Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Himanshu Arora | -- | 2287 | 2023-01-16 06:27:38 | | | |
| 2 | Catherine Yang | Meta information modification | 2287 | 2023-01-16 06:50:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mintz, J.; Vedenko, A.; Rosete, O.; Shah, K.; Goldstein, G.; Hare, J.M.; Ramasamy, R.; Arora, H. Nitric Oxide in Different Cancer Types. Encyclopedia. Available online: https://encyclopedia.pub/entry/40191 (accessed on 07 February 2026).
Mintz J, Vedenko A, Rosete O, Shah K, Goldstein G, Hare JM, et al. Nitric Oxide in Different Cancer Types. Encyclopedia. Available at: https://encyclopedia.pub/entry/40191. Accessed February 07, 2026.
Mintz, Joel, Anastasia Vedenko, Omar Rosete, Khushi Shah, Gabriella Goldstein, Joshua M. Hare, Ranjith Ramasamy, Himanshu Arora. "Nitric Oxide in Different Cancer Types" Encyclopedia, https://encyclopedia.pub/entry/40191 (accessed February 07, 2026).
Mintz, J., Vedenko, A., Rosete, O., Shah, K., Goldstein, G., Hare, J.M., Ramasamy, R., & Arora, H. (2023, January 16). Nitric Oxide in Different Cancer Types. In Encyclopedia. https://encyclopedia.pub/entry/40191
Mintz, Joel, et al. "Nitric Oxide in Different Cancer Types." Encyclopedia. Web. 16 January, 2023.
Copy Citation
Nitric oxide (NO) is a short-lived, ubiquitous signaling molecule that affects numerous critical functions in the body. There are markedly conflicting findings in the literature regarding the bimodal effects of NO in carcinogenesis and tumor progression, which has important consequences for treatment.
nitric oxide
prostate cancer
castration
1. Lung Cancer
Lung cancer has the second-highest incidence amongst all cancer types, with a poor 5-year survival rate of 4–17% [1][2]. Due to its high incidence and often aggressive course, it is no surprise that NO has been shown to function in the pathogenesis of lung cancer. In fact, population-based studies of NO and NO metabolites suggest that increased accumulation of NO metabolites were associated with an increased risk of lung cancer, even after controlling for relevant confounding factors such as smoking [3]. Furthermore, cigarette smoking, a notable risk factor for lung cancer, often contains NO and other ROS [4]. Such compounds may encourage the development of NO-mediated cellular changes, which can eventually accumulate in malignant lung tissue (Figure 1) [4]. Excess exposure of lung tissue to NO results in the accumulation of nitrosylated proteins, which were significantly higher in those with lung cancer [5]. Often, this effect is mediated via iNOS, which is often shown to be upregulated in lung cancer cell lines, similar to other cancers [6][7].
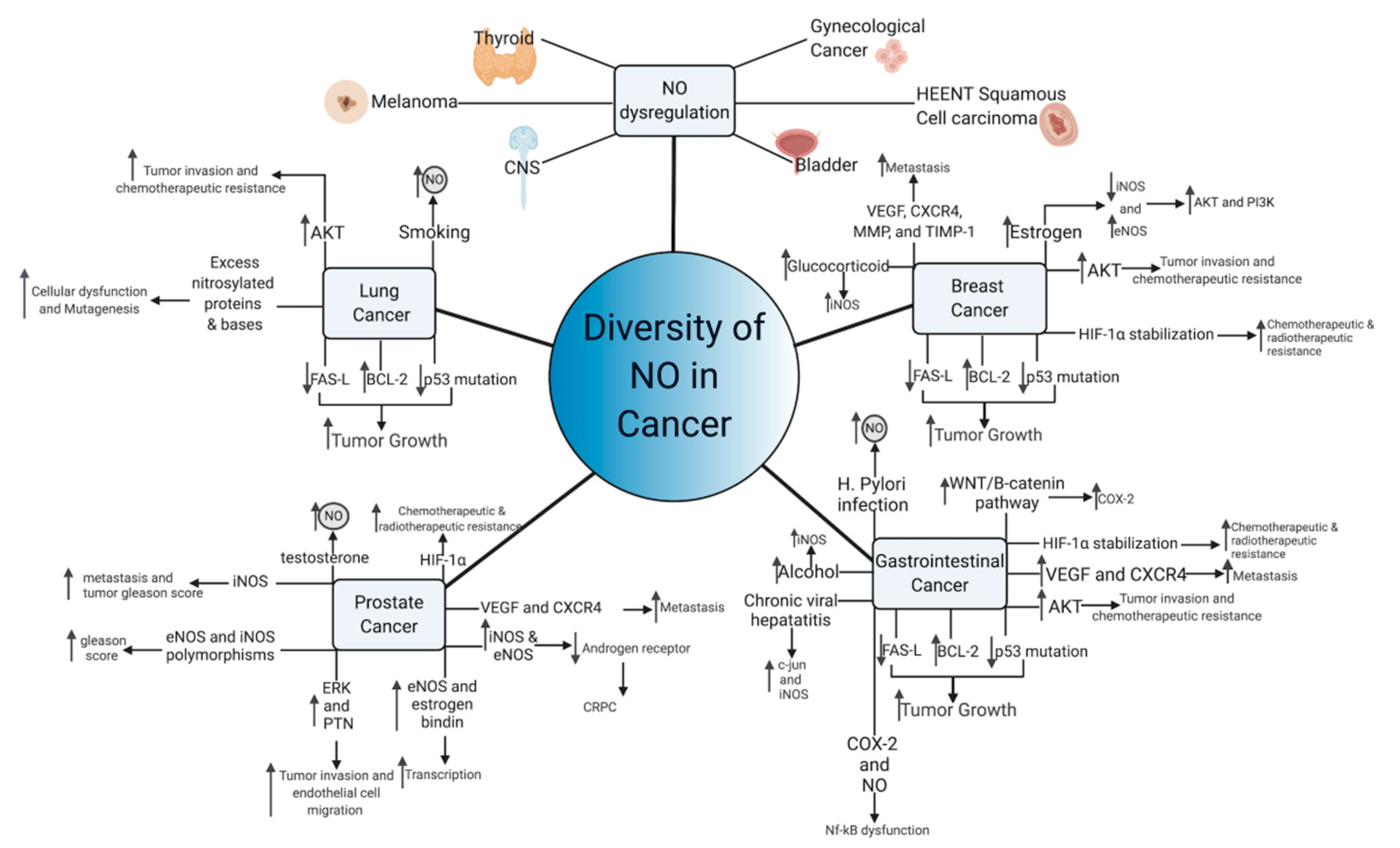
Figure 1. Diversity of NO functioning in cancer. Multiple NO-mediated cancer pathways contribute to cancer growth and metastasis. Common pathways of NO-mediated mutagenesis and cancer growth, include p53 mutation, AKT upregulation, and VEGF induction, among others. Aberrant NO signaling also occurs in response to common carcinogens, including viruses, alcohol, and tobacco, suggesting that NO may lie in a common carcinogenic pathway shared by these compounds. In hormone-sensitive tumors, NO paradoxically functions to transmit the hormonal growth signals and can make the tumor hormone-insensitive.
Excess NO in lung tissues may contribute to a number of effects that synergistically act to encourage cancer formation. For example, one study noted that gaseous nitric oxide and inducible NOS produced an increase of 8-nitroguanine, which may increase DNA damage and encourage mutagenesis [8]. Although protein nitrosylation is often viewed as a marker of oxidative stress, one group proposed that protein nitrosylation may also impair antioxidant proteins and those involved in cellular metabolism, which may further contribute to the development of non-small-cell lung cancer (NSCLC) [9]. Furthermore, elevated iNOS in NSCLC cells are linked to p53 mutations, removing growth checkpoint inhibition and creating cells with unlimited replicative potential, another notable effect of NO [10][11]. Within lung cancer, the antiapoptotic effect is hypothesized function through BCL-2 upregulation and modifications to FAS death-ligand signaling, which encourages aggressive growth [12]. Once established, NO also modulates integrin expression amongst NSCLC cells via protein kinase B (AKT) activity, and increased AKT activity is correlated with a poor prognosis and chemotherapeutic resistance [13]. Increased exposure to nitric oxide over time is itself correlated with enhanced cellular migration and chemotherapy resistance [14][15]. Nevertheless, a number of NO-donating compounds have been reported to have efficacy at inhibiting some of these effects [16][17][18]. As such, NO-donating drugs may raise the concentration of NO to cytotoxic levels, bypassing the physiological mechanisms underlying tumorigenesis and actually serve to inhibit cancer growth.
2. Breast Cancer
Amongst all non-skin cancer sites in females, breast cancer has the highest incidence and second-highest mortality, although the 5-year survival rate is over 90% [19]. Similar to NSCLC and other types of cancer, elevated levels of iNOS were noted from tissue samples of patients with breast cancer, which was significantly greater than normal breast tissue and benign breast diseases such as fibroadenomas [20]. NO was also shown to function through the same p53 and AKT pathways as those of lung cancer cells, suggesting common pathways for NO signaling to induce cancer growth and encourage progression [21]. Such pathways may work synergistically with other NO pathways in breast cancer, such as the epidermal growth factor receptor (EGFR)-mediated activation of ERK [22]. Furthermore, a number of studies suggest that the activity of NO pathways in breast cancer are concentration-dependent, which is backed by other NO studies. NO concentration of less than 100 nM activates cGMP-dependent pathways, while concentrations of 200–600 nM activate cGMP independent pathways [21]. Above these concentrations, NO has been suggested to phosphorylate p53 and halt the function of DNA repair enzymes [21]. cGMP dependent pathways were noted to be dysregulated within breast cancer, with lower concentration of cGMP being correlated to malignant disease, suggesting low physiological concentration of NO-mediated cGMP signaling may actually provide protection against breast cancer [23]. cGMP independent pathways have been discussed previously and are not unique to breast cancer. Nevertheless, NO-mediated HIF-1a stabilization, nitrosylation of metabolic enzymes, and induction of VEGF are major pathways of breast cancer growth and metastasis [21]. Additionally, NO-mediated activation of matrix metalloproteinases (MMP) and inhibition of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) may reorganize the tumor microenvironment to increase metastatic potential, while further stabilizing downstream cGMP-independent NO pathways [21]. Numerous studies have found that many of these NO-mediated effectors, such as VEGF and CXCR4, are correlated to lymph node metastasis and overall prognosis [24][25]. The growth arrest and p53 phosphorylation exhibited by supraphysiological concentrations of NO may explain the effect that NO-conjugated drugs have at inhibiting breast cancer growth and inducing apoptosis [26]. The underlying mechanisms behind how nitric oxide becomes upregulated in breast cancer remain unknown; however, similar to other cancer types, systemic effects from environmental, hereditary, and physiological processes all may play a role. For example, iNOS was noted to be upregulated in response to glucocorticoids, a stress hormone, suggesting multisystem processes may contribute to the pathogenesis of breast cancer [27]. Furthermore, cyclooxygenase-2 (COX-2) and NO are strongly linked in breast cancer, with one multivariate analysis suggesting that those with COX-2 and iNOS-positive tumors were associated with extremely poor prognosis [28]. Nevertheless, the exact role of NO in breast cancer remains controversial, especially in the context of estrogen- and progesterone-mediated signaling.
