Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marouane Mohaddab | -- | 2089 | 2023-01-10 15:30:58 | | | |
| 2 | Rita Xu | Meta information modification | 2089 | 2023-01-11 03:54:28 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mohaddab, M.; Goumi, Y.E.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Alternative System for Secondary Metabolite Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/39980 (accessed on 09 March 2026).
Mohaddab M, Goumi YE, Gallo M, Montesano D, Zengin G, Bouyahya A, et al. Alternative System for Secondary Metabolite Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/39980. Accessed March 09, 2026.
Mohaddab, Marouane, Younes El Goumi, Monica Gallo, Domenico Montesano, Gokhan Zengin, Abdelhakim Bouyahya, Malika Fakiri. "Alternative System for Secondary Metabolite Production" Encyclopedia, https://encyclopedia.pub/entry/39980 (accessed March 09, 2026).
Mohaddab, M., Goumi, Y.E., Gallo, M., Montesano, D., Zengin, G., Bouyahya, A., & Fakiri, M. (2023, January 10). Alternative System for Secondary Metabolite Production. In Encyclopedia. https://encyclopedia.pub/entry/39980
Mohaddab, Marouane, et al. "Alternative System for Secondary Metabolite Production." Encyclopedia. Web. 10 January, 2023.
Copy Citation
Medicinal plants are rich sources of bioactive compounds widely used as medicaments, food additives, perfumes, and agrochemicals. These secondary compounds are produced under stress conditions to carry out physiological tasks in plants. Secondary metabolites have a complex chemical structure with pharmacological properties. The widespread use of these metabolites in a lot of industrial sectors has raised the need to increase the production of secondary metabolites. Biotechnological methods of cell culture allow the conservation of plants, as well as the improvement of metabolite biosynthesis and the possibility to modify the synthesis pathways.
secondary metabolites
cell culture
biological effects
1. Introduction
Plants can synthesize chemical compounds either as primary or secondary metabolites according to their biosynthetic pathways and their functions. The primary metabolites ensure the vital function of the plant. However, the process of secondary metabolites is not directly involved in plant growth and development. Even so, they have major roles in interactions with the environment as a means of defense and adaptation to environmental conditions [1].
The biosynthesis of secondary metabolites is based on geographical area, genetics, climate, and environmental conditions [2]. Under plant growth conditions, many secondary metabolites are amassed in distinct sites (vacuoles, specialized glands, trichomes, and sometimes only during certain developmental stages) to enable functional flexibility under the impact of environmental factors without influencing the cellular and physiological developmental pathways [3]. Indeed, these substances have high values for humans as pharmaceuticals, nutraceuticals, and cosmetics, making them targets for metabolic engineering [4]. Phytochemical investigations have identified an arsenal of secondary metabolites such as flavonoids, phenolic acids, nitrogen compounds, and terpenes [5][6].
The therapeutic effects of plants have been known since time immemorial [7]. These molecules, which are made by plants, are now utilized by the pharmaceutical industry from used vegetable raw materials [8][9]. While secondary metabolites exhibit various biological properties [10][11][12][13], their distribution is very limited compared to primary metabolites. Many of these compounds occur in very low quantities in nature [14][15], necessitating massive harvesting. This over-harvesting can threaten the biodiversity of the plants from which these secondary metabolites originate.
Biotechnological approaches can be considered a key and powerful substitute in the production of secondary metabolites coming from medicinal plants to support industrial production and reduce the overexploitation of natural resources [16]. However, cell, tissue, and plant organ culture techniques have been used for the production of these natural substances [17]. In this regard, effort has been made towards optimizing the culture conditions for the production of secondary metabolites, as well as manipulating the synthesis of these phytoconstituents through the application of different technological approaches including cell line selection, elicitation, and precursor feeding [18]. These efforts have been carried out to increase secondary metabolite production to meet the demand of the pharmaceutical industry and to conserve natural sources [18][19][20][21][22].
