Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jaison Jeevanandam | -- | 3802 | 2023-01-16 12:30:34 | | | |
| 2 | Dean Liu | -9 word(s) | 3793 | 2023-01-17 03:54:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Thimmiah, B.R.; Chien, B.T.C.; Fui, K.S.; Yon, L.S.; Nallathambi, G.; Jeevanandam, J.; Danquah, M.K. Nanoformulation of Peptides. Encyclopedia. Available online: https://encyclopedia.pub/entry/40223 (accessed on 07 February 2026).
Thimmiah BR, Chien BTC, Fui KS, Yon LS, Nallathambi G, Jeevanandam J, et al. Nanoformulation of Peptides. Encyclopedia. Available at: https://encyclopedia.pub/entry/40223. Accessed February 07, 2026.
Thimmiah, Bhargavi Ram, Belinda Tang Chien Chien, Kiew Siaw Fui, Lau Sie Yon, Gobi Nallathambi, Jaison Jeevanandam, Michael K. Danquah. "Nanoformulation of Peptides" Encyclopedia, https://encyclopedia.pub/entry/40223 (accessed February 07, 2026).
Thimmiah, B.R., Chien, B.T.C., Fui, K.S., Yon, L.S., Nallathambi, G., Jeevanandam, J., & Danquah, M.K. (2023, January 16). Nanoformulation of Peptides. In Encyclopedia. https://encyclopedia.pub/entry/40223
Thimmiah, Bhargavi Ram, et al. "Nanoformulation of Peptides." Encyclopedia. Web. 16 January, 2023.
Copy Citation
Several polymeric nanoparticles have been utilized as potential carriers for peptides and are used for the peptide formulation in controlled and targeted delivery applications. Nanoformulated peptides are reported to improve drug administration, where the drugs are either dissolved, entrapped, encapsulated, or attached to drug carriers.
peptides
nanoformulation
drug design
pharmaceutical delivery
Nanoparticles
Nanomedicine
1. Lipid-Based Nanoformulations (LNFs)
LNFs have been focused on in the scientific arena due to their great biocompatibility and versatility. They are great candidates for peptide carriers due to their submicron sizes, encapsulation capabilities (in protecting peptides against degradation), biodegradability, and their ability to maintain the drug activity’s effectiveness [1]. Table 1 summarizes different types of LNFs that are used as peptide carriers.
Table 1. Summary of different LNF types as peptide carrier.
| LNFs | Descriptions | Advantages | Disadvantages | Applications | References |
|---|---|---|---|---|---|
| Nanoemulsions (NEs) | - Oil, water, and surfactant. - Long- and medium-chain glycerides, and fatty acids. - Peptide is solubilized within the dispersed nanoparticles. |
1. Excellent dispersity. 2. Prolonged stability. 3. Good penetration abilities. |
1. Require high concentrations of surfactant. 2. Choices of biocompatible surfactants are limited. |
1. Shah et al. (2014) discovered polyunsaturated fatty acid NEs able to encapsulate the analgesic peptide; demonstrated efficacy in the capsaicin. 2. Pattani et al. (2006) developed NEs of polymyxin B and found NEs capable of producing potent effects in short times. |
[2][3] |
| Liposomes | - Bilayer vesicles; aqueous compartment is enclosed entirely by the membranous lipid bilayer. - Involve phospholipids, phosphatyl, glycerol derivates, and saturated and unsaturated fatty acids. |
1. Consistent release of proteins and peptides. 2. Easy modification of surface attachment. 3. Increase membrane permeability, drugs with proteins 4. Toxic-free. 5. Amphiphilic character, enables self-assembly. |
1. Limited stability can cause possible drug leakage and lead to aggregation. 2. Extra steps are commonly needed to modify their sizes and structures. |
Omri et al. (2002) formulated a DPPC/Chol liposomal polymyxin B against Pseudomonas aeruginosa and discovered that it can decrease the pulmonary bacterial counts along with a higher level of polymyxin B in the lungs, compared with those treated with free drugs after administered in a rat model for 3 days. | [4][5] |
| Solid Lipid nanoparticles (SLNs) | - Consist of lipids in a solid state at both room and body temperature. - Solid hydrophobic core and single layer of the phospholipid coating. |
1. Better drug encapsulation efficiency. 2. Encapsulate both lipophilic and hydrophilic drugs. 3. Improve drug stability. 4. Control release drug formulation as drug mobility is low in a solid state and triglycerides are slower than other glycerides. 5. Suitable for large-scale production. |
1. Poor drug loading capacity. 2. Drug expulsion (burst release after intravenous injection). 3. Require stabilizer to prevent drug portioning to the outer aqueous phase. |
Yuan et al. (2008) reported that PEG-SA and conjugate FA-SA that are inserted into SLN become potential applications for tumor therapy as they show efficient cellular updates and cytotoxicity by endocytosis. 2. Garcia-Fuentes et al. (2005) developed new surface-modified SLNs coated with chitosan (CS) for peptide delivery and discovered the ability of the ready release of the peptide; provided continuous delivery of the associated peptide. |
[6][7][8][9] |
| Nanostructure lipid carriers (NLCs) | - Next generation of SLNs by improving the stability and capacity loading, and preventing drug expulsion. - Blend of solid lipids and liquid lipids (oil) in certain proportions. |
1. Simple preparation. 2. Larger drug loading capacity compare with SLN. 3. Sustained drug release properties. 4. Low water content in final particle suspension. |
