Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liborija Lugović-Mihić | -- | 2151 | 2023-01-12 11:16:27 | | | |
| 2 | Catherine Yang | Meta information modification | 2151 | 2023-01-12 11:43:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lugović-Mihić, L.; Špiljak, B.; Blagec, T.; Aždajić, M.D.; Franceschi, N.; Gašić, A.; Parać, E. Inflammation of the Lips (Cheilitis) and Perioral Skin. Encyclopedia. Available online: https://encyclopedia.pub/entry/40113 (accessed on 01 March 2026).
Lugović-Mihić L, Špiljak B, Blagec T, Aždajić MD, Franceschi N, Gašić A, et al. Inflammation of the Lips (Cheilitis) and Perioral Skin. Encyclopedia. Available at: https://encyclopedia.pub/entry/40113. Accessed March 01, 2026.
Lugović-Mihić, Liborija, Bruno Špiljak, Tadeja Blagec, Marija Delaš Aždajić, Nika Franceschi, Ana Gašić, Ena Parać. "Inflammation of the Lips (Cheilitis) and Perioral Skin" Encyclopedia, https://encyclopedia.pub/entry/40113 (accessed March 01, 2026).
Lugović-Mihić, L., Špiljak, B., Blagec, T., Aždajić, M.D., Franceschi, N., Gašić, A., & Parać, E. (2023, January 12). Inflammation of the Lips (Cheilitis) and Perioral Skin. In Encyclopedia. https://encyclopedia.pub/entry/40113
Lugović-Mihić, Liborija, et al. "Inflammation of the Lips (Cheilitis) and Perioral Skin." Encyclopedia. Web. 12 January, 2023.
Copy Citation
Lip inflammation may manifest as mainly reversible cheilitis, mainly irreversible, or cheilitis connected to dermatoses or systemic diseases. Therefore, knowing a patient’s medical history is important, especially whether their lip lesions are temporary, recurrent, or persistent. Sometimes temporary contributing factors, such as climate and weather conditions, can be identified and avoided—exposure to extreme weather conditions (e.g., dry, hot, or windy climates) may cause or trigger lip inflammation. Emotional and psychological stress are also mentioned in the etiology of some lip inflammations (e.g., exfoliative cheilitis) and may be associated with nervous habits such as lip licking.
lip inflammation
cheilitis
perioral dermatitis
comorbidities
atopic dermatitis
1. Psychiatric Diseases and Conditions and Common Behavioral Attitudes in Patients with Lip and Perioral Inflammation
The inflammation of the lips and perioral skin may be related to, or triggered by, some patients’ habits or psychological condition. Older literature data have already confirmed that licking of the lips is a crucial trigger for irritant contact cheilitis [1][2]. According to other literature data, among subjects with exfoliative cheilitis, mainly female, the main undesirable habit was lip licking (53%), and 40% of the patients had a history of a mental disorder [3].
Of other habits, one of the most unfavorable habits related to lip inflammation is frequent or prolonged sun exposure [4]. Actinic cheilitis is considered a premalignant lesion to carcinoma of the lip and has a high probability of developing into invasive squamous cell carcinoma (SCC) [4][5][6][7][8]. Almost all SCCs (95%) of the lower lip develop on pre-existing actinic cheilitis [6][7]. Other risk factors include fair skin, aging, occupation or daily activities which involve intense sun exposure (more than four hours daily), male gender, immunosuppression, latitude of residence, genetic predisposition, and others [4][9]. Actinic cheilitis often has asymptomatic lesions, but some patients report dryness, cracking, burning, stinging, pain, or even abnormal mobility of the lips [10]. In recent research, patients with actinic cheilitis reported that they were often or very often exposed to the sun, never or almost never use a hat or cap during sun exposure, and never/almost never use protective creams [11].
