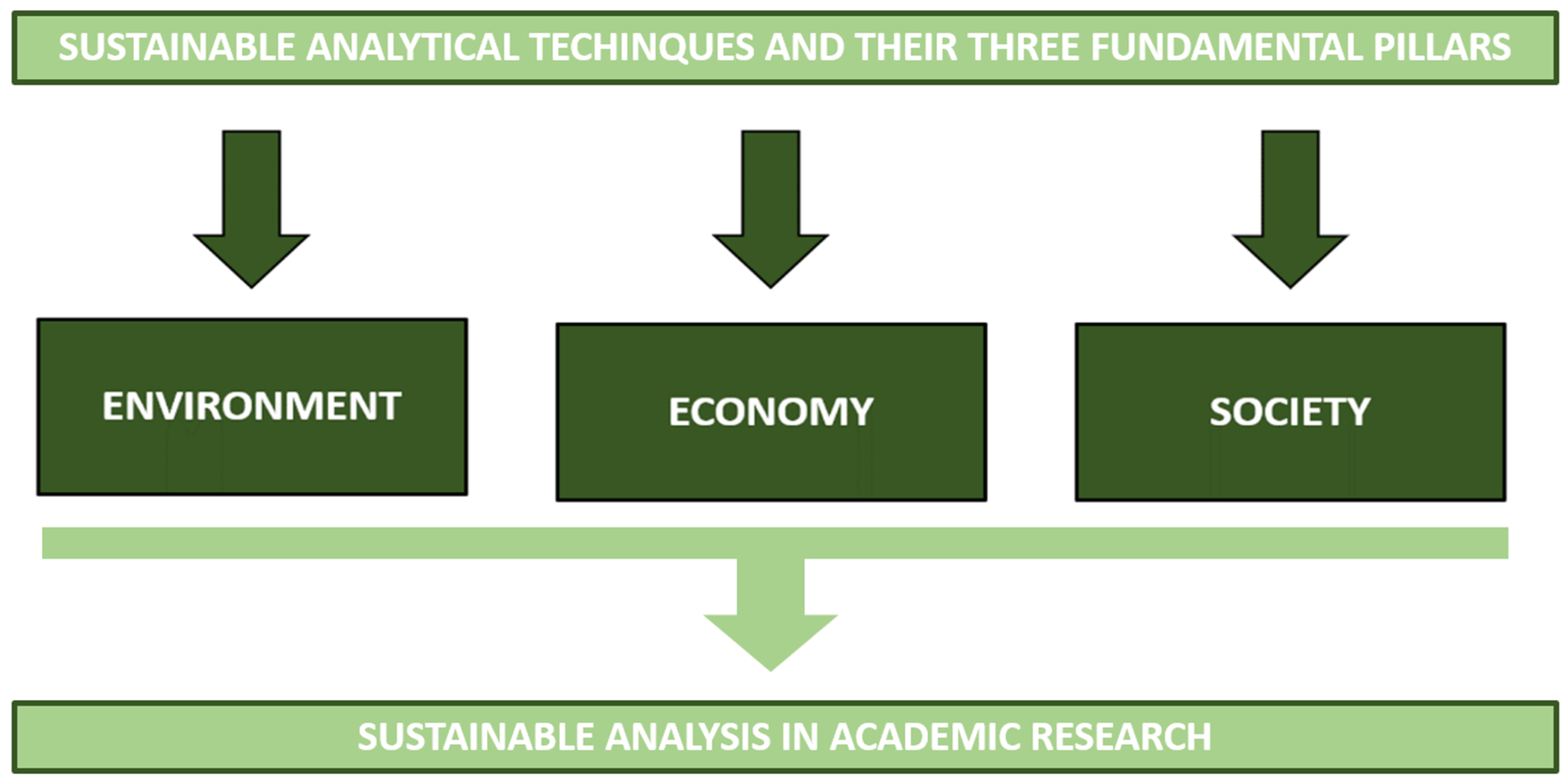
Figure 1. Sustainable analysis in academic research starts from sustainable analytical techniques that have to take into consideration three fundamental pillars: environment, economy, and society.
Pillar 1: Sustainability can be achieved by waste minimization, adopting green solvents, or focusing on solventless methods, energy savings, and trying to avoid the use of auxiliary reagents and chemicals [5]. Sustainable analytical techniques should also be considered to limit pollution.