1. Susceptible to degradation by gastrointestinal lipases. | - | [10] |
| Lipid nanocapsules (LNCs) | - Made of solvent-free process with biocompatible excipients. - Contain oily a core surrounded by hydrophilic surfactants. - Involve medium-chain mono-, di-, and triglycerides, and long-chain fatty acids. - Combination of polymeric nanoparticles and liposomes. |
Provide considerable drug encapsulation capacity and sustain release properties. | 1. Non-specificity. 2. Unable to cross the weakly permeable endothelia. |
Nada et al. (2019) demonstrated the ability of antimicrobial peptide loads in LNCs using different strategies and discovered that both absorption and encapsulation methods can protect the peptide from proteolytic degradation. However, the LNC encapsulation method is not suitable for peptides with great amphipathic abilities. | [11] |
2. Silk Fibroin Nanoparticles
Silk fibroin nanoparticles (SF-NPs) have been identified to possess excellent biocompatibility and degradability as well as conjugating abilities with other active molecules. The enhanced binding capacity toward distinct drugs, controlled release capability, and mild preparation approach make SF-NPs effective drug delivery carriers [12]. Recently, Hassanzadeh et al. (2021) successfully developed biomimetic SF-NPs against breast cancer by coating SF-NPs with polydopamine (PDA), paclitaxel (PTX), peptides (iRGD), and transformed them into iRGD-PDA-PTX-SF-NPs. This new biomimetic peptide-based nanoformulation acted as an effective drug nanocarrier to target cancer cells overexpressing integrin (selective targeting of tumor). The SF-NPs showed improved intra-tumoral penetration and accumulation. The better drug entrapment also helped to reduce the dose frequency and drug intake time into the human body for complete recovery, thus, lessening the burden to the patient. Further, Li et al. (2022) formulated SF-NPs, where doxorubicin and atovaquone were encapsulated with Arg-Gly-Asp-SF-polylactic acid (RSA) to improve the chemotherapy treatment. The treated mice (injected with breast 4T1 cancer cells) showed higher inhibition rates, compared to phosphate buffer saline (PBS) and RSA alone, with minimal changes to the weights exhibited in the treated mice. This SF-based targeted drug carrier can alleviate the hypoxia microenvironment by suppressing mitochondrial respiration, which means it may suppress tumor development [13].
3. Cavitands
Cavitands are synthetic macromolecules, which can act as stabilizers for peptides and protein formulations by binding to amino acids and preventing the recognition of peptidase. Cyclodextrins (CDs) and cucurbiturils (CBs) are the best example of cavitands. CDs are cyclic non-reducing oligosaccharides that are made up of glucopyranose units [14]. CDs have amphiphilic structures that allow them to form in the inclusion complex with the protein and peptide. Moreover, CDs are safe for medical administration with less side effects even after chemical modifications of their exterior parts. They possess increased bioavailability and have enhanced local tolerability to peptide formulations. Further, Jóhannsdóttir et al. (2017) formulated CD-based aqueous cyclosporin A (CyA) as an eyedrop formulation. CD was selected as a solubilizer due to its great solubility effect on CyA, with its ability to form a CyA/CD complex aggregation and nanoparticles for better ocular bioavailability of drugs [15]. However, there are adverse possible consequences of cyclodextrin intake. One study reported that the administration of 2-hydroxypropyl-β-CD, i.e., 200 mg/kg every day for the long-term, could cause bone loss [16]. Further, Li et al. (2016) compared the toxicity and solubilizing capacity of hydroxypropyl--β-CD with different degree(s) of substitution (DS). The study revealed that hydroxypropyl--β-CD with a high DS not only exhibited weak solubilizing capacity for steroids, but also low hemolytic activity, while hydroxypropyl--β-CD with a medium DS demonstrated slightly higher nephrotoxicity. On the other hand, CBs are macrocyclic co-polymers of formaldehyde and glycoluril [17]. Similarly, CBs have hydrophobic voids, whereas hydrophilic parts are located in the portal area. The CB[n] family, where n is the different number of glycoluril units, possesses high affinity, high selectivity, and constrictive binding interactions, which make them great choices for the nanoformulation of peptides. Thus, CBs can bind better together to cationic guests, compared with CDs [18].
The most ideal system among all nanoformulation is difficult to select as every system has specific advantages and limitations. For instances, SLN and NLC have the ability to improve the delivery of drugs in various ways, but the choice of lipids and surfactants can affect both the particle size and stability in the long term. Toxicity is a significant factor that needs to be considered when selecting the surfactants to prevent any adverse impacts to be included in biomedical applications. Therefore, the above-mentioned limitations must be considered when designing a novel nanoformulated peptide with enhanced stability and the drug release time for effective circulation, low toxicity, high biocompatibility, and improved biological response.