Aside from behavior, inflammation of the lips and perioral skin may also be associated with some psychiatric diseases and conditions [12]. For example, an association between exfoliative cheilitis and anxious conditions (le tic des lèvres) has been recorded in the research literature [12]. In addition, some researchers have singled out factitial cheilitis as a special type of cheilitis caused by habits patients are unaware of [12][13]. According to recent research on different types of cheilitis and psychiatric diseases associated with cheilitis, anxiety was more commonly recorded in patients with exfoliative cheilitis [11]. Previous research on the occurrence of oral lesions in patients with eating disorders (anorexia, bulimia, and nonspecific eating disorders) showed that oral lesions were observed in most of these patients (94%) [14]. Furthermore, the occurrence of exfoliative cheilitis caused by undesirable habits is associated with a higher incidence of stress and anxiety [15][16]. Other research on cheilitis patients showed that the largest percentage of them exhibiting high stress were those with exfoliative cheilitis [11]. In cheilitis simplex, low stress is somewhat more frequent in those who do not have recurrent lesions than in those who do. Moreover, mental stress and psychiatric disorders (e.g., personality disorders) can lead to undesirable habits and self-harm (biting, sucking, pulling, and licking) in factitial cheilitis, as in exfoliative cheilitis [17]. In addition, it is known that patients with atopic dermatitis (in which cheilitis is a typical manifestation) are prone to anxiety and depression [18][19].
In addition, patients with herpetic cheilitis often mention its association with stress. Recently, psychological stress levels were compared between patients with herpetic cheilitis and a healthy group, and significantly higher stress levels were seen in the patients with herpetic cheilitis [11]. In an older study on herpetic cheilitis, eighteen subjects completed a questionnaire on stressful events at two different times—during the latent phase of viral infection when they had no symptoms and in the active phase of disease. The results showed that, in the active phase of viral infection, patients experienced higher numbers of stressful events, as well as anxiety and daily falls, all of which differed significantly from the results recorded during the latent phase of the disease [20]. Despite these psychological factors related to lip inflammation, generally, psychiatrists and mental health professionals are rarely involved in a patient’s treatment for cheilitis, which could make a great difference in treatment efficacy. For example, one study showed that the use of an antidepressant, a selective serotonin reuptake inhibitor (fluoxetine), led to the improvement of lip inflammation [12].
2. Allergies Associated with Lip and Perioral Inflammation
Inflammation of the lips and perioral skin can be triggered by different allergens. Allergens associated with cheilitis are predominantly cosmetic and decorative products (e.g., lipstick, toothpaste, nail polish, and lip balm), dental materials, metals found in musical instruments, food, drugs, etc. [21][22][23][24][25]. Allergic contact cheilitis typically manifests with dryness, erythema, peeling, and fissures, most often on the lips, with occasional spreading to the perioral skin. Lip inflammation caused by food oftentimes spreads to the perioral skin [21][22]. Although contact allergic cheilitis is frequently caused by a reaction to balms and lipsticks, other items can also cause an allergic reaction (e.g., pencils or hairpins) [23]. Allergic contact cheilitis can occasionally leave residual lip hyperpigmentation that resolves over time [23][26].
Inflammation of the lips or surrounding skin may reflect gender-specific behavior—for instance, allergic contact cheilitis is more frequent among women because they use cosmetics more often, are more aware of changes in their appearance, and seek medical attention more often than men [27]. The prevalence of this type of cheilitis increases with age, given the more frequent use of hygienic and cosmetic products [25][28].
The prevalence of allergic reactions is generally more frequent in patients with cheilitis compared to patients with perioral diseases. Allergies to contact allergens more commonly manifest as cheilitis, while other allergy-related oral manifestations include gingivitis, stomatitis, perioral dermatitis, lichenoid reaction, etc. However, contact allergic reactions have been most frequently found in patients with cheilitis [26][29][30]. Previous studies in patients with oral and perioral manifestations have shown that 60% of patients with cheilitis had a positive patch test reaction, more often than in other conditions involving this area (angioedema, oral lichen planus, gingivostomatitis, and perioral dermatitis) [31]. A study of 121 dermatological patients also showed that contact allergies are relatively common in patients with cheilitis and perioral dermatitis (41.9% of the subjects had a positive patch test to allergens from the dental series), ranking them third, after orofacial granulomatosis and burning mouth syndrome [32].
