
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Leonel Pereira | -- | 1997 | 2023-01-16 10:18:18 | | | |
| 2 | Lindsay Dong | Meta information modification | 1997 | 2023-01-18 01:49:08 | | | | |
| 3 | Lindsay Dong | Meta information modification | 1997 | 2023-01-18 01:50:30 | | | | |
| 4 | Lindsay Dong | Meta information modification | 1997 | 2023-01-18 01:52:26 | | |
Video Upload Options
The ecosystem services can be divided using two major classification systems, the Millennium Ecosystem Assessment (MEA) and the Common International Classification of Ecosystem Services (CICES). In the MEA system, the ecosystem services are divided into four major service clusters: supporting, provisioning, regulating, and cultural. On the other hand, the CICES system regards the “MEA supporting services” as organism natural function (and not an ecosystem service). Thus, this function is the basis for all the three CICES ecosystem services (provisioning, regulating, and cultural) provided by one organism. These ecosystem services can be analyzed for the type of habitat, fauna or flora. Seaweeds, or marine macroalgae, are one of the key organisms in estuarine and seawater habitats ecosystems, which currently is of extreme importance due to the climate changes and the blue–green economy. Seaweeds and humankind have been interlinked from the beginning, mainly as a food source, fibers, biochemicals, natural medicine, ornamental resources, art inspiration, and esthetic values in several coastal communities. Moreover, currently they are being studied as green carbon, carbon sequestration, and as a possible source for the biomedical and pharmaceutical areas.
1. Introduction
2. Ecosystem Services Provided by Seaweeds
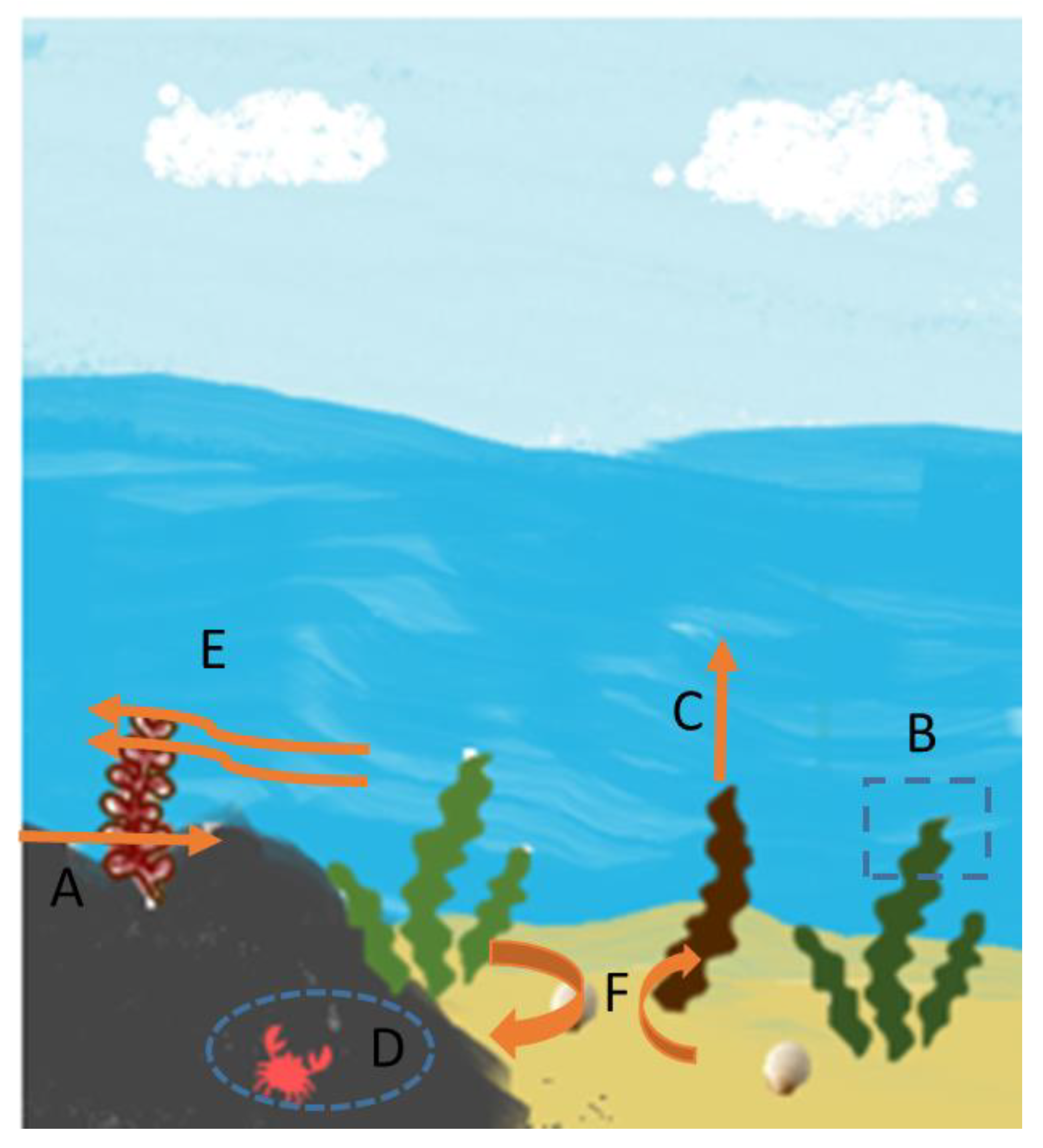
3. Seaweed Ecosystem Services
3.1. Seaweed Functions/Supporting Services

3.2. Regulating Services
Unlike supporting services, regulating services provide advantages that are more directly tied to human benefits; however, they are not always clearly recognized such as the climate regulation provided by seaweeds.