4. In Vitro Analysis of Nanoformulated Peptides
Recently, various peptide encapsulated nanoparticles were developed for anti-tumor applications with less/no toxicity toward normal or noncancerous cells, as listed in Table 2. Yu et al. (2007) synthesized nucleolin-encapsulated florescent silver nano clusters. Nucleolin acted as a protective layer to prevent silver clusters from rapid oxidization. In the study, the fibroblast cell line was utilized to observe the delivery of the encapsulant. The results revealed that the nucleolin can enter the cells via the nuclear membrane for a strong nuclear staining application [19]. Likewise, Jagani et al. (2013) developed chitosan nanoparticles, which are encapsulated by siRNA for anticancer applications. The resultant siRNA was identified to silence the overexpression of the anti-apoptotic Bcl-2 gene. The size ranges of the siRNA-encapsulated chitosan nanoparticles were identified to be 190–340 nm. The study showed that the encapsulation efficiency of the chitosan nanoparticles was ~80%. The cytotoxicity results showed that the cell viability was 95% after 48 h. After 72 h, 85% of cells were viable, which revealed that the encapsulated nanoparticles do not exhibit toxic reactions toward noncancerous cells [20]. Further, Bawa et al. (2012) compared the anticancer activities of the nano- and micro-sized formulations of a peptide drug named ellipticine, which is an anticancer drug. The particle size distributions of the peptide formulations were in the range of 40–300 nm due to the agglomeration of particles. The cytotoxicity results demonstrated that nanoformulated peptides possess enhanced cancer cell inhibition properties toward the human carcinoma cell line (A549) after 24 h via improved endocytosis-mediated cellular uptake [21]. Furthermore, Gomes et al. (2013) prepared a novel nanoemulsion with a peptide containing essential oil extracted from Dodonaea angustifolia Miers (DA) and D. brasiliensis Miers (DB). Transmission electron microscope (TEM) results showed homogeneous droplets of nanoemulsions with sizes less than 200 nm. The cytotoxicity analysis was performed toward human glioma (U-138 MG) and human bladder carcinoma (T24) cell lines. The results revealed that the essential oil from both DA and DB reduced the cell viability at a 500 μg mL−1 concentration, while the nanoemulsion containing 4% essential oil possesses the ability to reduce the cell viability at 250 μg mL−1. Thus, nanoemulsion has been identified to act as a promising candidate for cancer treatment at lower concentrations, compared to free essential oils [22]. Moreover, Kulsharova et al. (2013) synthesized doxorubicin (DOX)-coated gelatin nanoparticles, which are encapsulated with a specific peptide for targeting breast cancer cells. DOX is a chemotherapy drug that leads to certain side effects, such as vomiting, diarrhea, eye redness, and darkening of the skin. The goal of the study is to minimize the side effects of DOX via controlled drug delivery into cancer cells. Three cell lines were used for the in vitro analysis of the novel formulation, namely MCF7 mammary adenocarcinoma cells, 4T1 mouse mammary carcinoma cells, and 3T3 mouse fibroblasts. The results showed that the viability of tumor cells decreased to 50% after 5 h due to the targeted delivery of DOX. Meanwhile, the increase of the 3T3 mouse fibroblasts cell growth indicate that the peptide drug possesses the ability to selectively inhibit the tumor cells [23].
Nishikawa et al. (2009) successfully coated the (AG)-30 angiogenic peptide on gelatin nanoparticles (AG-30/gelatin NPs) to be utilized for the treatment of ischemic diseases. The cytotoxicity analysis of AG-30/gelatin NPs using HAECs (human aortic endothelial cells) and HASMCs (human aortic smooth muscle cells) revealed that the AG-30/gelatin NPs possess dose-dependent toxic effects [24]. Further, Imanparast et al. (2017) prepared the mZD7349 peptide, which is encapsulated with poly(lactic-co-glycolic acid) nanoparticles (of 200 nm), and loaded with simvastatin. HUVEC (human umbilical cord vascular endothelial cells) were used for the cytotoxicity analysis, which showed that an increment in the drug concentration decreased the viability of the cells. Thus, it is evident that the peptide-encapsulated nanoparticles possess concentration-dependent cell viability, compared to standalone nanoparticles [25]. Furthermore, Campos et al. (2004) prepared mucin-encapsulated chitosan fluorescein nanoparticles with the size range of 384.6 ± 8.5 nm. The encapsulated nanoparticles did not exhibit cytotoxicity at higher concentrations. Moreover, certain minor damages were identified in cells due to the usage of the acetate buffer as observed in the scanning electron micrograph (SEM) [26]. Moreover, Narayanan et al. (2012) encapsulated human parathyroid hormone 1-34 (PTH1-34) into chitosan nanoparticles with an average particle size of 40 nm. The chitosan nanoformulation is shown to possess negligible effect toward the NIH3T3 fibroblast cells [27].
The standalone peptide has certain limitations, such as being unable to reach intracellular targets, poor stability, and insufficient drug delivery into the cell. Thus, peptide ligands were decorated on the surface of double emulsion (mPEG-b-PCL) nanoparticles via co-encapsulation for targeted drug delivery. The release rate of the peptide was slow from the nanoparticles when the pH of the peptide solution was altered. Later, the mixed peptide ligand with TGN, COG133, and RXR on the surface of the nanoparticles increased the drug release rate. The cytotoxicity analysis results showed that the double-encapsulated nanoparticles at 1 µM have the ability to suppress cell death and achieve significant peptide ligand release into the cerebellum [28]. Further, Silva et al. (2013) synthesized long peptides (OVA24) and encapsulated them within poly(lactic-co-glycolic acid) (PLGA) nanoparticles via a solvent evaporation technique for cancer immunotherapy. OVA24-loaded nanoparticles resulted in a high burst release due to low encapsulation efficiency. The burst release rate decreased from 90% to less than 10%, when there was an increase in the inner emulsion volume. Peptide encapsulated nanoparticles with a low burst release enhanced the activation of B3Z CD8+ T cells and the dendritic cell uptake due to T cell immunity generation, and played a vital role in cancer immunotherapy [29].