Inflammation of the lips and perioral skin is relatively often caused by contact irritation and allergic reactions, especially to metals such as nickel, mercury, cobalt, chromium, impression materials, eugenol, and others. When a patient presents with lesions in this area, it is important to take a history of the patient’s use of these and other substances used in dentistry, as they can cause an allergic reaction [21][24]. In addition, allergies to cinnamon and benzoates are a potential predisposing factor for granulomatous cheilitis [22][25][33]. The prevalence of allergies to contact allergens ranges from 25.9% to 75% (according to previous studies, at least one positive patch test reaction was reported by Torgerson et al. in 25.9%, Budimir et al. in 26.7%, Khamaysi et al. in 41.9%, and Kim et al. in 75% of cases) [30][31][32][34]. Overall, the most common allergens confirmed by patch test were cobalt chloride, nickel sulfate, and mercury precipitate [30][31]. In the study conducted by Zoli et al., which performed patch tests with the standard series of allergens, nickel sulfate was the most common allergen, followed by thimerosal and cobalt chloride [28]. Other frequently mentioned contact allergens include balsam of Peru, fragrances, benzophenone, gold, and other allergens [35]. Cobalt chloride is widely used in the chemical and pharmaceutical industries (e.g., in dyes and vitamin products and as an additive to animal feed). It is important to note that cobalt hypersensitivity is often associated with nickel hypersensitivity, and patients should be informed of this [36]. Nickel is a metal found in drinking water, jewelry, fertilizer, food, crockery, paints, and cutlery, among other products. Thimerosal is used as a preservative in a variety of cosmetic and ophthalmic products as well as vaccines [25][36]. In older articles, reactions to gold have been reported in patients with oral diseases; however, this is not common nowadays.
Inflammation of the lips and perioral skin can also be caused by other, noncontact allergens [25]. According to research by Budimir et al., atopy-related reactions to inhalant allergens were found in 30% of patients with cheilitis, most commonly weed pollen, grass pollen, and dust [31]. An association between cheilitis and food allergies is also possible. According to literature data, 13.3% of patients with cheilitis showed evidence of a food allergy, most commonly to fruit, vegetables, and preservatives (e.g., glutaraldehyde, glutamate, and citric acid) [31]. Recent studies in patients with various oral and perioral skin diseases have shown that immediate-type allergic reactions (positive prick test) to food and additives were more frequently confirmed in patients with cheilitis (33.3%), whereas they were less frequent in patients with perioral dermatitis (10%) [37]. Patients with atopic dermatitis are also prone to lip inflammation (Figure 1). The percentage of atopic patients tested with allergy