Pillar 2: Academic research is generally recognized as curiosity-driven. However, there has recently been a shift in this, which was also influenced by the types of funding available. Research has become more industry-oriented and practical than conceptual. It has also started to suggest green solutions to problems and have an economic impact. Therefore, in the current context, food analysis and quality evaluation need to be supported by procedures and analytical techniques whose challenge is to reduce the environmental footprint [5].
Pillar 3: The main educational task is to transmit a clear message to society that would shed light on chemistry as a fundamental part of the solution to pollution problems and not just part of the problem. Information on the possibility of “making science” with low environmental impact is a fundamental leitmotif that society should have clearly in mind.
In the context of analytical chemistry, as well as in food analytical chemistry, some well-established methods require hazardous chemicals and/or a high energy demand for sample preservation, pretreatment, calibration, and analyte determination. Frequently, the analytical methods yield large amounts of waste with even higher toxicity than the target analytes. Thus, analytical chemistry plays an important role in the sustainable development of the planet [5]. For this reason, green chemistry (GC) is considered an important tool for achieving sustainability since it aims at the development of chemicals and chemical processes to reduce the impact on human health and the environment. But how can it be achieved? Poliakoff, et al. [6] suggest a deep change in attitudes and behavior in the chemical industry all along the chemical supply chain. For example, at the start of the chain, laboratories, where research is carried out, should be rethought and built to minimize the use of energy. At the other end of the chain, wherever possible, the amount of chemical(s) used to achieve a given effect should be decreased by a factor of two every five years [6], according to Moore’s Law for Chemistry (MLFC) [7]. In this scenario, scientists are thus encouraged to develop or adopt new green analytical methods that (1) contribute to the reduction of pollution, including real-time analysis of pollutants; (2) avoid sample pretreatment or involve greener approaches for sample pretreatment, including safe solvents and auxiliaries; (3) use miniaturization and automation, including flow analysis and microfluidics and (4) use green separation techniques.
Based on these aspects, it is now possible to answer the second question: how can NMR-based metabolomics play a key role in this scenario? The High Resolution (HR)-NMR is a successful and functional research technology because of both its peculiarities which are listed in pillar 1 above. HR-NMR spectroscopy is one of the main analytical technologies that operates following the green chemistry guidelines in sample preparation [8]. Mielko, et al. [9] recognized the NMR as a green method as it requires for metabolomics studies only 10% D2O (deuterated water) in 90% of distilled water (H2Odd). The solution has the characteristic of being versatile as it can be used in different studies. For example, this aqueous solution has been adopted for urine and serum fluid analysis, as shown by Trimigno, et al. [10] and Münger, et al. [11]. The authors used simply a phosphate-buffered saline (PBS) which also includes sodium azide (NaN3) as an antibacterial agent and a 20 mM 2-chloropyrimidine-5-carboxylic acid (2CLPYR5CA) as the reference standard. Also, in the case of food metabolomics analysis, the NMR spectroscopic method requires simplicity in the analysis of polar metabolites. Their extraction can be performed by using a solution of trichloroacetic acid (TCA) 7% including 10% D2O [12][13]. This application has been described in the work of Ciampa, et al. [14], where the authors show how the NMR satisfies, for the quantification of trimethylamine (TMA) content in fish, all the validation requirements at the same level as the most frequently used methods (as HPLC). Furthermore, the technique has the advantage of being faster and more repeatable, avoiding the use of solvents, such as toluene and formaldehyde, or dangerous reagents, such as picric acid.
2. The NMR-Based Metabolomics in Food Science and the Foodomics Approach
It is important, before exploring the potentiality of the NMR in metabolomics studies, to clarify what metabolomics studies are. The term “metabolomics” was introduced by Oliver Fiehn and defined as a comprehensive and quantitative analysis of all metabolites in a system [15]. On the other hand, the term “metabonomics” was coined by J. K. Nicholson in 1999 [16], and it represents “the quantitative measurement of the dynamic multiparametric metabolic response of living systems to pathophysiological stimuli or genetic modification.” Nowadays, modern metabolic phenotyping (metabotyping) is known as metabolomics, encompassing both the comprehensive analysis of the small molecule content of the tested samples, and the changes that occur in response to a stimulus of one sort or another (physiological, pharmacological, or toxicological) [17].