Highly prolific seaweed species may contribute significantly to the yearly biological reduction in CO2 and the global carbon cycle [39]. However, in order to comprehend the scope of this decrease, researchers must first estimate the amount and rate at which fixed carbon is recycled [40]. Even though marine seaweed communities comprise a very tiny portion of the coastal region (approximately 33%, 3,5 mill km2), these habitats are an important component of climatic change adaptation and mitigation strategy [41]. Thus, seaweed are responsible for the uptake of 173 million tons of carbon per year, making seaweed a vital player in the global carbon cycle [30]. Seaweed are carried to the deep ocean by drifting and sinking after being pulled away from the substrate [30]. Fresh Sargassum were discovered in the intestines of abyssal isopods (a kind of marine crustacean) residing on the bottom at 6.475 m depth, indicating the transfer of seaweed to the deep waters. Organic material is considered gone from the carbon cycle when it falls below 1.000 m [30]. Seaweed also mitigates acidification, deoxygenation, and other marine effects of global warming, which endanger sea biodiversity and the source of food and livelihood for hundreds of millions of people [42]. Thus, the promotion of sustainable seaweed cultivation can promote this service even more. Seaweed cultivation can assist to prevent ocean acidification and deoxygenation [43].
Erosion regulation is also linked to seaweed colonization. Seaweeds prevent erosion and sediment trapping, aiding in the maintenance of saltwater environments’ shorelines (Figure 3). Shore erosion is reduced because seaweeds act as physical barriers that minimize wave energy [21][44].This is also observed in estuarine seaweeds that trap sediment in the river margins [21].

3.3. Provisioning Services

3.4. Cultural Services
References
- Pfister, C.A.; Altabet, M.A.; Weigel, B. Kelp beds and their local effects on seawater chemistry, productivity, and microbial communities. Ecology 2019, 100, e02798.
- García-Poza, S.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Marine macroalgae as a feasible and complete resource to address and promote Sustainable Development Goals (SDGs). Integr. Environ. Assess. Manag. 2022, 18, 1148–1161.
- Butt, K.R.; Méline, C.; Pérès, G. Marine macroalgae as food for earthworms: Growth and selection experiments across ecotypes. Environ. Sci. Pollut. Res. 2020, 27, 33493–33499.
- Randall, J.; Johnson, C.R.; Ross, J.; Hermand, J.-P. Acoustic investigation of the primary production of an Australian temperate macroalgal (Ecklonia radiata) system. J. Exp. Mar. Biol. Ecol. 2020, 524, 151309.
- Hay, K.H.; Hays, G.; Orth, R. Critical evaluation of the nursery role hypothesis for seagrass meadows. Mar. Ecol. Prog. Ser. 2003, 253, 123–136.
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.; Pfirrmann, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.; Orth, R. Are coastal habitats important nurseries? A meta-analysis. Conserv. Lett. 2019, 12, e12645.
- García-Poza, S.; Cotas, J.; Morais, T.; Pacheco, D.; Pereira, L.; Marques, J.C.; Gonçalves, A.M.M. Global Trade of Seaweed Foods. In Sustainable Global Resources of Seaweeds Volume 2; Springer International Publishing: Cham, Switzerland, 2022; pp. 325–337.