tests ranges from 19% to 34.9% in the research literature. In a study by Freeman et al., atopy was found in 19% of patients, while studies by Lim et al. and Zoli et al. showed that approximately one-third of subjects had atopy [1][2][28]. However, the number of atopic patients in studies depends on the populations tested and the allergen sets used in the tests. According to research by Blagec et al. on patients with lip inflammation, the percentage of atopic patients was even higher—84% (positive prick test result to at least one allergen) [25].
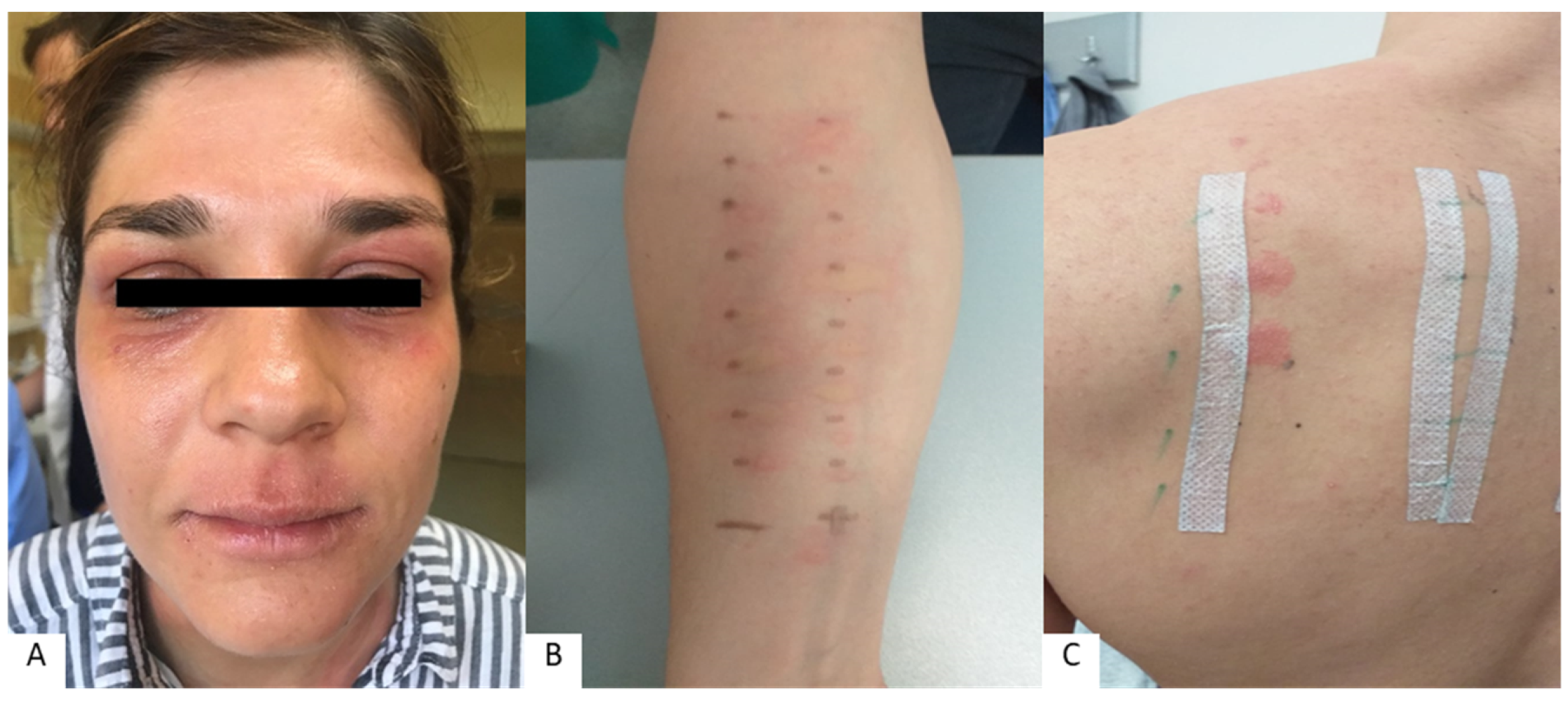
Figure 1. Cheilitis and periorificial lesions in patient with confirmed allergies (atopic dermatitis) (A), positive prick test to inhalant allergens (B), and positive patch test to contact allergens (C).
A patch test for contact allergens, performed with standard contact allergens, is essential in the assessment of contact cheilitis; however, possible allergens to personal products not included in the standard allergen series should also be considered [21]. In addition to patch tests, prick tests are also essential to confirm other mechanisms of IgE-mediated reaction (e.g., contact urticaria) and should be performed when indicated by the patient’s medical history [1][2][25][27][28][30][34][38]. Contact urticaria with lip manifestations is also possible on this part of the skin and can be triggered by food, preservatives, odors, and other factors (e.g., mint in toothpaste—with a positive prick test to mint leaves and negative patch test) [38]. Performing prick tests to assess and confirm food allergens is important for the diagnosis of so-called food cheilitis or lip manifestations in food allergic reactions [24]. In addition to patch tests, prick tests are sometimes needed to diagnose cheilitis, as they may be decisive in determining the cause of the lesions [1][2][25][27][28][30][34].
However, the clinical significance of positive allergy tests in patients remains to be established [25][31]. For this reason, patients are advised to avoid potential allergens in order to determine the relevance of the allergen to the disease itself [25].
3. Nutritional Deficiencies and Microbiome Changes in Patients with Lip and Perioral Inflammation
Inflammation of the lips and perioral skin can be related to various nutritional factors. Cheilitis can be linked to various vitamin and mineral deficiencies, such as iron (sideropenic anemia) [39][40][41]. Angular cheilitis can be caused by deficiencies of vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B6 (pyridoxine), vitamin B7 (biotin), vitamin B9 (folic acid), vitamin B12 (cyanocobalamin), and zinc, as well as protein deficiencies [21][23][39][40][41][42]. Additionally, studies have shown that angular cheilitis is a common clinical manifestation of sideropenic and megaloblastic anemia [41]. Exfoliative cheilitis can also be associated with iron deficiency (sideropenic anemia) and vitamin B12 deficiency [22].