The given definition points out the importance of the HR-NMR in “the augmentation and complementation of the information provided by measuring the genetic and proteomic responses to xenobiotic exposure” as it is appropriate “for investigating abnormal body fluid compositions”, as a wide range of metabolites can be quantified simultaneously with no sample preparation and “without prejudice” [16].
In this light, Nicholson describes de facto the NMR-based metabolomics approach. In summary, it represents a high-performance fingerprinting process that examines the entire collection of small molecules (typically <1800 Dalton) that are present in a concentration above 10 µM, including sugars, amino acids, organic acids, and lipids, and is called ‘metabolome’ [18]. In summary, metabolomics is the final step of a more structured pipeline that involves more omics platforms, such as proteomics, transcriptomics, and genomics [15], as represented in Figure 2.
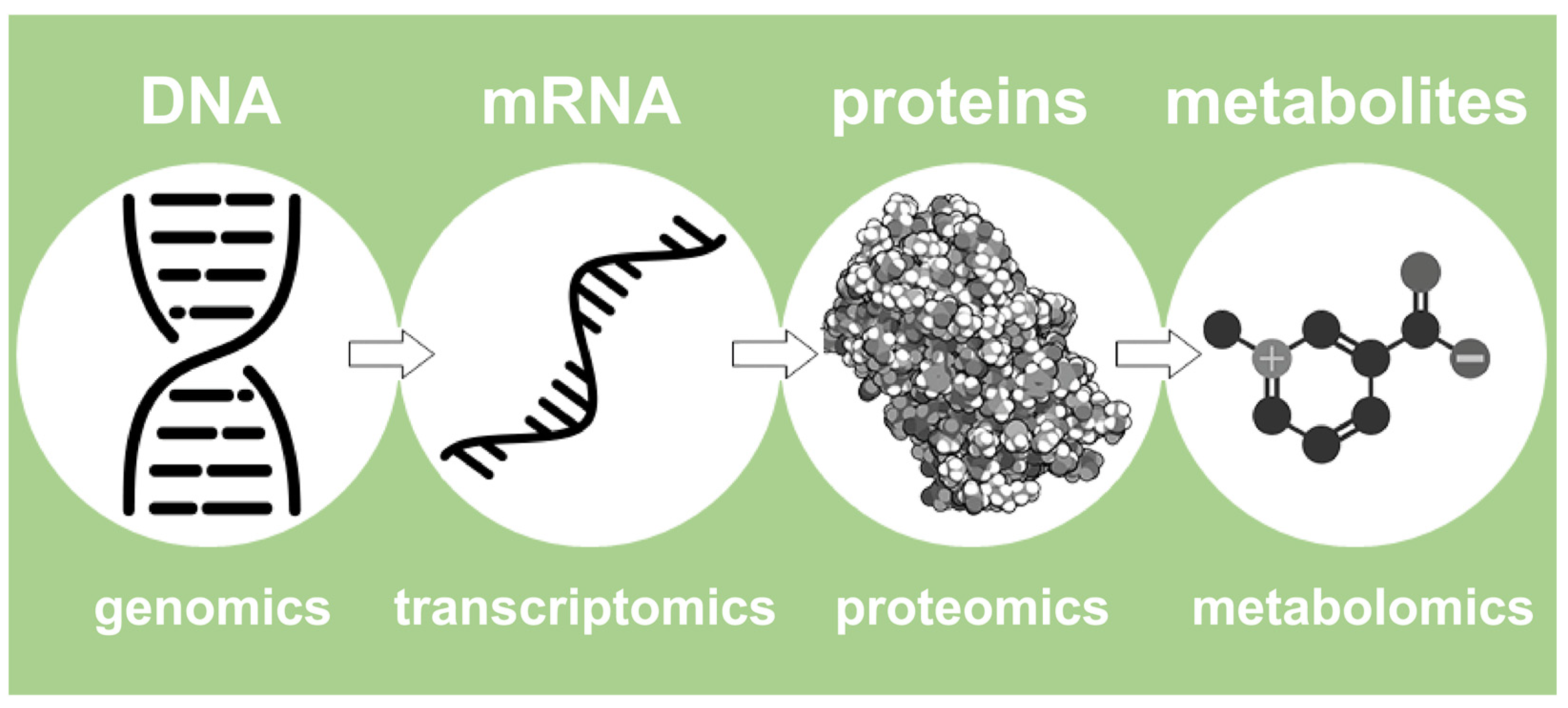
Figure 2. An outline of the four most important omics fields, ranging from genomics to metabolomics.