Several extraction methods can be applied, depending on the physicochemical nature of these compounds of interest [23]. These methods can be conventional or modern. Conventional methods are generally based on the extraction potential of the different solvents used before applying heat to them and/or mixing the solvents to obtain bioactive compounds, such as Soxhlet extraction, maceration, and hydrodistillation [24][25][26], while modern extraction techniques allow for shorter extraction time and reduced solvent consumption [27]. New extraction methods, including ultrasonic-assisted extraction [28][29][30], supercritical fluid extraction [29][30][31], and accelerated solvent extraction [32], are fast and efficient for extracting chemicals from plant matrices. In addition, in situ extraction is considered an efficient method to recover secondary metabolites; moreover, it allows both to improve the yield of the product and to orient the secondary metabolite pathway’s in vitro culture system [33][34][35]. As the results revealed, the use of perfluorodecalin in the in situ extraction system improved the performance of the cells’ culture as well as increased the production of targeted molecules [36][37]. The choice of an appropriate extraction method should be an essential consideration depending on the study objective, as the process of the extraction may fully influence the chemical composition and therefore the biological activity of the extract [38].
Plant extracts constitute a mixture of bioactive or phytochemical compounds of several polarities, and their separation is an important challenge that leads to identification and characterization processes [39]. In general, high-performance liquid chromatography (HPLC) and gas chromatography (GC) coupled with mass spectrometry (MS) or nuclear magnetic resonance spectroscopy (NMR) are widely used to characterize and quantify secondary metabolites in plant extracts.
For a long time, herbal treatments have been widely used for primary healthcare needs. Through time, and with progress in the field of pharmacopy, synthetic drugs have gradually started to be used instead of natural drugs, regardless of the side effects of the synthetic components [40]. Moreover, these natural products have lower hydrophobicity and higher stereochemical content than synthetic products [41]. Structural features of natural compounds can be effectively incorporated into synthetic drugs to increase chemical diversity, and molecular complicity is an important feature for drugs [42], as molecular complexity has been correlated with biological activity [43]. Indeed, in recent years, approval of synthetic drugs has declined substantially [40][43]. So far, many successes have been registered in the discovery of new active molecules in natural compounds. Some of these molecules have become medicines or new paths of inspiration in finding new ones [44]. On the other hand, medicinal plants and their natural products are still the best pharmaceutical lead and offer an opportunity to discover new structures effective in a variety of human diseases [38][44]. However, such property may threaten the biodiversity of these medicinal plants due to overexploitation and unsustainable harvesting techniques [45].
In addition, plant biotechnology has offered alternative ways to access and explore this chemical diversity through different in vitro culture techniques to produce natural products for the pharmaceutical industries [46][47][48]. The cell culture technique can be used as a platform for the production of high-value secondary compounds [46][48][49][50]. Different biotechnology approaches represent a beneficial alternative for the production of secondary metabolites under highly controlled conditions [51][52]. Therefore, in vitro culture techniques such as plant organ culture provide plant material as a source of natural products [38]. Multiple strategies using cell culture systems have been widely studied in the context of improving the production and manipulating the flow of the biosynthesis of desired secondary metabolites [46][53].
Plant cell and tissue culture offer an opportunity for the propagation of plants as well as the production of phytochemicals [54]. Many plant species can be regenerated in vitro through several approaches started by explants. Any part of the plant, such as meristems, nodes, leaves, stems, roots, buds, embryos, etc., can be used for a limitless multiplication of a plant and the production of bioactive compounds under sterile conditions [48][55][56][57]. Due to its various advantages, in vitro culture has been used as a powerful strategy for the production of secondary metabolites [22][58].
2. Plant Secondary Metabolites
Plant Secondary metabolites (PSMs) are low-weight molecules synthesized by the plant to protect itself against potential enemies, including pathogens and herbivore attacks. Even abiotic factors can affect the biosynthesis of secondary metabolites [59][60].
Due to their excellent biological activity, PSMs have been broadly used for centuries as an important resource for traditional medicine, perfumes, and industrial raw materials [61]. Subsequently, they have been widely applied as valuable compounds such as pharmaceuticals, cosmetics, and bio-pesticides [4][51][61][62]. PSMs have contributed greatly to the importance and commercial values of plants [63].
Phytochemical studies have identified an arsenal of secondary compounds such as flavonoids, phenols, nitrogen compounds, and terpenes [5][6]. Thus, a preview of the various biosynthetic pathways is represented in Figure 1.
