Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David Tarazona | -- | 1505 | 2023-01-12 22:24:10 | | | |
| 2 | Camila Xu | + 2 word(s) | 1507 | 2023-01-13 01:30:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sodhi, P.V.; Sidime, F.; Tarazona, D.D.; Valdivia, F.; Levano, K.S. ACE2 Signaling Pathway and Processing during COVID-19 Infection. Encyclopedia. Available online: https://encyclopedia.pub/entry/40135 (accessed on 28 February 2026).
Sodhi PV, Sidime F, Tarazona DD, Valdivia F, Levano KS. ACE2 Signaling Pathway and Processing during COVID-19 Infection. Encyclopedia. Available at: https://encyclopedia.pub/entry/40135. Accessed February 28, 2026.
Sodhi, Pia V., Francoise Sidime, David D. Tarazona, Faviola Valdivia, Kelly S. Levano. "ACE2 Signaling Pathway and Processing during COVID-19 Infection" Encyclopedia, https://encyclopedia.pub/entry/40135 (accessed February 28, 2026).
Sodhi, P.V., Sidime, F., Tarazona, D.D., Valdivia, F., & Levano, K.S. (2023, January 12). ACE2 Signaling Pathway and Processing during COVID-19 Infection. In Encyclopedia. https://encyclopedia.pub/entry/40135
Sodhi, Pia V., et al. "ACE2 Signaling Pathway and Processing during COVID-19 Infection." Encyclopedia. Web. 12 January, 2023.
Copy Citation
ACE2 (angiotensin-converting enzyme) can be described as an enzyme, a transporter, and through its role as a receptor.
COVID-19
ACE2
RAAS
1. ACE2 Protein
1.1. ACE2 and RAAS
ACE2 can be described as an enzyme, a transporter, and through its role as a receptor. As an enzyme, it is an important regulator of the RAAS, a major regulator system of human physiology, controlling blood pressure, volume, and electrolytes, thus affecting the heart, vasculature, and kidney, mainly