Table 2. In vitro analysis to evaluate the efficacy of nanoformulated peptides.
| Encapsulant | Nano Delivery System |
Cell Line | Applications | Reference |
|---|---|---|---|---|
| Nucleolin | Silver Nano clusters | NIH3T3 (Fibroblast cells) | Cancer | [19] |
| siRNA | Chitosan nanoparticles | HEp-2 (human epithelial laryngeal carcinoma), HeLa (human cervical carcinoma) |
Anti-tumor | [20] |
| Self-assembling peptide (EAK16-II) | Nanoformulation | A549 (lung carcinoma) | Anticancer | [21] |
| DA and DB oils | Nanoformulation | U-138 MG (human glioblastoma) and T24 (human bladder carcinoma) | Cancer | [22] |
| PEO and PPO blended | Poly (β-amino ester) nanoparticles | MDA-MB-231 (human breast adenocarcinoma cells) | Breast cancer | [30] |
| Cathepsin D | Gelatin | MCF7 (Human breast cancer cell) and HeLa cells | Breast cancer | [23] |
| (AG)-30 angiogenic peptide |
Gelatin | HAECs (human aortic endothelial cells) and HASMCs (human aortic smooth muscle cells) | Ischemic diseases | [24] |
| Simvastatin | Poly (lactic-co-glycolic acid) (PLGA) nanoparticles |
HUVEC (human umbilical cord vascular endothelial cells) | Cardiovascular disorders and cancer | [25] |
| Mucin | Chitosan nanoparticles | Conjunctival epithelial cells | Cornea | [26] |
| PTH 1-34 | Chitosan nanoparticles | NIH3T3 (Fibroblast cells) | Osteoporosis | [27] |
5. In Vivo Analysis of Nanoformulated Peptides
Recently, Fu and coworkers developed RGD peptide encapsulated doxorubicin-loaded selenium nanoparticles (146 nm) to target the tumor vasculature. Human breast cancer cells were injected into male nude mice. The in vivo experimental result showed that MCF-7 tumor growth was inhibited in the mice model by the RGD peptide selenium nanoparticles and the volume of the tumor decreased. However, there was no significant weight reduction in the body weights of the mice. Moreover, the RGD-selenium nanoparticles induced apoptosis and inhibited angiogenesis. Later, the mice were sacrificed, and their blood samples were obtained from different organs. The hematological analysis showed that the DOX could increase the levels of lactate dehydrogenase, aspartate aminotransferase, creatine kinase, and creatine, while peptide nanoparticles did not show any toxic effects. The in vivo results demonstrated that the peptide-decorated selenium nanoparticles were highly efficient for antitumor applications [31]. Further, Kaliaperumal et al. (2014) designed pACC1 peptide-encapsulated chitosan nanoparticles for controlled drug delivery toward breast cancer cells. Furthermore, 7,12-Dimethylbenz[α]anthracene (DMBA) carcinoma was induced in 6 groups of female rats for in vivo analysis. The results showed that the encapsulated nanoparticles could increase the level of antioxidant enzymes for the neutralization of the free radicals. ACC1 was shown to significantly increase the production of adenosine triphosphate (ATP) via mitochondria during glycolysis and the Krebs cycle. pACC1 has been shown to have enhanced abilities at controlling the membrane receptors of HER2 EGFR, where its over-expression can lead to breast cancer. Later, the pACC1-encapsulated chitosan nanoparticles were designed for dual functions, such as blocking lipogenesis and control membrane receptors for effective breast cancer treatment [32]. Furthermore, Wei et al. (2017) prepared doxorubicin-encapsulated mesoporous silica nanoparticles, which were surface-modified by polydopamine, and the CSNRDARRC peptide was decorated on the DOX-loaded MSNs@PDA for bladder cancer treatment. The HT-1376 cells were injected into male mice. It has been identified via histological studies that the DOX-loaded MSNs@ PDA and DOX-loaded MSNs@ PDA-PEP injected mice gained weight, where mice injected with DOX in the MSNs@ PDA-PEP sample remained healthy. After scarification of the mice, there were no morphological changes in the heart, liver, spleen, lung, and kidney. On the other hand, the mice injected with DOX-loaded MSNs@ PDA were weak and noticeable damages were identified in the organs. The results suggest that DOX-loaded MSNs@ PDA-PEP possesses antitumor effects and the peptide has the ability to control drug release toward the tumor cells [33]. Moreover, Sarangthem et al. (2020) incorporated the AP1 peptide with increased molecular weights of A38, A60, A86, and A100 on an elastin-like polypeptide polymer with a particle diameter of ~38–40 nm to exhibit antitumor activity. In vivo analysis results revealed that the peptide with a higher molecular weight retained for a longer period (up to 48 h) in the tumor cells; the peptide with a lower molecular weight retained for up to 12 h, where A86 and A100 reduced the volume of the tumor cells with low accumulation in the kidney, spleen, lungs, and heart of the mice model [34].