Estrogen and progesterone have also been shown to modulate nitric oxide expression, through the actions of eNOS and iNOS [29][30]. Estrogen is thought to downregulate the expression of iNOS and upregulate the expression of eNOS, which in turn activates phosphoinositide 3-kinase (PI3K) and AKT through direct activation and increased transcription, which have been shown to be critical for cancer proliferation [31]. Furthermore, upregulation of eNOS appears to be a unique feature of estrogen-dependent tumors, which are lacking in ER- breast cancers [32]. Interestingly, progesterone’s function within nitric oxide synthesis appears to be more controversial, with several studies suggesting that progesterone can affect eNOS production and others suggesting null or contradictory effects [31][33][34]. For example, one study suggested that progesterone-induced iNOS expression in vitro, which then induced cell apoptosis [35]. Additionally, progesterone has been proposed to affect and downregulate estrogen-mediated nitric oxide production [31]. These results suggest that breast cancer tumor progesterone status is a favorable prognostic factor, an effect that is backed by clinical studies [36]. Although it is currently unknown exactly how the surface marker human epidermal growth factor receptor 2 (HER2/neu) affects nitric oxide production, one group suggests that HER2/neu downregulates nitric oxide production, ablating the apoptotic effect of chemotherapeutics in vitro, potentially through a COX-2 mechanism [37][38]. Nevertheless, triple-negative breast cancer, the most aggressive form, appears to also be strongly correlated to iNOS [39]. Thus, nitric oxide expression appears to be central to the pathogenesis of breast cancer, regardless of receptor phenotype expression.
3. Prostate Cancer
Prostate cancer is highly prevalent in men and has an age-adjusted incidence of 453.8 per 100,000 and is highest amongst those 65–74 [40]. Similar to other cancers, iNOS expression was also significantly increased in prostate adenocarcinoma when compared to healthy prostate tissue [41]. Furthermore, previous work demonstrated that iNOS expression was greatest amongst those with metastasis and high Gleason scores, and one meta-analysis found that tumor iNOS expression may serve prognostic value [42][43]. These findings are backed by clinical studies, which suggest greater nitrosative stress in those with prostate cancer as compared to benign prostatic hyperplasia (BPH) and controls [44]. However, eNOS also appears to be linked to prostate cancer, as some but not all studies have shown that genetic polymorphisms of iNOS and eNOS carry an increased risk of high Gleason score prostate cancer [45][46][47]. eNOS may in turn upregulate pleiotrophin (PTN), expression through ERK activity, increasing tumor and endothelial migration, laying the groundwork for metastatic disease [48]. Additionally, the eNOS complex can interact with the estrogen receptor to transcriptionally alter other important gene products, such as Glutathione transferase P1, which are correlated to disease pathogenesis [49].
Prostate cancer is often initially androgen-sensitive, which is useful in androgen deprivation therapy, and is often a first-line therapy for advanced and metastatic disease [50]. This advanced form of prostate cancer is termed castration-resistant (CRPC) and is associated with poor outcomes [51]. Previous studies have implicated NO as a potential mediator of androgen resistance by androgen receptor transcriptional suppression and direct androgen receptor inhibition, which were mediated by iNOS and eNOS, respectively [52][53]. iNOS induction may encourage tumor growth, as two studies from the same lab found that NO promotes survival and accelerates tumor growth after oxidative stress [54][55]. However, testosterone, an androgen, also increases NO concentration and survival of prostate cancer cells, suggesting that NO’s mechanism in androgen sensitivity and resistance remains elusive [56][57][58][59]. These androgen-specific mechanisms may interact with previously defined effects of NO on hypoxia-induced HIF-1a and tumor angiogenesis, compounding the effect of nitric oxide on prostate cancer [60].