through the actions of the octapeptide hormone angiotensin II (Ang II) [1][2] (Figure 1). As expected, ACE2 is highly expressed in endothelial cells from the heart, kidney, upper airways, lungs, gut, liver, and testis [3]. Specifically, ACE2, as a zinc metallopeptidase with carboxypeptidase activity, hydrolyzes Ang II. Ang II is a vasoconstrictor that promotes inflammation and increases oxidative stress and apoptosis through the AT1 (angiotensin II type 1) receptors. ACE2, as a regulator, prevents its accumulation and thus minimizes its effect [3][4][5] (Figure 2a). When there is a reduced protein expression of ACE2 and, consequently, a buildup of Ang II, an increase in hypertension is observed, as shown in several animal models [3][6]. Additionally, ACE2 also plays an important role in glucose homeostasis. It has been shown that ACE2 overexpression in diabetic mice improves islet function and glycemic control [7][8].
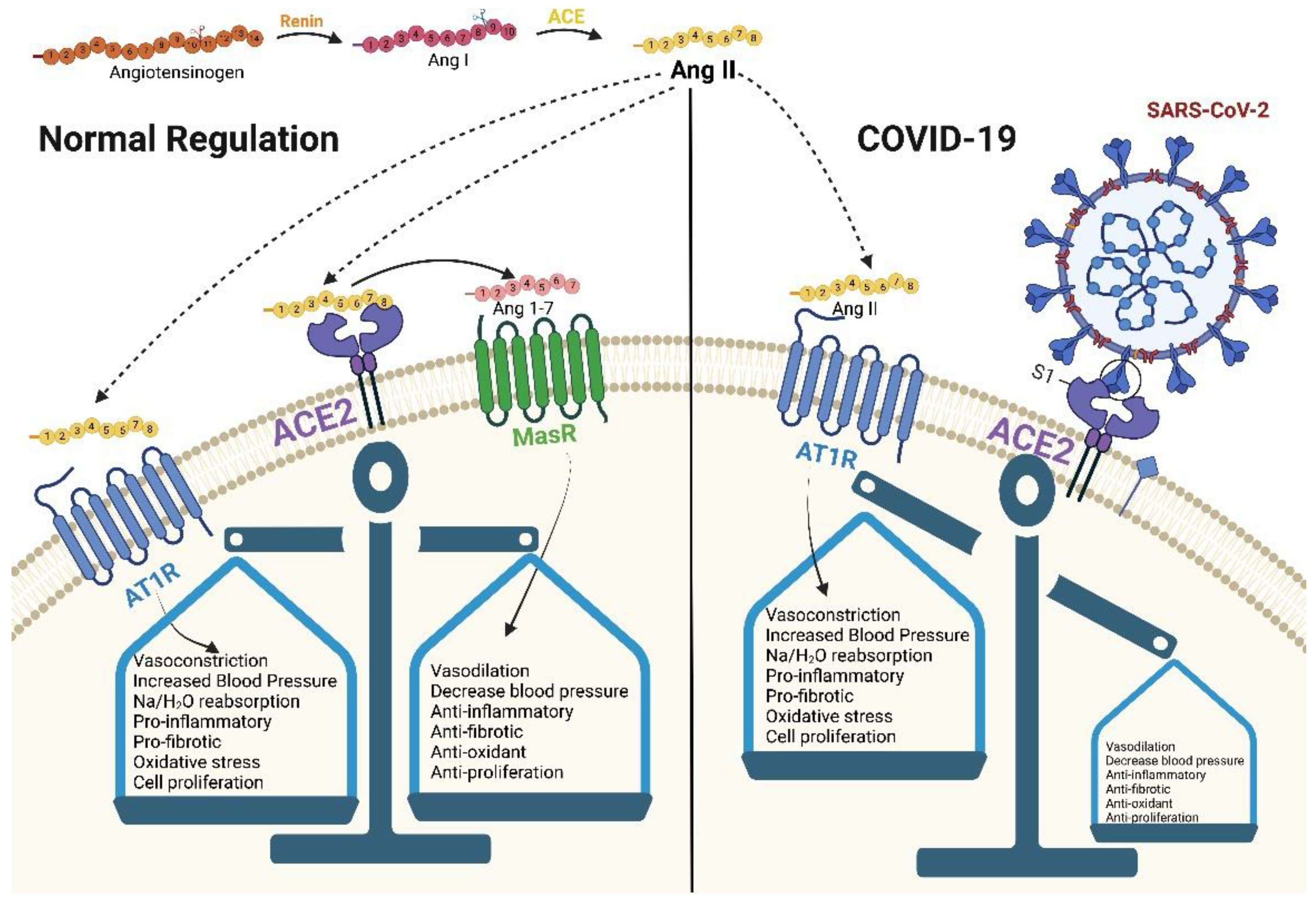
Figure 1. SARS-CoV-2 blocks ACE2 causing RAAS dysregulation. Created with BioRender.com.
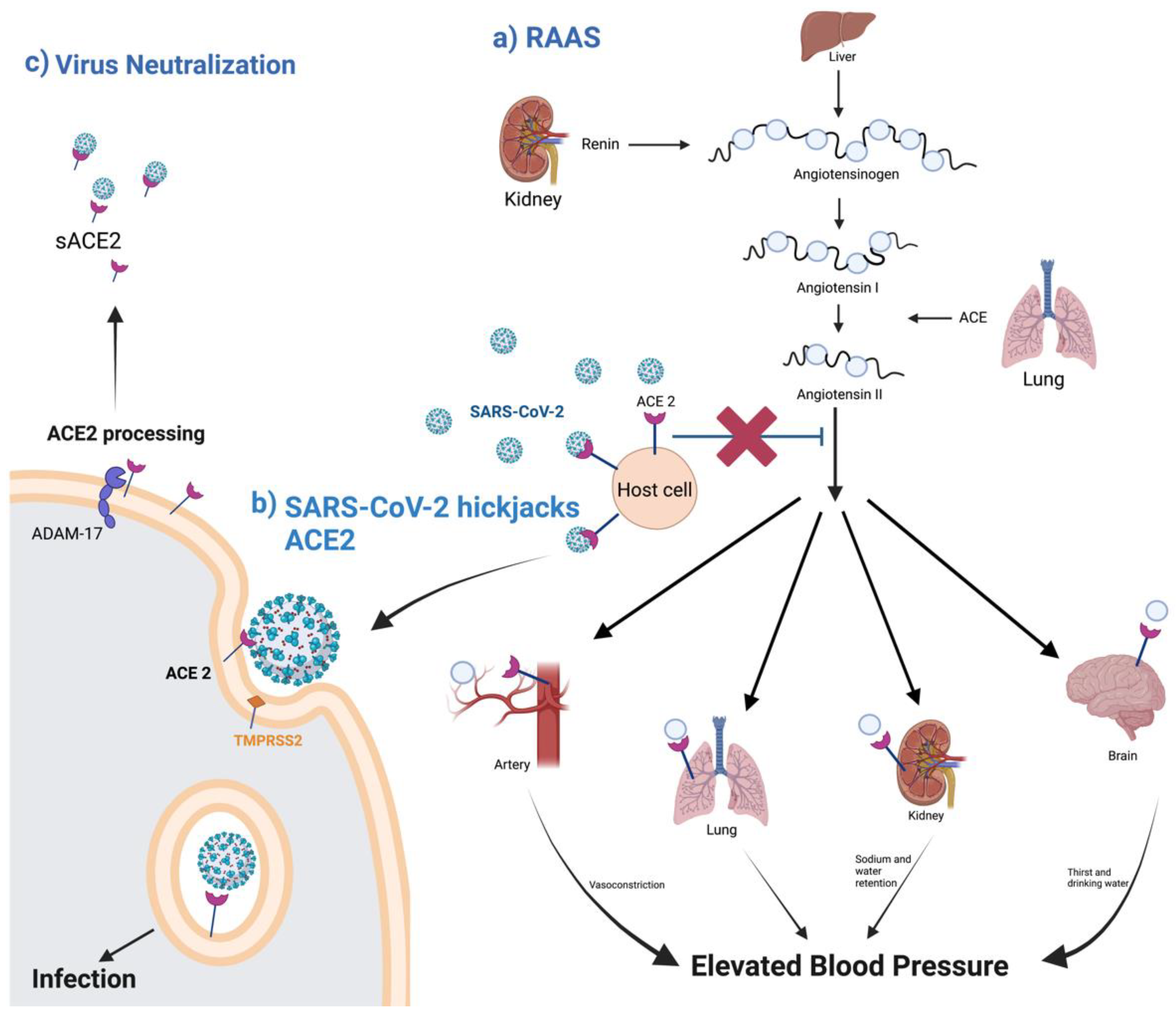
Figure 2. A schematic diagram of ACE2 pathways. (a) the regulation of the renin–angiotensin system. (b) ACE2 as SARS-CoV-2 receptor, leading to the internalization and infection of the virus. (c) ACE2 processing by ADAM-17, leading to the release of sACE2 and the possible blockage of SARS-CoV-2 infection. Created with BioRender.com.
1.2. ACE2 Processing during SARS-CoV-2 Infection