This integrated “snapshot” may change throughout the exposure to external factors or during organism development. When dealing with this technique, almost two kinds of approaches can be used for the analysis of the metabolome: (i) the target analysis, which is metabolic profiling, and (ii) pattern analysis, which is metabolic and metabonomic fingerprinting.
Target analysis, or metabolic profiling, is the identification and quantification of given and predefined metabolites [19]. It can be a specific metabolite or a selected number of metabolites. In the first case, a selective extraction is necessary before the NMR analysis to concentrate the selected metabolite. The latter case is used when the attention is focused on the specific role of a selected metabolic pathway; this approach is called metabolic profiling [20]. The pattern analysis is metabolic profiling obtained by identifying and quantifying all metabolites. This operation is led by using specific software, such as Chenomx [21][22], and it is called ‘metabonomics.’ On the other hand, metabolic profiling is used when sample classification without quantification of individual-specific metabolites is required [20]. In this case, the NMR spectrum becomes a fingerprint of the product, and all the NMR resonances/signals are measured without any identification [23], and it is called ‘fingerprinting’.
Due to the above-all-mentioned characteristics, it appears clear why in the last 15 years academic researchers have employed NMR-based metabolomics in food science more and more. With this technology, academics meet the consumers’ requests in one shot: a green approach that both pays attention to the environment in sample preparation and can assess and guarantee the quality of foodstuff. It is not a coincidence that with the opening of the food markets at a worldwide level, the number of research papers on the evaluation of food quality by using an NMR-metabolomics approach increased. Consonni and Cagliani [24] explain that the globalization phenomenon changes the definition of food quality that have to include also other aspects like geographical origin, sophisticated frauds, and adulteration practices, etc. The expansion of the food quality concept is also determined nowadays by the introduction on the market of new foods formulated to be much more sustainable and green [25]. These innovative foods are designed considering unconventional sources of nutrients, such as insects [26][27][28], for example, but also converting waste products into second-life products [29][30][31]. For this reason, consumers have to be reassured about food safety and from a nutritional value point of view. As these new aspects overcome the official analytical determination focused on specific compounds investigation, the role of metabolomics in this new quality assessment has become very important [24]. The importance is proved by the tremendous number of papers dealing with metabolomics and NMR that appear in the bibliography. Among them, geographical origin plays a key role as food quality is strongly affected by the particular conditions of production areas, which give unrepeatable organoleptic and nutritional properties to agricultural food products [32]. Extensive studies have been performed on several different foods to find out biomarkers able to classify them concerning their geographic origin: extra virgin olive oil (EVOO) [33][34][35][36], cheese [37][38][39], tomato [40][41][42], saffron [43][44], fruits and vegetables [45][46][47][48][49], honey [50][51][52][53] and wine [54][55][56][57][58], etc. Food frauds and adulteration have been largely taken in consideration in NMR-based metabolomics studies as well [59][60][61][62][63][64][65][66][67][68]. The application of NMR-based metabolomics in a food quality context can be included in the so-called foodomics, and we can talk about NMR-based foodomics. The first definition of this omics approach appeared in 2009 by Cifuentes [69], and it put attention on the investigation of all the possible connections among food, diet, and the individual, including health and ill impact [70]. These connections are included in the suffix “omics”, which comes from the Latin word “omne” and it means everything, totality, wholeness, and entirety [71]. A more holistic definition by Cifuentes [72] described foodomics as “the discipline that studies the food and nutrition domains through the application and integration of advanced omics technologies to improve consumer’s well-being, health, and confidence”. This new approach then had much more resonance during the first International Conference on Foodomics, held in Cesena in 2009, where scientists were invited to contribute to the holistic definition of food in a multidisciplinary environment [73]. The academics’ contribution to this new discipline is largely demonstrated by several papers describing in proper matter the aim and the applications of foodomics [71][74][75][76][77][78].
References
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2021, 61, 1448–1469.
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760.
- Nadeau, K.C.; Agache, I.; Jutel, M.; Annesi Maesano, I.; Akdis, M.; Sampath, V.; d’Amato, G.; Cecchi, L.; Traidl-Hoffmann, C.; Akdis, C.A. Climate change: A call to action for the united nations. Allergy 2022, 77, 1087–1090.
- Tobiszewski, M.; Mechlińska, A.; Namieśnik, J. Green analytical chemistry—Theory and practice. Chem. Soc. Rev. 2010, 39, 2869–2878.