Individuals with excess skin or wrinkles at the corner of the mouth may be prone to Candida overgrowth, leading to angular cheilitis [43]. Patients with Plummer–Vinson syndrome commonly present with angular cheilitis (along with anemia and dysphagia) mostly due to iron deficiency; therefore, iron supplementation is essential in the treatment of these patients [44]. A study analyzing children with vitamin B12 deficiency reported clinical features of cheilitis in 7.01% of patients. These patients were given cobalamin and saw complete resolution of lip inflammation [45]. A similar study analyzed dermatological signs of vitamin B12 deficiency in infants and found that 6.07% of participants presented with angular cheilitis. The most common sign of vitamin B12 deficiency on the skin, observed in almost all patients, was cutaneous hyperpigmentation [46]. Recent studies carried out in patients with cheilitis, analyzing levels of vitamin B9, vitamin B12, and iron, showed that only a small number of patients had abnormalities [11]. However, nutritional deficiencies should be considered in resistant cases. Abnormalities in zinc metabolism can cause disorders that usually manifest themselves on the skin, such as acrodermatitis enteropathica, an autosomal recessive genetic disorder of zinc deficiency. Acrodermatitis enteropathica presents with the triad of periorificial dermatitis, alopecia, and diarrhea. Oral zinc replacement therapy usually leads to a rapid clinical remission of this disorder [47]. In patients with perioral dermatitis and proven zinc deficiency, zinc supplementation can effectively improve the skin’s condition [48].
References
- Freeman, S.; Stephens, R. Cheilitis: Analysis of 75 cases referred to a contact dermatitis clinic. Am. J. Contact Dermat. 1999, 10, 198–200.
- Lim, S.W.; Goh, C.L. Epidemiology of eczematous cheilitis at a tertiary dermatological refferal centre in Singapore. Contact Dermat. 2000, 43, 322–326.
- Almazrooa, S.A.; Woo, S.B.; Mawardi, H.; Treister, N. Characterization and mangement of exfoliative cheilitis: A single-centre experience. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 485–489.
- Rodríguez-Blanco, I.; Flórez; Paredes-Suárez, C.; Rodríguez-Lojo, R.; González-Vilas, D.; Ramírez-Santos, A.; Paradela, S.; Conde, I.; Pereiro-Ferreirós, M. Actinic Cheilitis Prevalence and Risk Factors: A Cross-Sectional, Multicentre Study in a Population Aged 45 Years and over in North-West Spain. Acta Derm. Venereol. 2018, 98, 970–974.
- Gheno, J.N.; Martins, M.A.T.; Munerato, M.C.; Hugo, F.N.; Sant’ana Filho, M.; Weissheimer, C.; Carrard, V.C.; Martins, M.D. Oral Mucosal Lesions and Their Association with Sociodemographic, Behavioral, and Health Status Factors. Braz. Oral Res. 2015, 29, S1806-83242015000100289.
- Lopes, M.L.D.d.S.; da Silva Júnior, F.L.; Lima, K.C.; de Oliveira, P.T.; da Silveira, É.J.D. Clinicopathological Profile and Management of 161 Cases of Actinic Cheilitis. An. Bras. Dermatol. 2015, 90, 505–512.
- Bakirtzi, K.; Papadimitriou, I.; Andreadis, D.; Sotiriou, E. Treatment Options and Post-Treatment Malignant Transformation Rate of Actinic Cheilitis: A Systematic Review. Cancers 2021, 13, 3354.
- Vasilovici, A.; Ungureanu, L.; Grigore, L.; Cojocaru, E.; Şenilă, S. Actinic Cheilitis—From Risk Factors to Therapy. Front. Med. 2022, 9, 805425.
- De Lucena, I.M.; Santos, I.d.S.; Daroit, N.B.; Salgueiro, A.P.; Cavagni, J.; Haas, A.N.; Rados, P.V. Sun Protection as a Protective Factor for Actinic Cheilitis: Cross-Sectional Population-Based Study. Oral Dis. 2021, 28, 1802–1810.