ACE2 acts as the receptor for SARS-CoV and SARS-CoV-2. The binding of these viruses to the membrane-bound form of the ACE2 receptor is necessary for virus internalization [9]. The reason why COVID-19 has had a much bigger global impact than SARS is due to biding affinity. In SARS-CoV-2, the region that interacts with the metallopeptidase domain of ACE2 is the receptor-binding domain (RBD) in the S protein. This binding causes structural changes in the S protein, exposing the cleavage sites at the S1/S2 or adjacent regions, which are attacked by host cellular proteases [10]. The RBD of SARS-CoV-2 has a stronger binding affinity with the ACE2 receptor due to the five out of six changes of vital amino acids, enhancing the connection with stronger hydrophobic interactions, despite the close linkage of the viruses [3]. These five variations are in the amino acids Leu455, Phe486, Gln493, Ser494, and Asn501 in SARS-CoV-2. Out of the five, positions Gln493 and Asn501 have been highlighted as the most critical amino acid residues important for van der Waals interactions and hydrogen bonding [11].
In addition to ACE2, SARS-CoV-2 requires further processing to enter the host cell. Transmembrane Serine Protease 2 (TMPRSS2), a serine endopeptidase primes the S protein. This entails the cleaving of the S protein at the subunit 1 and 2 sites, as well as at the S2 site [12]. This allows for a fusing of both the cellular and viral membranes. SARS-CoV-2 also uses another protease for S protein priming, the endosomal cysteine proteases cathepsin B and L (CatB/L). SARS-CoV requires TMPRSS2 processing for viral spread, while CATB/L activity can be dispensable. This is not the case for SARS-CoV-2, which requires both TMPRSS2 and CATB/L activity for viral entry [12]. There are also data that suggest ACE2 is shed from membranes with the help of TMPRSS2, which leads to membrane fusion and the cellular uptake of the virus [13][14] (Figure 2b).