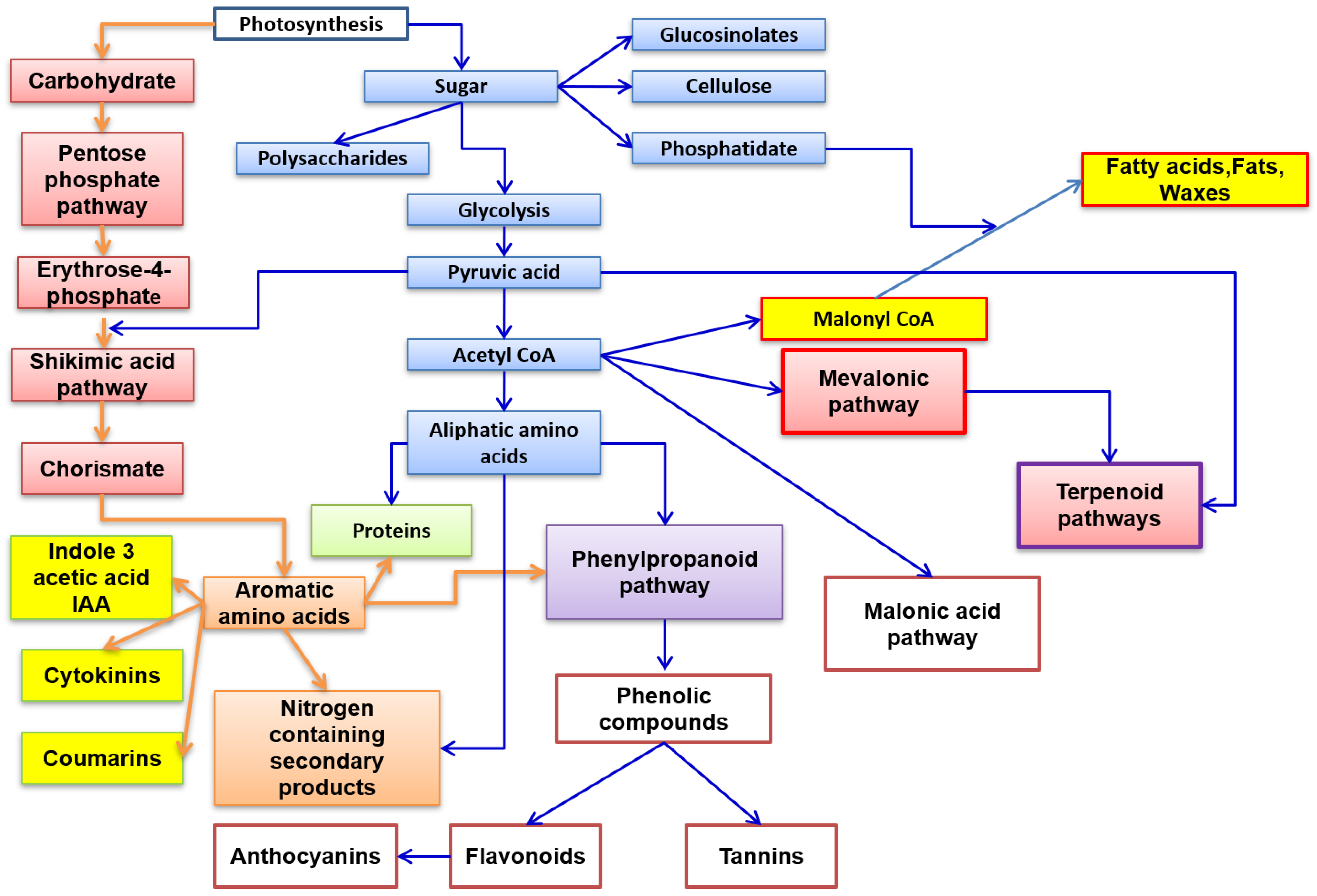
Figure 1. Principal biosynthetic pathways of major secondary metabolite plants’ classes.
3. Micropropagation as a Tool for the Production of Secondary Metabolites
Micropropagation is the reproduction of plants in vitro which leads to the multiplication of genetically identical copies of the parent plant asexually. Micropropagation offers the possibility of producing a limitless number of plants. Currently, this technique is applied for clone selection and rapid biomass production in several organizations or establishments for the large-scale production of higher plants.