- Lupu, M.; Caruntu, A.; Caruntu, C.; Boda, D.; Moraru, L.; Voiculescu, V.; Bastian, A. Non-Invasive Imaging of Actinic Cheilitis and Squamous Cell Carcinoma of the Lip. Mol. Clin. Oncol. 2018, 8, 640–646.
- Blagec, T.; Glavina, A.; Špiljak, B.; Bešlić, I.; Bulat, V.; Lugović-Mihić, L. Cheilitis: A Cross-Sectional Study—Multiple Factors Involved in the Aetiology and Clinical Features. Oral Dis. 2022. ahead of print.
- Nico, M.M.S.; Dwan, A.J.; Lourenço, S.V. Ointment pseudo-cheilitis: A disease distinct from factitial cheilitis. A series of 13 patients from São Paolo, Brazil. J. Cutan. Med. Surg. 2019, 23, 277–281.
- Brown, G.E.; Malakouti, M.; Sorenson, E.; Gupta, R.; Koo, J.Y. Psychodermatology. Adv. Psychosom. Med. 2015, 34, 123–134.
- Panico, R.; Piemonte, E.; Lazos, J.; Gilligan, G.; Zampini, A.; Lanfranchi, H. Oral mucosal lesions in anorexia nervosa, bulimia nervosa and EDNOS. J. Psychiatr. Res. 2018, 96, 178–182.
- Balighi, K.; Daneshpazhooh, M.; Lajevardi, V.; Talebi, S.; Azizpour, A. Cheilitis in acne vulgaris patients with no previous use of systemic retionoid products. Australas. J. Dermatol. 2017, 58, 211–213.
- Daley, T.D.; Gupta, A.K. Exfoliative cheilitis. J. Oral Pathol. Med. 1995, 24, 177–179.
- Girijala, R.L.; Falkner, L.; Dalton, S.R.; Martin, B.D. Exfoliative cheilitis as a manifestation of factitial cheilitis. Cureus 2018, 10, 2565.
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Ferček, I.; Pondeljak, N.; Lazić-Mosler, E.; Gašić, A. Atopic Dermatitis Severity, Patient Perception of the Disease, and Personality Characteristics: How Are They Related to Quality of Life? Life 2021, 11, 1434.
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101.
- Schmidt, D.D.; Zyzansky, S.; Ellner, J.; Kumar, M.L.; Arno, J. Stress as a precipitating factor in subjects with recurrent herpes labialis. J. Fam. Pract. 1985, 20, 359–366.
- Greenberg, S.A.; Schlosser, B.J.; Mirowski, G.W. Diseases of the lips. Clin. Dermatol. 2017, 35, e1–e14.
- Lugović-Mihić, L.; Pilipović, K.; Crnarić, I.; Šitum, M.; Duvančić, T. Differential diagnosis od cheilitis: How to classify cheilitis? Acta Clin. Croat. 2018, 57, 342–351.
- Scully, C. Dermatoses of the Oral Cavity and Lips. In Rook’s Textbook of Dermatology; Griffiths, C., Barker, J., Bleiker, T., Chalmers, R., Creamer, D., Eds.; Wiley Blackwell: Chichester, UK, 2016; pp. 110.1–110.94.
- Collet, E.; Jeudy, G.; Dalac, S. Cheilitis, perioral dermatitis and contact allergy. Eur. J. Dermatol. 2013, 23, 303–307.
- Blagec, T.; Crnarić, I.; Homolak, D.; Pondeljak, N.; Buljan, M.; Lugović-Mihić, L. The association between allergic reactions and lip inflammatory lesions (cheilitis). Acta Clin. Croat. 2022, in press.
- Lugović-Mihić, L.; Blagec, T.; Japundžić, I.; Skroza, N.; Delaš Adžajić, M.; Mravak-Stipetić, M. Diagnostic management of cheilitis: An approach based on a recent proposal for cheilitis classification. Acta Dermatovenerol. Alp. Pannonica Adriat. 2020, 29, 67–72.
- O’Gorman, S.M.; Torgerson, R.R. Contact allergy in cheilitis. Int. J. Dermatol. 2016, 55, 386–391.
- Zoli, V.; Silvani, S.; Vincenzi, C.; Tosti, A. Allergic contact cheilitis. Contact Dermat. 2006, 54, 296–297.