1.3. ACE Isoforms
ACE2 is a type I transmembrane protein of 805 amino acids containing an ectodomain (enzyme) and a C-terminal transmembrane anchor [15]. The extracellular N-terminal domain contains a zinc metallopeptidase catalytic site and the spike protein binding site where SARS-CoV and SARS-CoV-2 bind (amino acids 1–740), a short transmembrane domain (amino acids 741–763), and a C-terminal domain facing the cytosol [16][17][18] (Figure 3). ACE2 has two functional forms, as a membrane bound receptor and as a soluble form (sACE2) with 555 amino acids [19][20]. The enzyme ADAM-17 (a disintegrin and metallopeptidase domain 17) or TACE (tumor necrosis factor-converting enzyme) is responsible for cleaving ACE2 at amino acids 716–741 [21][22]. ADAM-17, a type I transmembrane protein belonging to the family of zinc-dependent metalloproteases, catalyzes ACE2 ectodomain shedding, compromising the role of ACE2 in regulating the RAAS. sACE2 maintains carboxypeptidase activity and thus the ability to bind to the RBD region in the viral S protein. In a study by Haga et al., in 2008, both the cytoplasmic tails of ADAM-17 and of ACE2 were shown to be necessary for SARS-CoV to infect host cells; however, the underlying mechanism is still unknown [23][24]. During SARS-CoV-2 infection, the binding of the ACE2 receptor to the viral S1 protein promotes the cleavage of the ACE2 ectodomain by ADAM-17 and the intracellular C-terminal domain by TMPRSS2, thus facilitating SARS-CoV-2 entry [25] (Figure 2c). ACE2 is internalized with the viral particles into endosomes. Together, ACE2 processing and internalization reduce its expression in the membrane, affecting the regulation of Ang II and promoting RAAS imbalance and the activation of the AT1 receptors [5][26]. In addition, ADAM-17 expression is also increased by ACE2 and SARS-CoV-2 internalization, further increasing ACE2 ectodomain proteolytic cleavage and sACE2 production.
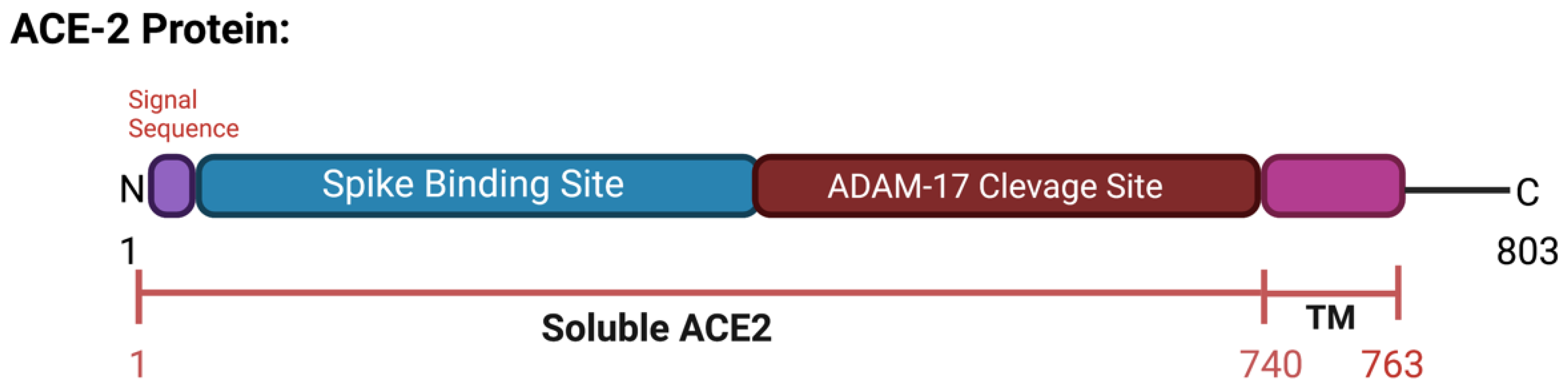
Figure 3. Schematic representation of the ACE2 protein. Includes spike binding site and ADAM-17 cleavage site and soluble ACE2. Created with BioRender.com.
sACE2 can be beneficial for preventing COVID-19. Many studies have reported its preventive role as a therapeutic agent due to its ability to bind to circulating SARS-CoV-2 and thus block viral entry [27][28][29] (Figure 2c). However, other researchers view sACE2 as another key to enter and infect non-ACE2-expressing cells. Yeung et al. showed that sACE2 facilitates SARS-CoV-2 entry through receptor-mediated endocytosis and thus enables its entrance in various tissues [30].