- Płotka-Wasylka, J.; Mohamed, H.M.; Kurowska-Susdorf, A.; Dewani, R.; Fares, M.Y.; Andruch, V. Green analytical chemistry as an integral part of sustainable education development. Curr. Opin. Green Sustain. Chem. 2021, 31, 100508.
- Poliakoff, M.; Licence, P.; George, M.W. UN sustainable development goals: How can sustainable/green chemistry contribute? By doing things differently. Curr. Opin. Green Sustain. Chem. 2018, 13, 146–149.
- Poliakoff, M.; Licence, P.; George, M.W. A new approach to sustainability: A Moor’s law for chemistry. Angew. Chem. Int. Ed. 2018, 57, 12590–12591.
- Gottlieb, H.E.; Graczyk-Millbrandt, G.; Inglis, G.G.; Nudelman, A.; Perez, D.; Qian, Y.; Shuster, L.E.; Sneddon, H.F.; Upton, R.J. Development of GSK’s NMR guides—A tool to encourage the use of more sustainable solvents. Green Chem. 2016, 18, 3867–3878.
- Mielko, K.A.; Pudełko-Malik, N.; Tarczewska, A.; Młynarz, P. NMR spectroscopy as a “green analytical method” in metabolomics and proteomics studies. Sustain. Chem. Pharm. 2021, 22, 100474.
- Trimigno, A.; Münger, L.; Picone, G.; Freiburghaus, C.; Pimentel, G.; Vionnet, N.; Pralong, F.; Capozzi, F.; Badertscher, R.; Vergères, G. GC-MS based metabolomics and NMR spectroscopy investigation of food intake biomarkers for milk and cheese in serum of healthy humans. Metabolites 2018, 8, 26.
- Münger, L.H.; Trimigno, A.; Picone, G.; Freiburghaus, C.; Pimentel, G.g.; Burton, K.J.; Pralong, F.o.P.; Vionnet, N.; Capozzi, F.; Badertscher, R. Identification of urinary food intake biomarkers for milk, cheese, and soy-based drink by untargeted GC-MS and NMR in healthy humans. J. Proteome Res. 2017, 16, 3321–3335.
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M. NMR spectroscopy for metabolomics research. Metabolites 2019, 9, 123.
- Laghi, L.; Picone, G.; Capozzi, F. Nuclear magnetic resonance for foodomics beyond food analysis. TrAC Trends Anal. Chem. 2014, 59, 93–102.
- Ciampa, A.; Laghi, L.; Picone, G. Validation of a 1H-NMR Spectroscopy Quantitative Method to Quantify Trimethylamine Content and K-Index Value in Different Species of Fish. J. Food Qual. 2022, 2022, 3612095.
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. In Functional Genomic; Springer: Cham, Switzerland, 2002; pp. 155–171.
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189.
- Wilson, I.D.; Theodoridis, G.; Virgiliou, C. A perspective on the standards describing mass spectrometry-based metabolic phenotyping (metabolomics/metabonomics) studies in publications. J. Chromatogr. B 2021, 1164, 122515.
- Zhu, Z.; Camargo, C.A., Jr.; Hasegawa, K. Metabolomics in the prevention and management of asthma. Expert Rev. Respir. Med. 2019, 13, 1135–1138.
- Sturm, S. Analytical Aspects of Plant Metabolite Profiling Platforms: Current Standings and Future Aims. J. Proteome Res. 2007, 6, 480–497.
- Mannina, L.; Sobolev, A.P.; Capitani, D.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Reale, A.; Sorrentino, E.; D’Amico, I.; Coppola, R. NMR metabolic profiling of organic and aqueous sea bass extracts: Implications in the discrimination of wild and cultured sea bass. Talanta 2008, 77, 433–444.
- Kim, H.S.; Kim, E.T.; Eom, J.S.; Choi, Y.Y.; Lee, S.J.; Lee, S.S.; Chung, C.D.; Lee, S.S. Exploration of metabolite profiles in the biofluids of dairy cows by proton nuclear magnetic resonance analysis. PLoS ONE 2021, 16, e0246290.
- Antonelo, D.S.; Cônsolo, N.R.; Gómez, J.F.; Beline, M.; Pavan, B.; Souza, C.; Goulart, R.S.; Colnago, L.A.; Silva, S.L. Meat metabolomic pathway of Nellore and crossbred Angus x Nellore cattle. In Proceedings of the 65th International Congress of Meat Scienece and Technology, Berlin, Germany, 4–9 August 2019.