4. Gastrointestinal Cancers
When summed, digestive system cancers have the highest incidence amongst all organ systems with colorectal cancer having the third-highest incidence amongst all non-skin types, regardless of gender [19]. Interestingly, one analysis of gastrointestinal cancers suggested that most had adherent iNOS expression, although whether iNOS expression was upregulated or downregulated was dependent upon cancer type [61]. Similar to other cancers, gastrointestinal cancers may also use previously discussed NO signaling pathways such as PI3-K, p53, AKT, PTEN, NF-kB, MMPs, and HIF-1a in their carcinogenic pathogenesis [61]. Malignant transformation of colorectal and other cancers are often dependent upon the epithelial–mesenchymal transition (EMT) in which cancer cells express genes that are normally associated with connective tissue [62]. Although a number of cell signals can stimulate this pathway, one particularly important player is the APC/Wnt/β-catenin pathway, which is often dysregulated in colorectal cancer [63]. In normal healthy colonic tissue, APC downregulates B-catenin, which prevents polyp formation [63]. NO has also been shown to upregulate the Wnt/β-catenin pathways, potentially through negative feedback NF-κB response elements on a Dickkopf-1 gene promoter that reduces gene silencing [64]. iNOS has been previously suggested to influence the function of NF-κB, and these findings correlate to the increased iNOS expression, which is often noted in colorectal carcinomas. However, increased iNOS expression is not a ubiquitous finding across all studies [65]. In addition, APC and the wingless-related integration site (WNT)/B-catenin pathways also serve to regulate COX-2 in a similar manner to NO, suggesting that NO may work in synchrony with COX-2 to promote its pro-cancer effects [61][66]. COX-2 has been previously linked to many of the same pro-cancer effects as NO [67]. Nevertheless, the underlying mechanisms linking COX-2 to NO remain elusive, although both undergo NF-kB regulation [68]. Taken together, these findings suggest that regulation of NO and APC may occur simultaneously, and treatment strategies targeting these pathways may prevent WNT/B-catenin upregulation, preventing the development of cancer.
Similar to cigarette smoking, the processing of digested metabolites and chronic infectious agents may also lead to cancer through nitric oxide-mediated mechanisms. For example, one of the major risk factors for gastric cancer is dietary consumption of nitro-compounds, with a relative risk of 1.31 (95% confidence interval (CI), 1.13–1.52) for nitrites, and 1.34 (95% CI, 1.02–1.76) for NDMA, although nitrate consumption was associated with a lower risk of gastric cancer 0.80 (95% CI, 0.69–0.93) [69]. Dietary consumption of salivary nitrites exposes nitrites to stomach acid and ascorbic acid, which then produces nitric oxide, which can diffuse rapidly to surrounding tissue [70]. A similar increase in NO production was noted with chronic Helicobacte pylori infection, suggesting that enhanced nitric oxide exposure from environmental and infectious agents may be responsible for the development of cancer, especially when there is high or chronic levels of exposure to these agents [71]. Viruses were also not immune to NO, as Hepatitis B was shown to be inhibited by NO via INF-γ, and chronic infection with Hepatitis B may induce increased NO, which can predispose hepatocytes to mutagenesis, potentially through a c-jun, an n-terminal protein kinase (JNK) [72][73]. Furthermore, Hepatitis C was also shown to induce DNA damage through upregulation of iNOS and nitrosylation of DNA glycosylase [73][74]. The effects of chronic viral-induced upregulation of NO may be compounded by mutations to proteins regulating nitrosylation, like GSNOR, which can cause the buildup of formaldehyde, a well-known carcinogen [73], increasing the risk of liver cancer. Evidence also suggests that iNOS and NO may be dysregulated upon alcohol exposure, as iNOS and NO are upregulated in response to ingested toxins [73][75]. When combined with studies on NO, such results suggest that increased nitric oxide dysregulation in response to cigarettes and alcohol may be one mechanism for the increased cancer risk amongst patients who engage in these behaviors.
5. Other Cancers
NO dysregulation is linked to a wide range of malignancies, including those of the brain [76], genitourinary system [77][78], skin [79], thyroid [80], and head and neck [81][82] cancers. Due to the complexity of nitric oxide and its wide number of potential interactions, it is suggested that the multifactorial effects of NO are cancer-specific. Although NO may often work through a common pathway such as p53 to induce mutagenesis, the exact mechanisms often differ between cancer types. Furthermore, in cases of sporadic cancers with minimal risk factors and no underlying conditions, it is unknown exactly what causes the underlying upregulation of NO or if the elevated NO is in response to another effect. However, chronic unchecked NO signaling is clearly beneficial to tumors and can encourage progression and metastasis. Therefore, therapies that can control NO growth signals have great promise, although delivering such therapies to the tumor without affecting nearby or distant healthy cells remains a significant problem.
References
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311.
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020.
- Gào, X.; Xuan, Y.; Benner, A.; Anusruti, A.; Brenner, H.; Schöttker, B. Nitric Oxide Metabolites and Lung Cancer Incidence: A Matched Case-Control Study Nested in the ESTHER Cohort. Oxid. Med. Cell. Longev. 2019, 2019, 6470950.
- Masri, F. Role of nitric oxide and its metabolites as potential markers in lung cancer. Ann. Thorac. Med. 2010, 5, 123–127.