2. ACE2 Variants and COVID-19 Susceptibility
It is crucial to identify at risk individuals in specific populations. The various ACE2 variants differ in how they affect a given population’s susceptibility to SARS-CoV-2. Globally, in 2021 the WHO has reported a total of 178,837,204 million cases with fewer cases reported in Africa and Western Pacific [31]. ACE2 genetic variation, especially deleterious missense variants in ACE2 flexible regions (regions that change between an open and close state when bound to the virus), may affect its function and structure, and thus may alter its affinity towards SARS-CoV-2 [32]. The ACE2 gene is a highly polymorphic gene [26][33] containing 18 exons and 20 introns and is located in the chromosome Xp22 (different location from the ACE gene, which encodes for the ACE protein) [34]. ACE2 variants that are only present in specific populations are presented in Table 1. There are some variants, such as rs1244687367 (I21T), that have been shown to improve binding and hence susceptibility to the virus in all populations and regions [35]. From previously reported structural data, different research groups have predicted the effect of various ACE2 variants on ACE2–SARS-CoV-2 interaction and thus host susceptibility. Some of these predictions were further confirmed using biochemical assays [36].
Table 1. ACE2 variants: interindividual variability in different populations.
| dbSNP ID | Substitution | Description | References | |
|---|---|---|---|---|
| AFR | rs73635825 | S19P |
|
[37] |
| [38] | ||||
| AMR | rs781255386 | T27A |
|
[35][39] |
| rs924799658 | F40L |
|
[37] | |
| NFE | rs778030746 | I21V |
|
[35] |
| rs756231991 | D23K |
|
[35] | |
| ALL Al |
rs1244687367 | I21T |
|
[35] |
Some variants differ even within populations. In African and African American populations, the variant rs73635825 (S19P) has been shown to both enhance affinity for the S protein of the SARS-CoV-2 and in some provide a lower binding affinity for the spike protein due to levels of resistance. This variant is located at a crucial site where the virus S-protein interacts, at the beginning of the helix Ser19-Ile54, helping stabilize the helical structure through hydrogen bonding and hydrophilic interactions. Thus, the change from Serine to Proline (having poor helix-forming properties) could lead to either breaks or kins in the helix structure [36].
In American populations, there are two predominant variants, rs781255386 (T27A) and rs924799658 (F40L) that have been found to increase binding affinity and thus increasing susceptibility. With variant T27A, the change from Threonine to Alanine leads to an increased hydrophobic environment that could explain an increase in binding affinity due to this mutation [36].
In European non-Finnish populations, two variants, rs778030746 (I21V) and rs756231991 (D23K) have been associated with enhance binding and increase susceptibility. In contrast, two other variants in these same populations, rs1192192618 (Y50F) and rs1325542104 (M62V), have exhibited lower binding affinity to the SARS-CoV-2 spike protein. In South Asian populations, the variant rs760159085 (N51D) has been shown to have a lower affinity for the coronavirus. While many variants have been shown to affect specific populations, there are many whose effects are not yet known.
Although limited, ACE2 variants among different populations could partially begin to explain differences in COVID-19 susceptibility.
References
- Keidar, S.; Kaplan, M.; Gamliel-Lazarovich, A. ACE2 of the heart: From angiotensin I to angiotensin (1–7). Cardiovasc. Res. 2007, 73, 463–469.
- Chambers, S.; Bhatia, M. ACE and ACE2 in Inflammation: A Tale of Two Enzymes. Inflamm. Allergy-Drug Targets 2014, 13, 224–234.
- Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System. Circ. Res. 2020, 126, 1456–1474.
- Palau, V.; Pascual, J.; Soler, M.J.; Riera, M. Role of Adam17 in Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2019, 317, F333–F342.
- Stephany, B.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-Converting Enzyme 2 (Ace2) Expression and Tissue Susceptibility to SARS-CoV-2 Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919.