Zhao et al. (2014) constructed nano-lipid formulations of monomeric and trimeric peptides for the treatment of atherosclerosis. The drug was administrated through oral and intraperitoneal (IP) injection toward the low-density lipoprotein receptor (LDLr)-containing mice model. The mice were fed with chow diet for 10 weeks to increase their blood cholesterol levels and to induce atherosclerotic plaque in the aorta. The plasma cholesterol level study revealed that the administration of the nano-lipid formulation has led to a 50% decrease in the total cholesterol level for reducing the atherosclerotic plaque. Ip-administrated trimeric nanoparticles reduced the area of atherosclerotic lesions more than the monomeric lipid nanoparticles. It is evident from the in vivo studies that both administration routes helped to improve the effectiveness of the nano-lipid formulation in reducing the total cholesterol level [35]. Similarly, Wang et al. (2016) studied the PEG-PLA nanoparticles, which were decorated by the iRGD peptide with a particle size distribution of 39 nm. In vivo studies showed that the peptide-decorated polymer nanoparticles possess the ability to enhance the antitumor activity by ~60% by inhibiting the cancer cell proliferation via the binding of αv integrins with tumor endothelial cells, compared to normal cells [36]. Likewise, Wang et al. (2014) conjugated iRGD-PPCD and prepared the CRGDKGPDC cyclopeptide, which was encapsulated in a PEGylated polyamidoamine (PAMAM) dendrimer. The in vivo studies revealed that the peptide-conjugated sample was released in the tumor blood vessels and their accumulation in the tumor cells were higher compared to nonconjugated samples. The peptide-conjugated drug induced the highest inhibition of tumor vascular growth and effectively reduced the volume of vascular tumor [37]. Further, Kang and co-workers (2014) developed iNGR peptide-coated PEGylated PLGA nanoparticles for glioma treatment. In vivo studies showed that peptide-coated polymer nanoparticles possess high florescence intensity and anti-glioma efficacy, compared to uncoated nanoparticles. The nanoformulated samples were identified to be accumulated in the tumor sites and they penetrated deeply into the parenchyma of cancer cells due to their proteolytic cleavage into CRNGR and binding with NRP-1 [38].
Recently, Feng and co-workers (2016) designed a pyropheophorbide-a-conjugated polymeric drug, decorated with the F3 peptide. The peptide was shown to possess the ability to bind with nucleolin and it is expressed in tumor cells. The in vivo pharmacodynamic analysis showed that the inhibition of a tumor by the peptide-decorated nanoparticles was high, compared to free nanoparticles (79.92%). The apoptosis of tumor cells was shown to be induced after the treatment of the peptide-decorated nanoparticles, which indicates that the peptide effectively helps in targeting the tumor cells to control their growth. The histopathological analysis results revealed that there was no toxicity on normal tissues or the organs of the mice model treated with the peptide containing the nanoparticle [39]. Further, Liang et al. (2015) introduced tLyP-1-functionalized nanoparticles into the mice model to evaluate their antitumor properties. The peptide-functionalized nanoparticle was injected though the tail vein of the animal models for equal distribution throughout their bodies. TLyP-1-functionalized tLPTS/HATS nanoparticles were identified to be completely penetrable into the tumor tissues and reduced the accumulation of nanoparticles in the liver, spleen, and kidney. The growth of the tumor was suppressed at around 74%, compared to standalone nanoparticles. Additionally, the study demonstrated that the lipid-loaded nanoparticles did not exhibit a loss of net body weight, indicating its nontoxicity. However, the cell apoptosis rate increased after the incorporation of peptide-functionalized nanoparticles [40]. Furthermore, Xiao et al. (2012) prepared the OA02 peptide encapsulated in micellar nanoparticles for cancer treatment by releasing the peptide against the a-3 integrin, which is overexpressed in ovarian cancer. In vivo bio distribution studies showed that the peptide-loaded nanoparticles possess the ability to target tumor sites faster than unloaded nanoparticles. Moreover, the peptide-coated nanoparticles were identified to penetrate deeper into the ovarian cancer cells and bind with the a-3 integrin to exhibit enhanced cancer cell inhibition [41]. Moreover, Miyano et al. (2017) synthesized a novel cyclic Arg-Gly-Asp (cRGD) peptide and coated it on the surface of cisplatin-loaded micellar nanoparticles to target the SAS-L1-Luc cells, which were inoculated onto the mice tongues. An in vivo antitumor analysis showed that there was no distinct weight loss in the peptide-encapsulated nanoparticle-treated mice and it has a high capacity to inhibit tumor growth. Moreover, the cRGD nanoparticles were identified to be rapidly accumulating on the tumor sites and interacting with αvβ3 integrins to be expressed in the endothelial cancer cells [42].
Fang and co-workers (2017) developed a micelle nanoparticle (below 50 nm) using the cyclic RGD peptide cross-linked with poly(ethylene glycol)-b-poly(e-caprolactone) (PEG-PCL) to target glioma cancer. In vivo results indicated that cRGD-RCCMs had the potential to inhibit tumor growth and their efficiencies were enhanced by increasing the concentration of cRGD. No weight loss was identified in cRGD-RCCM-treated mice and their survival time prolonged. However, slight toxicities was observed in the liver, spleen, and kidney due to an increment in the cRGD concentration [43]. Moreover, Bi et al. (2016) prepared carmustine-loaded polymeric nanoparticles using the T7 peptide, encapsulated on the surfaces of loaded micelles for targeting the tumor cells in the central nervous system. The results indicate that the T7 peptide-encapsulated nanoparticles possess enhanced abilities to highly accumulate in the tumor cells and penetrate deeper into brain tumors. Moreover, T7 peptides have been identified to potentially target Tf receptors, which could be overexpressed in the blood–brain barrier. Luciferin was injected into the tumor and it has been identified that the intensity of the luminescence decreased due to the inhibition of the tumor cell growth. Weight was not observed in the animal model, compared to the uncoated T7 peptide-polymeric nanoparticles, with the longest survival time of the peptide. Histopathology results showed that there were no toxic effects on the spleen, liver, kidneys, and lungs. It is evident from the results that the T7 peptide-encapsulated polymeric nanoparticles can target the tumor cells for the controlled delivery of drug candidates without inhibiting the normal noncancerous cells [44]. Figure 1 presents the summary of the in vivo analyses, which were used to evaluate the biological applications of nanoformulated peptides.