In vitro propagation has become a crucial method for the mass production of medicinal plants and various protocols of the micropropagation of numerous medicinal species that have been successfully achieved either by organogenesis [64][65][66][67][68] or by somatic embryogenesis [69][70][71]. The micropropagation of many medicinal species has been revealed to be similar and with a little variation in their phytochemical content [72].
Organogenesis is a micropropagation way that consists in the development of organs derived from cells or tissues. Plant regeneration through organogenesis involves specifically the induction and development of a shoot from an explant which is then transferred to a different medium for root induction [73]. Several studies have demonstrated that a successful application of organogenesis on medicinal plants can be achieved by the correct establishment of the medium components and the selection of an adequate explant under highly controlled conditions (Table 1).
Table 1. Micropropagation of medicinal plants by organogenesis methods.
| Plant | Explant Source | Shoot Multiplication | Rooting | Phytochemical Analysis | Key Findings | References | ||
|---|---|---|---|---|---|---|---|---|
| MS Medium | Phytohormone | MS Medium | Phytohormone | |||||
| Zingiber officinale Roscoe | Rhizome sproutedbud | solid | Zeatin (10 µM) | solid | NAA (7.5 µM) | Flavonoids and phenolic acids |
The total content of phytochemical components is not very different from those of conventionally propagated plants. | [74] |
| Plectranthusamboinicus | Axillarybuds | semi-solid | BAP (0.4 mg/L) | semi-solid | Without PGR | Carvacrol γ-Terpinene |
Essential oil yield was improved with a higher quantity of chemical compounds in vitro cultures. The in vitro regeneration was chemically true to the parent plant type. | [64] |
| Lavandula coronopifolia | Shoot tips | solid | BA (0.5 mg/L) | solid | IBA (10 mg/L) | Caffeic acid androsmarinic acid | Micropropagation was regenerated from plants with genetic fidelity to the parent plant. A remarkable difference in the chemical profiles of the in vitro culture and the wild-type plants. |
[75] |
| Tanacetum vulgare | Shoot tips | solid | without PGR | liquid half-strength | Without PGR | Monoterpenes Sesquiterpene Chlorogenic acid 3,5-O-Dicaffeoylquinic acid |
Spontaneously rooted seedlings at the time of propagation. Terpenes are the most abundant in essential oils. In vitro grown roots are richest in 3,5-O-dicaffeoylquinic acid. |
[76] |
| Cannabis sativa | Nodal segments | solid | mT (2 µM) | solid | mT (2 µM) | Cannabinoids | Rooting was performed on the same propagation medium. Auxin was not necessary for root induction. cannabinoid level in the micropropagated plants is comparable to the mother plant. In vitro propagated plants are identical to the mother plant. |
[77] |
| Eryngiumalpinum | Shoots | solid | BAP, IAA, and GA3 (each 1.0 mg/L) | __ | __ | Phenolic acids and flavonoids | The solid MS medium with BAP, IAA, and GA3 (each 1.0 mg/L) is the optimal system for micropropagation and accumulation of phenolic acids and flavonoids. An important variability in phytochemicals between the intact plant and different in vitro culture. |
[6] |
| Spiraeabetulifoliasubsp. aemiliana | Axillarybuds | solid | S1 = BAP (1.0 μM) S2 = (BAP 5.0 μM) + (NAA 1.0 μM) |
half-strength | S1 = S2= IBA (0.1 µM) | Phenolic acids and flavonoids | Many differences in chemical profile between in vitro culture and intact plants. Interpopulation genotypic differences in the activity of morphogenic processes have been identified in S. betulifolia in vitro culture. |
[78] |
| Salvia sclarea | Nodal segments | solid | mT (2.0 mg/L) + IAA (0.2 mg/L) | solid | NAA (1.0 mg/L) | A multitude of secondary metabolites | High genetic stability of micropropagated plants. N-alkanes, tetradecanal, octadecanal, and hentriacontane are the major components from micropropagated plants. PGRs have caused variability in the content of secondary metabolite. |
[79] |
| Lippiaoriganoides | Nodal segments | solid | KIN (4.6 μM) | solid | KIN (2.3 μM) | Myrcene, p-cymene, γ-terpinene, linalool, thymol, carvacrol and (E)-caryophyllene. | The presence of PGR changed the chemical profile of the volatile organic compound. | [80] |
Somatic cells can produce somatic embryos, which are similar to zygotic embryos, through a process called somatic embryogenesis. These somatic embryos can be developed into seedlings in an appropriate medium [81]. Plant regeneration via embryogenesis occurs in two steps: the callus is grown on an auxin-rich embryogenic induction medium, sometimes combined with cytokinins, and is then transferred to an auxin-free medium, which results in the formation of mature embryos [82]. The embryonic-like structure can be produced either directly on the explant or indirectly from the callus or cell suspension culture (Table 2). This technique has also allowed genetic, morphological, and physiological manipulations to be performed [83].