- Osman, I.O.; Melenotte, C.; Brouqui, P.; Million, M.; Lagier, J.-C.; Parola, P.; Stein, A.; La Scola, B.; Meddeb, L.; Mege, J.-L.; et al. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients. Front. Immunol. 2021, 12, 625732.
- Bindom, S.M.; Hans, C.P.; Xia, H.; Boulares, A.H.; Lazartigues, E. Angiotensin I–Converting En-zyme Type 2 (Ace2) Gene Therapy Improves Glycemic Control in Diabetic Mice. Diabetes 2010, 59, 2540–2548.
- Chhabra, K.H.; Chodavarapu, H.; Lazartigues, E. Angiotensin converting enzyme 2: A new important player in the regulation of glycemia. IUBMB Life 2013, 65, 731–738.
- Bosso, M.; Thanaraj, T.A.; Abu-Farha, M.; Alanbaei, M.; Abubaker, J.; Al-Mulla, F. The Two Faces of ACE2: The Role of ACE2 Receptor and Its Polymorphisms in Hypertension and COVID-19. Mol. Ther. Methods Clin. Dev. 2020, 18, 321–327.
- Jaehwan, Y.; Seok, J.H.; Joo, M.; Bae, J.-Y.; Kim, J.I.; Park, M.-S.; Kim, K. Multifactorial Traits of SARS-CoV-2 Cell Entry Related to Diverse Host Proteases and Proteins. Biomol. Ther. 2021, 29, 249–262.
- Hatmal, M.M.; Alshaer, W.; Al-Hatamleh, M.A.I.; Hatmal, M.; Smadi, O.; Taha, M.O.; Oweida, A.J.; Boer, J.C.; Mohamud, R.; Plebanski, M. Comprehensive Structural and Molecular Comparison of Spike Proteins of SARS-CoV-2, Sars-Cov and Mers-Cov, and Their Interactions with Ace2. Cells 2020, 9, 2638.
- Markus, H.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on Ace2 and Tmprss2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8.
- Patrick, B.; Refae, S.; Mograbi, B.; Hofman, P.; Milano, G. Host Polymorphisms May Impact SARS-CoV-2 Infectivity. Trends Genet. 2020, 36, 813–815.
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pohlmann, S. Tmprss2 and Adam17 Cleave Ace2 Differentially and Only Proteolysis by Tmprss2 Augments Entry Driven by the Severe Acute Respiratory Syndrome Corona-virus Spike Protein. J. Virol. 2014, 88, 1293–1307.
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A Human Homolog of Angiotensin-converting Enzyme. J. Biol. Chem. 2000, 275, 33238–33243.
- Turner, A.J.; Nalivaeva, N.N. Angiotensin-converting enzyme 2 (ACE2): Two decades of revelations and re-evaluation. Peptides 2022, 151, 170766.
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A Novel Angiotensin-Converting Enzyme–Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circ. Res. 2000, 87, e1–e9.
- García-Escobar, A.; Jiménez-Valero, S.; Galeote, G.; Jurado-Román, A.; García-Rodríguez, J.; Moreno, R. The soluble catalytic ectodomain of ACE2 a biomarker of cardiac remodelling: New insights for heart failure and COVID19. Hear. Fail. Rev. 2021, 26, 961–971.
- Bakhshandeh, B.; Sorboni, S.G.; Javanmard, A.-R.; Mottaghi, S.S.; Mehrabi, M.-R.; Sorouri, F.; Abbasi, A.; Jahanafrooz, Z. Variants in ACE2; potential influences on virus infection and COVID-19 severity. Infect. Genet. Evol. 2021, 90, 104773.
- Chen, F.; Zhang, Y.; Li, X.; Li, W.; Liu, X.; Xue, X. The Impact of Ace2 Polymorphisms on COVID-19 Disease: Susceptibility, Severity, and Therapy. Front. Cell Infect. Microbiol. 2021, 11, 753721.
- Daniel, W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor Necrosis Factor-A Convertase (Adam17) Mediates Regulated Ectodomain Shedding of the Se-vere-Acute Respiratory Syndrome-Coronavirus (Sars-Cov) Receptor, Angiotensin-Converting Enzyme-2 (Ace2). J. Biol. Chem. 2005, 280, 30113–30119.
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L84–L96.
- Monika, G. Adam-17: The Enzyme That Does It All. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169.
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of Tnf- -Converting Enzyme by the Spike Protein of Sars-Cov and Ace2 Induces Tnf- Production and Facilitates Viral Entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814.