- Pignatelli, B.; Li, C.Q.; Boffetta, P.; Chen, Q.; Ahrens, W.; Nyberg, F.; Mukeria, A.; Bruske-Hohlfeld, I.; Fortes, C.; Constantinescu, V.; et al. Nitrated and oxidized plasma proteins in smokers and lung cancer patients. Cancer Res. 2001, 61, 778–784.
- Liu, C.Y.; Wang, C.H.; Chen, T.C.; Lin, H.C.; Yu, C.T.; Kuo, H.P. Increased level of exhaled nitric oxide and up-regulation of inducible nitric oxide synthase in patients with primary lung cancer. Br. J. Cancer 1998, 78, 534–541.
- Liu, P.-F.; Zhao, D.-H.; Qi, Y.; Wang, J.-G.; Zhao, M.; Xiao, K.; Xie, L.-X. The clinical value of exhaled nitric oxide in patients with lung cancer. Clin. Respir. J. 2018, 12, 23–30.
- Hsieh, Y.S.; Wang, H.C.; Tseng, T.H.; Chang, W.C.; Wang, C.J. Gaseous nitric oxide-induced 8-nitroguanine formation in human lung fibroblast cells and cell-free DNA. Toxicol. Appl. Pharmacol. 2001, 172, 210–216.
- Masri, F.A.; Comhair, S.A.A.; Koeck, T.; Xu, W.; Janocha, A.; Ghosh, S.; Dweik, R.A.; Golish, J.; Kinter, M.; Stuehr, D.J.; et al. Abnormalities in nitric oxide and its derivatives in lung cancer. Am. J. Respir. Crit. Care Med. 2005, 172, 597–605.
- Yongsanguanchai, N.; Pongrakhananon, V.; Mutirangura, A.; Rojanasakul, Y.; Chanvorachote, P. Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am. J. Physiol. Cell Physiol. 2015, 308, C89–C100.
- Fujimoto, H.; Sasaki, J.; Matsumoto, M.; Suga, M.; Ando, Y.; Iggo, R.; Tada, M.; Saya, H.; Ando, M. Significant correlation of nitric oxide synthase activity and p53 gene mutation in stage I lung adenocarcinoma. Jpn. J. Cancer Res. 1998, 89, 696–702.
- Luanpitpong, S.; Chanvorachote, P. Nitric Oxide and Aggressive Behavior of Lung Cancer Cells. Anticancer Res. 2015, 35, 4585–4592.
- Saisongkorh, V.; Maiuthed, A.; Chanvorachote, P. Nitric oxide increases the migratory activity of non-small cell lung cancer cells via AKT-mediated integrin αv and β1 upregulation. Cell. Oncol. 2016, 39, 449–462.
- Wongvaranon, P.; Pongrakhananon, V.; Chunhacha, P.; Chanvorachote, P. Acquired resistance to chemotherapy in lung cancer cells mediated by prolonged nitric oxide exposure. Anticancer Res. 2013, 33, 5433–5444.
- Sanuphan, A.; Chunhacha, P.; Pongrakhananon, V.; Chanvorachote, P. Long-term nitric oxide exposure enhances lung cancer cell migration. Biomed. Res. Int. 2013, 2013, 186972.
- Maciag, A.E.; Chakrapani, H.; Saavedra, J.E.; Morris, N.L.; Holland, R.J.; Kosak, K.M.; Shami, P.J.; Anderson, L.M.; Keefer, L.K. The Nitric Oxide Prodrug JS-K Is Effective against Non–Small-Cell Lung Cancer Cells In Vitro and In Vivo: Involvement of Reactive Oxygen Species. J. Pharmacol. Exp. Ther. 2011, 336, 313–320.
- Song, J.M.; Upadhyaya, P.; Kassie, F. Nitric oxide-donating aspirin (NO-Aspirin) suppresses lung tumorigenesis in vitro and in vivo and these effects are associated with modulation of the EGFR signaling pathway. Carcinogenesis 2018, 39, 911–920.
- Ye, S.; Yang, W.; Wang, Y.; Ou, W.; Ma, Q.; Yu, C.; Ren, J.; Zhong, G.; Shi, H.; Yuan, Z.; et al. Cationic liposome-mediated nitric oxide synthase gene therapy enhances the antitumor effects of cisplatin in lung cancer. Int. J. Mol. Med. 2013, 31, 33–42.
- Hare, J.M.; Nguyen, G.C.; Massaro, A.F.; Drazen, J.M.; Stevenson, L.W.; Colucci, W.S.; Fang, J.C.; Johnson, W.; Givertz, M.M.; Lucas, C. Exhaled nitric oxide: A marker of pulmonary hemodynamics in heart failure. J. Am. Coll. Cardiol. 2002, 40.
- Bulut, A.S.; Erden, E.; Sak, S.D.; Doruk, H.; Kursun, N.; Dincol, D. Significance of inducible nitric oxide synthase expression in benign and malignant breast epithelium: An immunohistochemical study of 151 cases. Virchows Arch. 2005, 447, 24–30.