- Picone, G.; Mezzetti, B.; Babini, E.; Capocasa, F.; Placucci, G.; Capozzi, F. Unsupervised Principal Component Analysis of NMR Metabolic Profiles for the Assessment of Substantial Equivalence of Transgenic Grapes (Vitis vinifera). J. Agric. Food Chem. 2011, 59, 9271–9279.
- Consonni, R.; Cagliani, L.R. The potentiality of NMR-based metabolomics in food science and food authentication assessment. Magn. Reson. Chem. MRC 2019, 57, 558–578.
- Horlings, L.G.; Marsden, T.K. Towards the real green revolution? Exploring the conceptual dimensions of a new ecological modernisation of agriculture that could ‘feed the world’. Glob. Environ. Change 2011, 21, 441–452.
- Collins, C.M.; Vaskou, P.; Kountouris, Y. Insect food products in the western world: Assessing the potential of a new ‘green’ market. Ann. Entomol. Soc. Am. 2019, 112, 518–528.
- Lamsal, B.; Wang, H.; Pinsirodom, P.; Dossey, A.T. Applications of insect-derived protein ingredients in food and feed industry. J. Am. Oil Chem. Soc. 2019, 96, 105–123.
- Varelas, V. Food wastes as a potential new source for edible insect mass production for food and feed: A review. Fermentation 2019, 5, 81.
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive oil industry by-products. Effects of a polyphenol-rich extract on the metabolome and response to inflammation in cultured intestinal cell. Food Res. Int. 2018, 113, 392–400.
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940.
- Pampuri, A.; Casson, A.; Alamprese, C.; Di Mattia, C.D.; Piscopo, A.; Difonzo, G.; Conte, P.; Paciulli, M.; Tugnolo, A.; Beghi, R.; et al. Environmental Impact of Food Preparations Enriched with Phenolic Extracts from Olive Oil Mill Waste. Foods 2021, 10, 980.
- Masetti, O.; Sorbo, A.; Nisini, L. NMR Tracing of Food Geographical Origin: The Impact of Seasonality, Cultivar and Production Year on Data Analysis. Separations 2021, 8, 230.
- Longobardi, F.; Ventrella, A.; Napoli, C.; Humpfer, E.; Schütz, B.; Schäfer, H.; Kontominas, M.G.; Sacco, A. Classification of olive oils according to geographical origin by using 1H NMR fingerprinting combined with multivariate analysis. Food Chem. 2012, 130, 177–183.
- Mannina, L.; Marini, F.; Gobbino, M.; Sobolev, A.; Capitani, D. NMR and chemometrics in tracing European olive oils: The case study of Ligurian samples. Talanta 2010, 80, 2141–2148.
- Mannina, L.; Patumi, M.; Proietti, N.; Bassi, D.; Segre, A.L. Geographical characterization of Italian extra virgin olive oils using high-field 1H NMR spectroscopy. J. Agric. Food Chem. 2001, 49, 2687–2696.
- Ingallina, C.; Cerreto, A.; Mannina, L.; Circi, S.; Vista, S.; Capitani, D.; Spano, M.; Sobolev, A.P.; Marini, F. Extra-virgin olive oils from nine Italian regions: An 1H NMR-chemometric characterization. Metabolites 2019, 9, 65.
- Shintu, L.; Caldarelli, S. Toward the determination of the geographical origin of emmental (er) cheese via high resolution MAS NMR: A preliminary investigation. J. Agric. Food Chem. 2006, 54, 4148–4154.
- Brescia, M.; Monfreda, M.; Buccolieri, A.; Carrino, C. Characterisation of the geographical origin of buffalo milk and mozzarella cheese by means of analytical and spectroscopic determinations. Food Chem. 2005, 89, 139–147.
- Piras, C.; Marincola, F.C.; Savorani, F.; Engelsen, S.B.; Cosentino, S.; Viale, S.; Pisano, M.B. A NMR metabolomics study of the ripening process of the Fiore Sardo cheese produced with autochthonous adjunct cultures. Food Chem. 2013, 141, 2137–2147.
- Abreu, A.C.; Fernández, I. NMR metabolomics applied on the discrimination of variables influencing tomato (Solanum lycopersicum). Molecules 2020, 25, 3738.