- Fredericq, S. The Wonderful World of Seaweeds. Available online: https://oceanexplorer.noaa.gov/explorations/03mex/background/seaweeds/seaweeds.html#:~:text=Because%20seaweeds%20are%20photosynthetic%20organisms,1) (accessed on 14 August 2022).
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528.
- Pardilhó, S.; Cotas, J.; Gonçalves, A.M.M.; Dias, J.M.; Pereira, L. Seaweeds Used in Wastewater Treatment: Steps to Industrial Commercialization. In Phycology-Based Approaches for Wastewater Treatment and Resource Recovery; CRC Press: Boca Raton, FL, USA, 2021; pp. 247–262.
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559.
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates—A General Overview. In Alginates—Recent Uses of This Natural Polymer; IntechOpen: London, UK, 2020.
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384.
- Watson, R.T.; Rosswall, T.; Steiner, A.; Töpfer, K.; Arico, S.; Bridgewater, P. Ecosystems and Human Well-Being; Island Press: Washington, DC, USA, 2003; ISBN 1559634022.
- Carpenter, S.R.; DeFries, R.; Dietz, T.; Mooney, H.A.; Polasky, S.; Reid, W.V.; Scholes, R.J. Millennium Ecosystem Assessment: Research Needs. Science 2006, 314, 257–258.
- Gomes, E.; Inácio, M.; Bogdzevič, K.; Kalinauskas, M.; Karnauskaitė, D.; Pereira, P. Future land-use changes and its impacts on terrestrial ecosystem services: A review. Sci. Total Environ. 2021, 781, 146716.
- Haines-Young, R.; Potschin-Young, M. Revision of the Common International Classification for Ecosystem Services (CICES V5.1): A Policy Brief. One Ecosyst. 2018, 3, e27108.
- Thomaz, S.M. Ecosystem services provided by freshwater macrophytes. Hydrobiologia 2021, 1–21.
- Garden, C.J.; Smith, A.M. Voyages of seaweeds: The role of macroalgae in sediment transport. Sediment. Geol. 2015, 318, 1–9.
- Morris, R.L.; Graham, T.D.J.; Kelvin, J.; Ghisalberti, M.; Swearer, S. Kelp beds as coastal protection: Wave attenuation of Ecklonia radiata in a shallow coastal bay. Ann. Bot. 2019, 125, 235–246.
- Smith, S. Climatic Factors—Wild seaweed harvesting: Strategic environmental assessment—Environmental report—Gov.scot. In Wild Seaweed Harvesting: Strategic Environmental Assessment—Environmental Report; Scottish Government: Edinburgh, UK, 2016; ISBN 9781786526229.
- Airoldi, L.; Ballesteros, E.; Buonuomo, R.; van Belzen, J.; Bouma, T.; Cebrian, E.; de Clerk, O.; Engelen, A.; Ferrario, F.; Fraschetti, S.; et al. Marine forests at risk: Solutions to halt the loss and promote the recovery of Mediterranean canopy-forming seaweeds. In Proceedings of the 5th Mediterranean Symposium on Marine Vegetation, Portorož, Slovenia, 27–28 October 2014; Labgar, H., Bouafif, C., Ouerghi, A., Eds.; United Nations Environment Programme, Mediterranean Action Plan, Regional Activity Center for Specially Protected Areas: Portoroz, Slovenia, 2014; pp. 27–28.
- Field, C.B.; Barros, V.; Stocker, T.F.; Dahe, Q.; Jon Dokken, D.; Ebi, K.L.; Mastrandrea, M.D.; Mach, K.J.; Plattner, G.K.; Allen, S.K.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Cambridge University Press: Cambridge, UK, 2012; ISBN 9781139177245.
- Duarte, C.M.; Losada, I.J.; Hendriks, I.E.; Mazarrasa, I.; Marbà, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang. 2013, 3, 961–968.
- Barbier, E.B.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193.
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2000.
- Temmerman, S.; Govers, G.; Wartel, S.; Meire, P. Modelling estuarine variations in tidal marsh sedimentation: Response to changing sea level and suspended sediment concentrations. Mar. Geol. 2004, 212, 1–19.
- Duarte, C.M.; Chiscano, C.L. Seagrass biomass and production: A reassessment. Aquat. Bot. 1999, 65, 159–174.
- Smale, D.A.; Pessarrodona, A.; King, N.; Burrows, M.T.; Yunnie, A.; Vance, T.; Moore, P. Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Sci. Rep. 2020, 10, 1–12.
- Krause-Jensen, D.; Duarte, C.M. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742.
- Duarte, C.M. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences 2017, 14, 301–310.