- Basudhar, D.; Somasundaram, V.; de Oliveira, G.A.; Kesarwala, A.; Heinecke, J.L.; Cheng, R.Y.; Glynn, S.A.; Ambs, S.; Wink, D.A.; Ridnour, L.A. Nitric Oxide Synthase-2-Derived Nitric Oxide Drives Multiple Pathways of Breast Cancer Progression. Antioxid. Redox Signal. 2017, 26, 1044–1058.
- Garrido, P.; Shalaby, A.; Walsh, E.M.; Keane, N.; Webber, M.; Keane, M.M.; Sullivan, F.J.; Kerin, M.J.; Callagy, G.; Ryan, A.E.; et al. Impact of inducible nitric oxide synthase (iNOS) expression on triple negative breast cancer outcome and activation of EGFR and ERK signaling pathways. Oncotarget 2017, 8, 80568–80588.
- Windham, P.F.; Tinsley, H.N. cGMP signaling as a target for the prevention and treatment of breast cancer. Semin. Cancer Biol. 2015, 31, 106–110.
- Yasuoka, H.; Tsujimoto, M.; Yoshidome, K.; Nakahara, M.; Kodama, R.; Sanke, T.; Nakamura, Y. Cytoplasmic CXCR4 expression in breast cancer: Induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC Cancer 2008, 8, 340.
- Nakamura, Y.; Yasuoka, H.; Tsujimoto, M.; Yoshidome, K.; Nakahara, M.; Nakao, K.; Nakamura, M.; Kakudo, K. Nitric oxide in breast cancer: Induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin. Cancer Res. 2006, 12, 1201–1207.
- Nath, N.; Chattopadhyay, M.; Rodes, D.B.; Nazarenko, A.; Kodela, R.; Kashfi, K. Nitric Oxide-Releasing Aspirin Suppresses NF-κB Signaling in Estrogen Receptor Negative Breast Cancer Cells in Vitro and in Vivo. Molecules 2015, 20, 12481–12499.
- Flaherty, R.L.; Intabli, H.; Falcinelli, M.; Bucca, G.; Hesketh, A.; Patel, B.A.; Allen, M.C.; Smith, C.P.; Flint, M.S. Stress hormone-mediated acceleration of breast cancer metastasis is halted by inhibition of nitric oxide synthase. Cancer Lett. 2019, 459, 59–71.
- Davila-Gonzalez, D.; Chang, J.C.; Billiar, T.R. NO and COX2: Dual targeting for aggressive cancers. Proc. Natl. Acad. Sci. USA 2017, 114, 13591–13593.
- Duckles, S.P.; Miller, V.M. Hormonal modulation of endothelial NO production. Pflug. Arch. 2010, 459, 841–851.
- Yallampalli, C.; Dong, Y.L. Estradiol-17beta inhibits nitric oxide synthase (NOS)-II and stimulates NOS-III gene expression in the rat uterus. Biol. Reprod. 2000, 63, 34–41.
- Pance, A. Nitric oxide and hormones in breast cancer: Allies or enemies? Future Oncol. 2006, 2, 275–288.
- Martin, J.H.J.; Alalami, O.; van den Berg, H.W. Reduced expression of endothelial and inducible nitric oxide synthase in a human breast cancer cell line which has acquired estrogen independence. Cancer Lett. 1999, 144, 65–74.
- Vakkala, M.; Paakko, P.; Soini, Y. eNOS expression is associated with the estrogen and progesterone receptor status in invasive breast carcinoma. Int. J. Oncol. 2000, 17, 667–671.
- Simoncini, T.; Fu, X.-D.; Caruso, A.; Garibaldi, S.; Baldacci, C.; Giretti, M.S.; Mannella, P.; Flamini, M.I.; Sanchez, A.M.; Genazzani, A.R. Drospirenone increases endothelial nitric oxide synthesis via a combined action on progesterone and mineralocorticoid receptors. Hum. Reprod. 2007, 22, 2325–2334.
- Bentrari, F.; Arnould, L.; Jackson, A.P.; Jeannin, J.-F.; Pance, A. Progesterone enhances cytokine-stimulated nitric oxide synthase II expression and cell death in human breast cancer cells. Lab. Investig. 2005, 85, 624–632.
- Bardou, V.-J.; Arpino, G.; Elledge, R.M.; Osborne, C.K.; Clark, G.M. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003, 21, 1973–1979.
- Simeone, A.-M.; Broemeling, L.D.; Rosenblum, J.; Tari, A.M. HER2/neu reduces the apoptotic effects of N-(4-hydroxyphenyl)retinamide (4-HPR) in breast cancer cells by decreasing nitric oxide production. Oncogene 2003, 22, 6739–6747.
- Simeone, A.-M.; Li, Y.-J.; Broemeling, L.D.; Johnson, M.M.; Tuna, M.; Tari, A.M. Cyclooxygenase-2 is essential for HER2/neu to suppress N- (4-hydroxyphenyl)retinamide apoptotic effects in breast cancer cells. Cancer Res. 2004, 64, 1224–1228.
- Granados-Principal, S.; Liu, Y.; Guevara, M.L.; Blanco, E.; Choi, D.S.; Qian, W.; Patel, T.; Rodriguez, A.A.; Cusimano, J.; Weiss, H.L.; et al. Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 2015, 17, 1169.