- Bakula, A.; Lugović-Mihić, L.; Šitum, M.; Turčin, J.; Sinković, A. Contact allergy in the mouth: Diversity of clinical presentations and diagnosis of common allergens relevant to dental practice. Acta Clin. Croat. 2011, 50, 553–561.
- Kim, T.W.; Kim, W.I.; Mun, J.H.; Song, M.; Kim, H.S.; Kim, B.S.; Kim, M.B.; Ko, H.C. Patch testing with dental screening series in oral disease. Ann. Dermatol. 2015, 27, 389–393.
- Budimir, J.; Mravak-Stipetić, M.; Bulat, V.; Ferček, I.; Japundžić, I.; Lugović-Mihić, L. Allergic reactions in oral and perioral diseases- what do allergy skin test results show? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 40–48.
- Khamaysi, Z.; Bergman, R.; Weltfriend, S. Positive patch test reactions to allergens of the dental series and the relation to the clinical presentations. Contact Dermat. 2006, 55, 216–218.
- Critchlow, W.A.; Chang, D. Cheilitis granulomatosa: A review. Head Neck Pathol. 2014, 8, 209–213.
- Torgerson, R.R.; Davis, M.D.P.; Bruce, A.J.; Farmer, S.A.; Rogers, R.S., 3rd. Contact allergy in oral disease. J. Am. Acad. Dermatol. 2007, 57, 315–321.
- Cheng, H.S.; Konya, J.; Lobel, E.; Fernandez-Penas, P. Patch testing for cheilitis: A 10-year series. Dermatitis 2019, 30, 347–351.
- Tomljanović-Veselski, M.; Jovanović, I. Najčešći kontaktni alergeni u bolesnika s kontaktnim dermatitisima u području Slavonskog Broda. Med. Jadertina 2006, 36, 45–52.
- Domić, I.; Budimir, J.; Novak, I.; Mravak-Stipetić, M.; Lugović-Mihić, L. Assessment of allergies to food and additives in patients with angioedema, burning mouth syndrome, cheilitis, gingivostomatitis, oral lichenoid reactions, and perioral dermatitis. Acta Clin. Croat. 2021, 60, 276–281.
- Holmes, G.; Freeman, S. Cheilitis caused by contact urticaria to mint flavoured toothpaste. Australas J. Dermatol. 2001, 42, 43–45.
- Oakley, A. Cheilitis. Avaliable online: https://www.dermnetnz.org/topics/cheilitis/ (accessed on 17 November 2022).
- Ayesh, M.H. Angular cheilitis induced by iron deficiency anemia. Cleve Clin. J. Med. 2018, 85, 581–582.
- Schlosser, B.J.; Pirigyi, M.; Mirowski, G.W. Oral manifestations of hematologic and nutritional diseases. Otolaryngol. Clin. N. Am. 2011, 44, 183–203.
- Bhutta, B.S.; Hafsi, W. Cheilitis. Available online: https://www.statpearls.com/articlelibrary/viewarticle/37546/ (accessed on 17 November 2022).
- Baumgardner, D.J. Oral Fungal Microbiota: To Thrush and Beyond. J. Patient Cent. Res. Rev. 2019, 6, 252–261.
- Phatak, S.; Redkar, N.; Patil, M.A.; Kuwar, A. Plummer-Vinson Syndrome. Case Rep. 2012, 2012, bcr2012006403.
- Demir, N.; Doğan, M.; Koç, A.; Kaba, S.; Bulan, K.; Ozkol, H.U.; Doğan, S.Z. Dermatological findings of vitamin B12 deficiency and resolving time of these symptoms. Cutan. Ocul. Toxicol. 2014, 33, 70–73.
- Kaur, S.; Goraya, J.S. Dermatologic findings of vitamin B(12) deiciency in infants. Pediatr. Dermatol. 2018, 35, 796–799.
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and Skin: An Update. J. Dtsch. Dermatol. Ges. 2019, 17, 589–596.
- Gürtler, A.; Laurenz, S. The Impact of Clinical Nutrition on Inflammatory Skin Diseases. J. Dtsch. Dermatol. 2022, 20, 185–202.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
2 times
(View History)
Update Date:
12 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