- Masetti, O.; Nisini, L.; Ciampa, A.; Dell’Abate, M.T. 1H NMR spectroscopy coupled with multivariate analysis was applied to investigate Italian cherry tomatoes metabolic profile. J. Chemom. 2020, 34, e3191.
- Capozzi, F.; Savorani, F.; Engelsen, S.; Dell’Abate, M.; Sequi, P. Pomodoro di Pachino: An authentication study using 1H-NMR and chemometrics-protecting its PGI European certification. Magn. Reson. Food Sci. 2009, 106–166.
- Sobolev, A.P.; Carradori, S.; Capitani, D.; Vista, S.; Trella, A.; Marini, F.; Mannina, L. Saffron samples of different origin: An NMR study of microwave-assisted extracts. Foods 2014, 3, 403–419.
- Consonni, R.; Ordoudi, S.A.; Cagliani, L.R.; Tsiangali, M.; Tsimidou, M.Z. On the traceability of commercial saffron samples using 1H-NMR and FT-IR metabolomics. Molecules 2016, 21, 286.
- Di Matteo, G.; Spano, M.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L.; Ingallina, C.; Sobolev, A.P. Nmr characterization of ten apple cultivars from the piedmont region. Foods 2021, 10, 289.
- Hamid, N.A.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Park, Y.S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Gorinstein, S. Characterization of metabolites in different kiwifruit varieties by NMR and fluorescence spectroscopy. J. Pharm. Biomed. Anal. 2017, 138, 80–91.
- Do Prado Apparecido, R.; Lopes, T.I.B.; Alcantara, G.B. NMR-based foodomics of common tubers and roots. J. Pharm. Biomed. Anal. 2022, 209, 114527.
- Lee, D.; Kim, M.; Kim, B.H.; Ahn, S. Identification of the geographical origin of Asian red pepper (Capsicum annuum L.) powders using 1H NMR spectroscopy. Bull. Korean Chem. Soc. 2020, 41, 317–322.
- Lau, H.; Laserna, A.K.C.; Li, S.F.Y. 1H NMR-based metabolomics for the discrimination of celery (Apium graveolens L. var. dulce) from different geographical origins. Food Chem. 2020, 332, 127424.
- Spiteri, C.; Lia, F.; Farrugia, C. Determination of the geographical origin of Maltese honey using 1H NMR fingerprinting. Foods 2020, 9, 1455.
- Schievano, E.; Stocchero, M.; Morelato, E.; Facchin, C.; Mammi, S. An NMR-based metabolomic approach to identify the botanical origin of honey. Metabolomics 2012, 8, 679–690.
- Gerginova, D.; Simova, S.; Popova, M.; Stefova, M.; Stanoeva, J.P.; Bankova, V. NMR profiling of North Macedonian and Bulgarian honeys for detection of botanical and geographical origin. Molecules 2020, 25, 4687.
- Karabagias, I.K.; Vlasiou, M.; Kontakos, S.; Drouza, C.; Kontominas, M.G.; Keramidas, A.D. Geographical discrimination of pine and fir honeys using multivariate analyses of major and minor honey components identified by 1H NMR and HPLC along with physicochemical data. Eur. Food Res. Technol. 2018, 244, 1249–1259.
- Brescia, M.; Caldarola, V.; de Giglio, A.; Benedetti, D.; Fanizzi, F.; Sacco, A. Characterization of the geographical origin of Italian red wines based on traditional and nuclear magnetic resonance spectrometric determinations. Anal. Chim. Acta 2002, 458, 177–186.
- Ogrinc, N.; Košir, I.J.; Kocjančič, M.; Kidrič, J. Determination of authenticy, regional origin, and vintage of Slovenian wines using a combination of IRMS and SNIF-NMR analyses. J. Agric. Food Chem. 2001, 49, 1432–1440.
- Mascellani, A.; Hoca, G.; Babisz, M.; Krska, P.; Kloucek, P.; Havlik, J. 1H NMR chemometric models for classification of Czech wine type and variety. Food Chem. 2021, 339, 127852.
- Gougeon, L.; da Costa, G.; Guyon, F.; Richard, T. 1H NMR metabolomics applied to Bordeaux red wines. Food Chem. 2019, 301, 125257.
- Magdas, D.A.; Pirnau, A.; Feher, I.; Guyon, F.; Cozar, B.I. Alternative approach of applying 1H NMR in conjunction with chemometrics for wine classification. LWT 2019, 109, 422–428.