- Lomartire, S.; Cotas, J.; Pacheco, D.; Marques, J.; Pereira, L.; Gonçalves, A. Environmental Impact on Seaweed Phenolic Production and Activity: An Important Step for Compound Exploitation. Mar. Drugs 2021, 19, 245.
- Mishra, S.; Maiti, A. The efficacy of bacterial species to decolourise reactive azo, anthroquinone and triphenylmethane dyes from wastewater: A review. Environ. Sci. Pollut. Res. 2018, 25, 8286–8314.
- Koelmans, A. Integrated Modelling of Eutrophication and Organic Contaminant Fate & Effects in Aquatic Ecosystems. A Review. Water Res. 2001, 35, 3517–3536.
- Wetzel, R.G. Limnology: Lake and River Ecosystem, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; Volume 35, ISBN 9780127447605.
- Miranda, L.E.; Driscoll, M.P.; Allen, M.S. Transient physicochemical microhabitats facilitate fish survival in inhospitable aquatic plant stands. Freshw. Biol. 2000, 44, 617–628.
- Hasselström, L.; Visch, W.; Gröndahl, F.; Nylund, G.M.; Pavia, H. The impact of seaweed cultivation on ecosystem services—A case study from the west coast of Sweden. Mar. Pollut. Bull. 2018, 133, 53–64.
- Bak, U.G. Seaweed Cultivation in the Faroe Islands: An Investigation of the Biochemical Composition of Selected Macroalgal Species, Optimised Seeding Technics, and Open-Ocean Cultivation Methods from a Commercial Perspective. Ph.D. Thesis, Technical University of Denmark, Kgs. Lyngby, Denmark, 2019; p. 157.
- Turan, G.; Neori, A. Intensive seaweed aquaculture: A potent solution against global warming. In Seaweeds and Their Role in Globally Changing Environments; Israel, A., Einav, R., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 359–372.
- Chung, I.K.; Oak, J.H.; Lee, J.A.; Shin, J.A.; Kim, J.G.; Park, K.-S. Installing kelp forests/seaweed beds for mitigation and adaptation against global warming: Korean Project Overview. ICES J. Mar. Sci. 2013, 70, 1038–1044.
- Wernberg, T.; Krumhansl, K.; Filbee-Dexter, K.; Pedersen, M.F. Status and Trends for the World’s Kelp Forests. In World Seas: An Environmental Evaluation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 57–78.
- Forests of Seaweed Can Help Climate Change—Without Risk of Fire. Available online: https://www.nationalgeographic.com/environment/article/forests-of-seaweed-can-help-climate-change-without-fire (accessed on 1 April 2022).
- Duarte, C.M.; Wu, J.; Xiao, X.; Bruhn, A.; Krause-Jensen, D. Can Seaweed Farming Play a Role in Climate Change Mitigation and Adaptation? Front. Mar. Sci. 2017, 4, 00100.
- Alvera-Azcárate, A.; Ferreira, J.; Nunes, J. Modelling eutrophication in mesotidal and macrotidal estuaries. The role of intertidal seaweeds. Estuarine, Coast. Shelf Sci. 2003, 57, 715–724.
- Pacheco, D.; Araújo, G.S.; Cotas, J.; Gaspar, R.; Neto, J.M.; Pereira, L. Invasive Seaweeds in the Iberian Peninsula: A Contribution for Food Supply. Mar. Drugs 2020, 18, 560.
- Pereira, L.; Cotas, J. Historical Use of Seaweed as an Agricultural Fertilizer in the European Atlantic Area. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–22.
- Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T.; Pérez-Lloréns, J.L. Saved by seaweeds: Phyconomic contributions in times of crises. J. Appl. Phycol. 2021, 33, 443–458.
- Pérez-Lloréns, J.L.; Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T. Seaweeds in mythology, folklore, poetry, and life. J. Appl. Phycol. 2020, 32, 3157–3182.
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & amp Mohr). J. Appl. Phycol. 2013, 25, 1777–1791.
- O’Connor, K. Seaweed: A Global History (Edible); Reaktion Books, Limited: London, UK, 2017; ISBN 9781780237534.
- Pérez Lloréns, J.L.; Hernández Carrero, I.; Vergara Oñate, J.J.; Brun Murillo, F.G.; León González, A. Those Curious and Delicious Seaweeds: A Fascinating Voyage from Biology to Gastronomy; Pérez Lloréns, J.L., Hernández Carrero, I., Vergara Oñate, J.J., Brun Murillo, F.G., León González, A., de Harland, A.B., Eds.; Editorial UCA: Cadiz, Spain, 2018; ISBN 978-84-9828-666-3.