- Li, J.; Siegel, D.A.; King, J.B. Stage-specific incidence rates and trends of prostate cancer by age, race, and ethnicity, United States, 2004-2014. Ann. Epidemiol. 2018, 28, 328–330.
- Uotila, P.; Valve, E.; Martikainen, P.; Nevalainen, M.; Nurmi, M.; Härkönen, P. Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol. Res. 2001, 29, 23–28.
- Aaltoma, S.H.; Lipponen, P.K.; Kosma, V.M. Inducible nitric oxide synthase (iNOS) expression and its prognostic value in prostate cancer. Anticancer Res. 2001, 21, 3101–3106.
- Liao, W.; Ye, T.; Liu, H. Prognostic Value of Inducible Nitric Oxide Synthase (iNOS) in Human Cancer: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2019, 2019, 6304851.
- Arsova-Sarafinovska, Z.; Eken, A.; Matevska, N.; Erdem, O.; Sayal, A.; Savaser, A.; Banev, S.; Petrovski, D.; Dzikova, S.; Georgiev, V.; et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin. Biochem. 2009, 42, 1228–1235.
- Lee, K.-M.; Kang, D.; Park, S.K.; Berndt, S.I.; Reding, D.; Chatterjee, N.; Chanock, S.; Huang, W.-Y.; Hayes, R.B. Nitric oxide synthase gene polymorphisms and prostate cancer risk. Carcinogenesis 2009, 30, 621–625.
- Dianat, S.S.; Margreiter, M.; Eckersberger, E.; Finkelstein, J.; Kuehas, F.; Herwig, R.; Ayati, M.; Lepor, H.; Djavan, B. Gene polymorphisms and prostate cancer: The evidence. BJU Int. 2009, 104, 1560–1572.
- Jacobs, E.J.; Hsing, A.W.; Bain, E.B.; Stevens, V.L.; Wang, Y.; Chen, J.; Chanock, S.J.; Zheng, S.L.; Xu, J.; Thun, M.J.; et al. Polymorphisms in angiogenesis-related genes and prostate cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 972–977.
- Polytarchou, C.; Hatziapostolou, M.; Poimenidi, E.; Mikelis, C.; Papadopoulou, A.; Parthymou, A.; Papadimitriou, E. Nitric oxide stimulates migration of human endothelial and prostate cancer cells through up-regulation of pleiotrophin expression and its receptor protein tyrosine phosphatase beta/zeta. Int. J. Cancer 2009, 124, 1785–1793.
- Re, A.; Aiello, A.; Nanni, S.; Grasselli, A.; Benvenuti, V.; Pantisano, V.; Strigari, L.; Colussi, C.; Ciccone, S.; Mazzetti, A.P.; et al. Silencing of GSTP1, a prostate cancer prognostic gene, by the estrogen receptor-β and endothelial nitric oxide synthase complex. Mol. Endocrinol. 2011, 25, 2003–2016.
- Sharifi, N. Androgen Deprivation Therapy for Prostate Cancer. JAMA 2005, 294, 238.
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192.
- Cronauer, M.V.; Ince, Y.; Engers, R.; Rinnab, L.; Weidemann, W.; Suschek, C.V.; Burchardt, M.; Kleinert, H.; Wiedenmann, J.; Sies, H.; et al. Nitric oxide-mediated inhibition of androgen receptor activity: Possible implications for prostate cancer progression. Oncogene 2007, 26, 1875–1884.
- Yu, S.; Jia, L.; Zhang, Y.; Wu, D.; Xu, Z.; Ng, C.-F.; To, K.K.W.; Huang, Y.; Chan, F.L. Increased expression of activated endothelial nitric oxide synthase contributes to antiandrogen resistance in prostate cancer cells by suppressing androgen receptor transactivation. Cancer Lett. 2013, 328, 83–94.
- Bhowmick, R.; Girotti, A.W. Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photodynamic therapy model. Cancer Lett. 2014, 343, 115–122.
- Fahey, J.M.; Girotti, A.W. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: Role of nitric oxide. Nitric Oxide 2015, 49, 47–55.
- Soni, Y.; Softness, K.; Arora, H.; Ramasamy, R. The Yin Yang Role of Nitric Oxide in Prostate Cancer. Am. J. Mens. Health 2020, 14, 1557988320903191.
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512.
- Thomas, L.N.; Morehouse, T.J.; Too, C.K.L. Testosterone and prolactin increase carboxypeptidase-D and nitric oxide levels to promote survival of prostate cancer cells. Prostate 2012, 72, 450–460.
- Arora, H.; Panara, K.; Kuchakulla, M.; Kulandavelu, S.; Burnstein, K.L.; Schally, A.V.; Hare, J.M.; Ramasamy, R. Alterations of tumor microenvironment by nitric oxide impedes castration-resistant prostate cancer growth. Proc. Natl. Acad. Sci. USA 2018, 115, 11298–11303.
- Stewart, G.D.; Ross, J.A.; McLaren, D.B.; Parker, C.C.; Habib, F.K.; Riddick, A.C.P. The relevance of a hypoxic tumour microenvironment in prostate cancer. BJU Int. 2010, 105, 8–13.