- Lobelia, S.; Uhal, B.D. Ace2, Much More Than Just a Receptor for SARS-CoV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317.
- Zipeto, D.; Palmeira, J.D.F.; Arganaraz, G.A.; Arganaraz, E.R. Ace2/Adam17/Tmprss2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745.
- Rahman, M.M.; Hasan, M.; Ahmed, A. Potential detrimental role of soluble ACE2 in severe COVID-19 comorbid patients. Rev. Med. Virol. 2021, 31, 1–12.
- Chamsi-Pasha, M.A.R.; Shao, Z.; Tang, W.H.W. Angiotensin-Converting Enzyme 2 as a Therapeutic Target for Heart Failure. Curr. Hear. Fail. Rep. 2013, 11, 58–63.
- Shahnawaz, M.; Nasrin, N.; Alotaibi, F.O.; Prasad, G.; Singh, S.K.; Alam, I.; Mustafa, G. Treatment Options Available for COVID-19 and an Analysis on Possible Role of Combination of Rhace2, Angioten-sin (1-7) and Angiotensin (1-9) as Effective Therapeutic Measure. SN Compr. Clin. Med. 2020, 2, 1761–1766.
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chan, K.H.; Zhao, H.; Zhu, L.; et al. Soluble Ace2-Mediated Cell Entry of SARS-CoV-2 Via Interaction with Proteins Related to the Renin-Angiotensin System. Cell 2021, 184, 2212–2228 e12.
- WHO. Coronavirus Disease (COVID-19) Situations Reports; WHO: Geneva, Switzerland, 2021.
- Guo, X.; Chen, Z.; Xia, Y.; Lin, W.; Li, H. Investigation of the Genetic Variation in Ace2 on the Structural Recognition by the Novel Coronavirus (SARS-CoV-2). J. Transl. Med. 2020, 18, 321.
- Yi, L.; Liu, C.; Guan, T.; Li, Y.; Lai, Y.; Li, F.; Zhao, H.; Maimaiti, T.; Zeyaweiding, A. Association of Ace2 Genetic Polymorphisms with Hypertension-Related Target Organ Damages in South Xin-jiang. Hypertens. Res. 2019, 42, 681–689.
- Aneta, A.; Gagno, G.; Sinagra, G.; Beltrami, A.P.; Janjusevic, M.; Ippolito, G.; Zumla, A.; Fluca, A.L.; Ferro, F. Effects of SARS-CoV-2 on Cardiovascular System: The Dual Role of Angiotensin-Converting Enzyme 2 (Ace2) as the Virus Receptor and Homeostasis Regulator-Review. Int. J. Mol. Sci. 2021, 22, 4526.
- Antony, P.; Vijayan, R. Role of SARS-CoV-2 and Ace2 Variations in COVID-19. Biomed. J. 2021, 44, 235–244.
- Suryamohan, K.; Diwanji, D.; Stawiski, E.W.; Gupta, R.; Miersch, S.; Liu, J.; Chen, C.; Jiang, Y.P.; Fellouse, F.A.; Sathirapongsasuti, J.F.; et al. Human Ace2 Receptor Polymorphisms and Altered Susceptibility to SARS-CoV-2. Commun. Biol. 2021, 4, 475.
- Macgowan, S.A.; Barton, M.I.; Kutuzov, M.; Dushek, O.; van der Merwe, P.A.; Barton, G.J. Missense Variants in Human Ace2 Strongly Affect Binding to SARS-CoV-2 Spike Providing a Mechanism for Ace2 Mediated Genetic Risk in COVID-19: A Case Study in Affinity Predictions of Interface Variants. PLoS Comput. Biol. 2022, 18, e1009922.
- Mushtaq, H.; Jabeen, N.; Raza, F.; Shabbir, S.; Baig, A.A.; Amanullah, A.; Aziz, B. Structural Variations in Human Ace2 May Influence Its Binding with SARS-CoV-2 Spike Protein. J. Med. Virol. 2020, 92, 1580–1586.
- Teng, S.; Tang, Q. ACE2 enhance viral infection or viral infection aggravate the underlying diseases. Comput. Struct. Biotechnol. J. 2020, 18, 2100–2106.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
COVID-19
Revisions:
2 times
(View History)
Update Date:
13 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