- Girelli, C.R.; Del Coco, L.; Fanizzi, F.P. Tunisian Extra Virgin Olive Oil Traceability in the EEC Market: Tunisian/Italian (Coratina) EVOOs Blend as a Case Study. Sustainability 2017, 9, 1471.
- Šmejkalová, D.; Piccolo, A. High-power gradient diffusion NMR spectroscopy for the rapid assessment of extra-virgin olive oil adulteration. Food Chem. 2010, 118, 153–158.
- Petrakis, E.A.; Cagliani, L.R.; Tarantilis, P.A.; Polissiou, M.G.; Consonni, R. Sudan dyes in adulterated saffron (Crocus sativus L.): Identification and quantification by 1H NMR. Food Chem. 2017, 217, 418–424.
- Sobolev, A.P.; Thomas, F.; Donarski, J.; Ingallina, C.; Circi, S.; Marincola, F.C.; Capitani, D.; Mannina, L. Use of NMR applications to tackle future food fraud issues. Trends Food Sci. Technol. 2019, 91, 347–353.
- Milani, M.I.; Rossini, E.L.; Catelani, T.A.; Pezza, L.; Toci, A.T.; Pezza, H.R. Authentication of roasted and ground coffee samples containing multiple adulterants using NMR and a chemometric approach. Food Control 2020, 112, 107104.
- Yong, C.-H.; Muhammad, S.A.; Nasir, F.I.; Mustafa, M.Z.; Ibrahim, B.; Kelly, S.D.; Cannavan, A.; Seow, E.-K. Detecting adulteration of stingless bee honey using untargeted 1H NMR metabolomics with chemometrics. Food Chem. 2022, 368, 130808.
- Schmitt, C.; Bastek, T.; Stelzer, A.; Schneider, T.; Fischer, M.; Hackl, T. Detection of peanut adulteration in food samples by nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2020, 68, 14364–14373.
- Rysova, L.; Legarova, V.; Pacakova, Z.; Hanus, O.; Nemeckova, I.; Klimesova, M.; Havlik, J. Detection of bovine milk adulteration in caprine milk with N-acetyl carbohydrate biomarkers by using 1H nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 9583–9595.
- Horn, B.; Esslinger, S.; Fauhl-Hassek, C.; Riedl, J. 1H NMR spectroscopy, one-class classification and outlier diagnosis: A powerful combination for adulteration detection in paprika powder. Food Control 2021, 128, 108205.
- Kuballa, T.; Brunner, T.S.; Thongpanchang, T.; Walch, S.G.; Lachenmeier, D.W. Application of NMR for authentication of honey, beer and spices. Curr. Opin. Food Sci. 2018, 19, 57–62.
- Cifuentes, A. Food analysis and foodomics. J. Chromatogr. A 2009, 1216, 7109.
- Picone, G.; Mengucci, C.; Capozzi, F. The NMR added value to the green foodomics perspective: Advances by machine learning to the holistic view on food and nutrition. Magn. Reson. Chem. 2022, 60, 590–596.
- Balkir, P.; Kemahlioglu, K.; Yucel, U. Foodomics: A new approach in food quality and safety. Trends Food Sci. Technol. 2021, 108, 49–57.
- Cifuentes, A. Advanced separation methods in food analysis. J. Chromatogr. A 2009, 1216, 7109–7358.
- Capozzi, F.; Bordoni, A. Foodomics: A new comprehensive approach to food and nutrition. Genes Nutr. 2013, 8, 1–4.
- Valdés, A.; Álvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Foodomics: Analytical opportunities and challenges. Anal. Chem. 2021, 94, 366–381.
- Cifuentes, A. Food analysis: Present, future, and foodomics. Int. Sch. Res. Not. 2012, 2012, 801607.
- García-Cañas, V.; Simó, C.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Present and future challenges in food analysis: Foodomics. Anal. Chem. 2012, 84, 10150–10159.
- Cifuentes, A. Comprehensive Foodomics; Elsevier: Amsterdam, The Netherlands, 2020.
- Gallo, M.; Ferranti, P. The evolution of analytical chemistry methods in foodomics. J. Chromatogr. A 2016, 1428, 3–15.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Nuclear Magnetic Resonance
Revisions:
2 times
(View History)
Update Date:
19 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