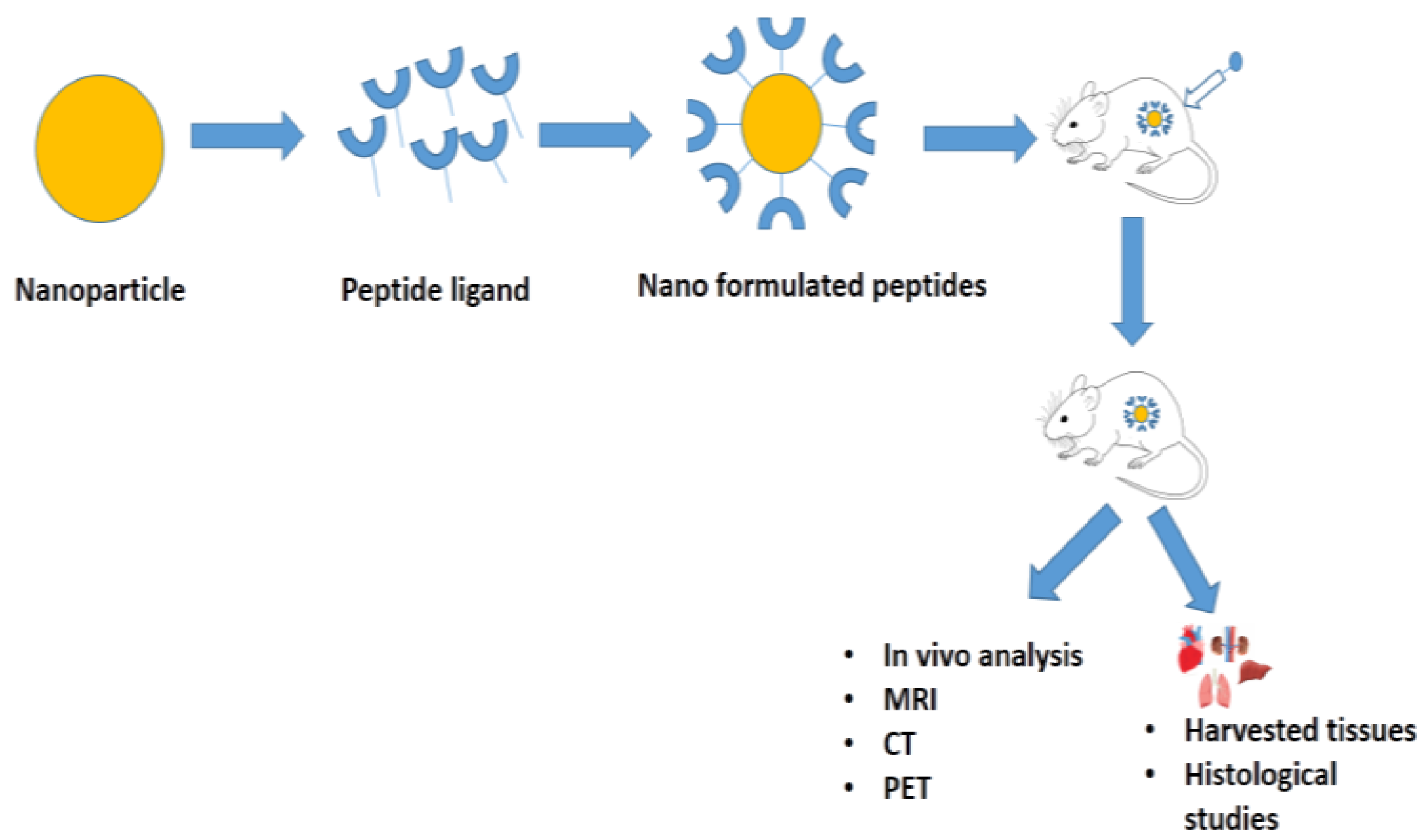
Figure 1. Types of in vivo analyses to evaluate the biological applications of nanoformulated peptides or peptide-conjugated/functionalized nanoparticles.
References
- Matougui, N.; Boge, L.; Groo, A.-C.; Umerska, A.; Ringstad, L.; Bysell, H.; Saulnier, P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016, 502, 80–97.
- Shah, L.; Kulkarni, P.; Ferris, C.; Amiji, M.M. Analgesic efficacy and safety of DALDA peptide analog delivery to the brain using oil-in-water nanoemulsion formulation. Pharm. Res. 2014, 31, 2724–2734.
- Pattani, A.S.; Mandawgade, S.D.; Patravale, V.B. Development and comparative anti-microbial evaluation of lipid nanoparticles and nanoemulsion of polymyxin B. J. Nanosci. Nanotechnol. 2006, 6, 2986–2990.
- Niu, Z.; Zhao, W.; Zhang, Z.; Xiao, F.; Tang, X.; Yang, J. The Molecular Structure of Alzheimer β-Amyloid Fibrils Formed in the Presence of Phospholipid Vesicles. Angew. Chem. 2014, 126, 9448–9451.
- Omri, A.; Suntres, Z.E.; Shek, P.N. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 2002, 64, 1407–1413.
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490.
- Müller, R.; Maaben, S.; Weyhers, H.; Mehnert, W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J. Drug Target. 1996, 4, 161–170.
- Yuan, H.; Miao, J.; Du, Y.-Z.; You, J.; Hu, F.-Q.; Zeng, S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int. J. Pharm. 2008, 348, 137–145.