- De Oliveira, G.A.; Cheng, R.Y.S.; Ridnour, L.A.; Basudhar, D.; Somasundaram, V.; McVicar, D.W.; Monteiro, H.P.; Wink, D.A. Inducible Nitric Oxide Synthase in the Carcinogenesis of Gastrointestinal Cancers. Antioxid. Redox Signal. 2017, 26, 1059–1077.
- Monteiro, H.P.; Rodrigues, E.G.; Reis, A.K.C.A.; Longo, L.S., Jr.; Ogata, F.T.; Moretti, A.I.S.; da Costa, P.E.; Teodoro, A.C.S.; Toledo, M.S.; Stern, A. Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: A redox signaling perspective. Nitric Oxide 2019, 89, 1–13.
- Bienz, M.; Clevers, H. Linking Colorectal Cancer to Wnt Signaling. Cell 2000, 103, 311–320.
- Du, Q.; Zhang, X.; Liu, Q.; Zhang, X.; Bartels, C.E.; Geller, D.A. Nitric oxide production upregulates Wnt/β-catenin signaling by inhibiting Dickkopf-1. Cancer Res. 2013, 73, 6526–6537.
- Ambs, S.; Merriam, W.G.; Bennett, W.P.; Felley-Bosco, E.; Ogunfusika, M.O.; Oser, S.M.; Klein, S.; Shields, P.G.; Billiar, T.R.; Harris, C.C. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998, 58, 334–341.
- Araki, Y.; Okamura, S.; Hussain, S.P.; Nagashima, M.; He, P.; Shiseki, M.; Miura, K.; Harris, C.C. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003, 63, 728–734.
- Goradel, N.H.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699.
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11.
- Song, P.; Wu, L.; Guan, W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients 2015, 7, 9872–9895.
- Iijima, K.; Grant, J.; McElroy, K.; Fyfe, V.; Preston, T.; McColl, K.E.L. Novel mechanism of nitrosative stress from dietary nitrate with relevance to gastro-oesophageal junction cancers. Carcinogenesis 2003, 24, 1951–1960.
- Rieder, G.; Hofmann, J.A.; Hatz, R.A.; Stolte, M.; Enders, G.A. Up-regulation of inducible nitric oxide synthase in Helicobacter pylori-associated gastritis may represent an increased risk factor to develop gastric carcinoma of the intestinal type. Int. J. Med. Microbiol. 2003, 293, 403–412.
- Guidotti, L.G.; McClary, H.; Loudis, J.M.; Chisari, F.V. Nitric oxide inhibits hepatitis B virus replication in the livers of transgenic mice. J. Exp. Med. 2000, 191, 1247–1252.
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536.
- Jaiswal, M.; LaRusso, N.F.; Shapiro, R.A.; Billiar, T.R.; Gores, G.J. Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology 2001, 120, 190–199.
- Toh, Y.; Oki, E.; Ohgaki, K.; Sakamoto, Y.; Ito, S.; Egashira, A.; Saeki, H.; Kakeji, Y.; Morita, M.; Sakaguchi, Y.; et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: Molecular mechanisms of carcinogenesis. Int. J. Clin. Oncol. 2010, 15, 135–144.
- Altieri, R.; Fontanella, M.; Agnoletti, A.; Panciani, P.P.; Spena, G.; Crobeddu, E.; Pilloni, G.; Tardivo, V.; Lanotte, M.; Zenga, F.; et al. Role of Nitric Oxide in Glioblastoma Therapy: Another Step to Resolve the Terrible Puzzle? Transl. Med. UniSa 2015, 12, 54–59.
- Thomsen, L.L.; Lawton, F.G.; Knowles, R.G.; Beesley, J.E.; Riveros-Moreno, V.; Moncada, S. Nitric oxide synthase activity in human gynecological cancer. Cancer Res. 1994, 54, 1352–1354.
- Seabra, A.B.; Durán, N. Nitric oxide donors for prostate and bladder cancers: Current state and challenges. Eur. J. Pharmacol. 2018, 826, 158–168.
- Yarlagadda, K.; Hassani, J.; Foote, I.P.; Markowitz, J. The role of nitric oxide in melanoma. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 500–509.
- Choe, W.; Kim, S.; Hwang, T.S.; Lee, S.S. Expression of inducible nitric oxide synthase in thyroid neoplasms: Immunohistochemical and molecular analysis. Pathol. Int. 2003, 53, 434–439.
- Gallo, O.; Masini, E.; Morbidelli, L.; Franchi, A.; Fini-Storchi, I.; Vergari, W.A.; Ziche, M. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl. Cancer Inst. 1998, 90, 587–596.
- Utispan, K.; Koontongkaew, S. High Nitric Oxide Adaptation in Isogenic Primary and Metastatic Head and Neck Cancer Cells. Anticancer Res. 2020, 40, 2657–2665.
More
Information
Subjects:
Endocrinology & Metabolism
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Nitric Oxide: Physiology, Pharmacology, and Therapeutic Applications
Revisions:
2 times
(View History)
Update Date:
16 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