- Garcia-Fuentes, M.; Torres, D.; Alonso, M.J. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int. J. Pharm. 2005, 296, 122–132.
- Martin, N.I.; Breukink, E. The expanding role of lipid II as a target for lantibiotics. Future Med. 2007, 2, 513–525.
- Matougui, N.; Groo, A.-C.; Umerska, A.; Cassisa, V.; Saulnier, P. A comparison of different strategies for antimicrobial peptides incorporation onto/into lipid nanocapsules. Nanomedicine 2019, 14, 1647–1662.
- Zhao, Z.; Li, Y.; Xie, M.-B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903.
- Li, B.; Yang, Y.; Wang, F.; Wang, R.; Fei, H.; Duan, S.; Huang, L.; Liao, N.; Zhao, S.; Ma, X. Biodegradable silk fibroin nanocarriers to modulate hypoxia tumor microenvironment favoring enhanced chemotherapy. Front. Bioeng. Biotechnol. 2022, 10, 1246.
- Yeguas, V.; Altarsha, M.; Monard, G.; López, R.; Ruiz-López, M.F. Peptide binding to β-cyclodextrins: Structure, dynamics, energetics, and electronic effects. J. Phys. Chem. A 2011, 115, 11810–11817.
- Jóhannsdóttir, S.; Kristinsson, J.K.; Fülöp, Z.; Ásgrímsdóttir, G.; Stefánsson, E.; Loftsson, T. Formulations and toxicologic in vivo studies of aqueous cyclosporin A eye drops with cyclodextrin nanoparticles. Int. J. Pharm. 2017, 529, 486–490.
- Kantner, I.; Erben, R.G. Long-term parenteral administration of 2-hydroxypropyl-β-cyclodextrin causes bone loss. Toxicol. Pathol. 2012, 40, 742–750.
- Li, Z.; Zheng, Z.; Su, S.; Yu, L.; Wang, X. Hydroxypropyl-β-CD vs. its α-homologue for a 3D modified polyrotaxane network formation and properties: The relationship between modified CD and polymer revealed through comparison. Soft Matter 2016, 12, 7089–7101.
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological aspects of the design of nanocarriers for therapeutic peptides and proteins. Pharmaceutics 2019, 11, 91.
- Yu, J.; Patel, S.A.; Dickson, R.M. In vitro and intracellular production of peptide-encapsulated fluorescent silver nanoclusters. Angew. Chem. Int. Ed. 2007, 46, 2028–2030.
- Jagani, H.; Rao, J.; Palanimuthu, V.; Hariharapura, R.; Gang, S. A nanoformulation of siRNA and its role in cancer therapy: In vitro and in vivo evaluation. Cell. Mol. Biol. Lett. 2013, 18, 120–136.
- Bawa, R.; Fung, S.-Y.; Shiozaki, A.; Yang, H.; Zheng, G.; Keshavjee, S.; Liu, M. Self-assembling peptide-based nanoparticles enhance cellular delivery of the hydrophobic anticancer drug ellipticine through caveolae-dependent endocytosis. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 647–654.
- Gomes, M.R.F.; Schuh, R.S.; Jacques, A.L.B.; Augustin, O.A.; Bordignon, S.A.L.; Dias, D.O.; Kelmann, R.G.; Koester, L.S.; Gehring, M.P.; Morrone, F.B. Citotoxic activity evaluation of essential oils and nanoemulsions of Drimys angustifolia and D. brasiliensis on human glioblastoma (U-138 MG) and human bladder carcinoma (T24) cell lines in vitro. Rev. Bras. Farmacogn. 2013, 23, 259–267.
- Kulsharova, G.K.; Lee, M.B.; Cheng, F.; Haque, M.; Choi, H.; Kim, K.; O’Brien, W.D.; Liu, G.L. In vitro and in vivo imaging of peptide-encapsulated polymer nanoparticles for cancer biomarker activated drug delivery. IEEE Trans. Nanobiosci. 2013, 12, 304–310.
- Nishikawa, T.; Nakagami, H.; Maeda, A.; Morishita, R.; Miyazaki, N.; Ogawa, T.; Tabata, Y.; Kikuchi, Y.; Hayashi, H.; Tatsu, Y. Development of a novel antimicrobial peptide, AG-30, with angiogenic properties. J. Cell. Mol. Med. 2009, 13, 535–546.
- Imanparast, F.; Faramarzi, M.A.; Vatannejad, A.; Paknejad, M.; Deiham, B.; Kobarfard, F.; Amani, A.; Doosti, M. mZD7349 peptide-conjugated PLGA nanoparticles directed against VCAM-1 for targeted delivery of simvastatin to restore dysfunctional HUVECs. Microvasc. Res. 2017, 112, 14–19.
- De Campos, A.M.; Diebold, Y.; Carvalho, E.L.S.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles as new ocular drug delivery systems: In vitro stability, in vivo fate, and cellular toxicity. Pharm. Res. 2004, 21, 803–810.
- Narayanan, D.; Anitha, A.; Jayakumar, R.; Nair, S.V.; Chennazhi, K.P. Synthesis, characterization and preliminary in vitro evaluation of PTH 1-34 loaded chitosan nanoparticles for osteoporosis. J. Biomed. Nanotechnol. 2012, 8, 98–106.
- Kim, R.M.; Feng, T.; Zhang, Q.; Chan, Y.H.; Chau, Y. Co-Encapsulation and Co-Delivery of Peptide Drugs via Polymeric Nanoparticles. Polymers 2019, 11, 288.
- Silva, A.L.; Rosalia, R.A.; Sazak, A.; Carstens, M.G.; Ossendorp, F.; Oostendorp, J.; Jiskoot, W. Optimization of encapsulation of a synthetic long peptide in PLGA nanoparticles: Low-burst release is crucial for efficient CD8+ T cell activation. Eur. J. Pharm. Biopharm. 2013, 83, 338–345.
- Shenoy, D.; Little, S.; Langer, R.; Amiji, M. Poly (ethylene oxide)-modified poly (β-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol. Pharm. 2005, 2, 357–366.
- Fu, X.; Yang, Y.; Li, X.; Lai, H.; Huang, Y.; He, L.; Zheng, W.; Chen, T. RGD peptide-conjugated selenium nanoparticles: Antiangiogenesis by suppressing VEGF-VEGFR2-ERK/AKT pathway. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1627–1639.
- Kaliaperumal, J.; Padarthi, P.; Elangovan, N.; Hari, N. Anti-tumorigenic effect of nano formulated peptide pACC1 by diminishing de novo lipogenisis in DMBA induced mammary carcinoma rat model. Biomed. Pharmacother. 2014, 68, 763–773.
- Wei, Y.; Gao, L.; Wang, L.; Shi, L.; Wei, E.; Zhou, B.; Zhou, L.; Ge, B. Polydopamine and peptide decorated doxorubicin-loaded mesoporous silica nanoparticles as a targeted drug delivery system for bladder cancer therapy. Drug Deliv. 2017, 24, 681–691.
- Sarangthem, V.; Seo, B.-Y.; Yi, A.; Lee, Y.-J.; Cheon, S.-H.; Kim, S.K.; Singh, T.D.; Lee, B.-H.; Park, R.-W. Effects of molecular weight and structural conformation of multivalent-based elastin-like polypeptides on tumor accumulation and tissue biodistribution. Nanotheranostics 2020, 4, 57.
- Zhao, Y.; Black, A.S.; Bonnet, D.J.; Maryanoff, B.E.; Curtiss, L.K.; Leman, L.J.; Ghadiri, M.R. In vivo efficacy of HDL-like nanolipid particles containing multivalent peptide mimetics of apolipoprotein AI. J. Lipid Res. 2014, 55, 2053–2063.
- Wang, J.; Wang, H.; Li, J.; Liu, Z.; Xie, H.; Wei, X.; Lu, D.; Zhuang, R.; Xu, X.; Zheng, S. iRGD-decorated polymeric nanoparticles for the efficient delivery of vandetanib to hepatocellular carcinoma: Preparation and in vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 19228–19237.
- Wang, K.; Zhang, X.; Liu, Y.; Liu, C.; Jiang, B.; Jiang, Y. Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials 2014, 35, 8735–8747.
- Kang, T.; Gao, X.; Hu, Q.; Jiang, D.; Feng, X.; Zhang, X.; Song, Q.; Yao, L.; Huang, M.; Jiang, X. iNGR-modified PEG-PLGA nanoparticles that recognize tumor vasculature and penetrate gliomas. Biomaterials 2014, 35, 4319–4332.
- Feng, X.; Jiang, D.; Kang, T.; Yao, J.; Jing, Y.; Jiang, T.; Feng, J.; Zhu, Q.; Song, Q.; Dong, N. Tumor-homing and penetrating peptide-functionalized photosensitizer-conjugated PEG-PLA nanoparticles for chemo-photodynamic combination therapy of drug-resistant cancer. ACS Appl. Mater. Interfaces 2016, 8, 17817–17832.
- Liang, D.-S.; Su, H.-T.; Liu, Y.-J.; Wang, A.-T.; Qi, X.-R. Tumor-specific penetrating peptides-functionalized hyaluronic acid-d-α-tocopheryl succinate based nanoparticles for multi-task delivery to invasive cancers. Biomaterials 2015, 71, 11–23.
- Xiao, K.; Li, Y.; Lee, J.S.; Gonik, A.M.; Dong, T.; Fung, G.; Sanchez, E.; Xing, L.; Cheng, H.R.; Luo, J. “OA02” peptide facilitates the precise targeting of paclitaxel-loaded micellar nanoparticles to ovarian cancer in vivo. Cancer Res. 2012, 72, 2100–2110.
- Miyano, K.; Cabral, H.; Miura, Y.; Matsumoto, Y.; Mochida, Y.; Kinoh, H.; Iwata, C.; Nagano, O.; Saya, H.; Nishiyama, N. cRGD peptide installation on cisplatin-loaded nanomedicines enhances efficacy against locally advanced head and neck squamous cell carcinoma bearing cancer stem-like cells. J. Control. Release 2017, 261, 275–286.
- Fang, Y.; Jiang, Y.; Zou, Y.; Meng, F.; Zhang, J.; Deng, C.; Sun, H.; Zhong, Z. Targeted glioma chemotherapy by cyclic RGD peptide-functionalized reversibly core-crosslinked multifunctional poly (ethylene glycol)-b-poly (ε-caprolactone) micelles. Acta Biomater. 2017, 50, 396–406.
- Bi, Y.; Liu, L.; Lu, Y.; Sun, T.; Shen, C.; Chen, X.; Chen, Q.; An, S.; He, X.; Ruan, C. T7 peptide-functionalized PEG-PLGA micelles loaded with carmustine for targeting therapy of glioma. ACS Appl. Mater. Interfaces 2016, 8, 27465–27473.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
17 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