Table 2. Micropropagation of medicinal plants by somatic embryogenesis (SE).
| Family | Plant | Explant Source | Phytohormone (mg/L) for Induction SE | Basal Medium |
Somatic Embryogenesis |
References | |
|---|---|---|---|---|---|---|---|
| Direct | Indirect | ||||||
| Apiaceae | Ferulajaeschkeana | Petiole | 2,4-D (4.0) | MS | - | X | [84] |
| Asteraceae | Seriphidiumherba-album | Leaves | 2,4-D (1.5) + BA (0.5) | MS | - | X | [85] |
| Fumariaceae | Lamprocapnosspectabilis | Leaves | 2,4-D (0.5) + BA (0.5) | ½ MS | - | X | [86] |
| Petioles | PIC (1.0) + BA (0.5) | ||||||
| Plantaginaceae | Digitalislanata | Leaves | 2,4-D (1.0) + Kin (1.0) | MS | - | X | [87] |
| IBA (2.0) + Kin (2.0) | X | - | |||||
| Root | IBA (2.0) + Kin (2.0) | X | - | ||||
Micropropagation could be an attractive commercial activity for the production of high-quality plants and offers advantages over conventional propagation practices [88]. Thus, in vitro propagation is a sustainable alternative to the large-scale production of medicinal species with economic value. Castilho et al. [80] allowed the use of an automated micropropagation system using bioreactors for industrial plant propagation as a possible way to reduce micropropagation costs [89]. This can provide a means of supplying plant material capable of providing plant material that is able to produce phytocompounds [19][38][48][90] throughout the year without seasonal constraints [16].
References
- Harborne, J.B. Classes and Functions of Secondary Products from Plants. Chem. Plants 1999, 26, 1–25.
- Aboukhalid, K.; Lamiri, A.; Agacka-Moldoch, M.; Doroszewska, T.; Douaik, A.; Bakha, M.; Casanova, J.; Tomi, F.; Machon, N.; Faiz, C.A. Chemical Polymorphism of Origanum Compactum Grown in All Natural Habitats in Morocco. Chem. Biodivers. 2016, 13, 1126–1139.
- Yang, W.-C.; Bao, H.-Y.; Liu, Y.-Y.; Nie, Y.-Y.; Yang, J.-M.; Hong, P.-Z.; Zhang, Y. Depsidone Derivatives and a Cyclopeptide Produced by Marine Fungus Aspergillus unguis under Chemical Induction and by Its Plasma Induced Mutant. Molecules 2018, 23, 2245.
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487.
- Elazzouzi, H.; Soro, A.; Elhilali, F.; Bentayeb, A.; El Belghiti, M.A.; Zair, T. Phytochemical Study of Anacyclus pyrethrum (L.) of Middle Atlas (Morocco), and in Vitro Study of Antibacterial Activity of Pyrethrum. Adv. Nat. Appl. Sci. 2014, 8, 131–141.
- Kikowska, M.; Thiem, B.; Szopa, A.; Ekiert, H. Accumulation of Valuable Secondary Metabolites: Phenolic Acids and Flavonoids in Different in Vitro Systems of Shoot Cultures of the Endangered Plant Species-Eryngium alpinum L. Plant Cell Tissue Organ Cult. 2020, 141, 381–391.
- Gurib-Fakim, A. Medicinal Plants: Traditions of Yesterday and Drugs of Tomorrow. Mol. Asp. Med. 2006, 27, 1–93.
- Isah, T. Anticancer Alkaloids from Trees: Development into Drugs. Pharmacogn. Rev. 2016, 10, 90.
- Park, S.-Y.; Paek, K.-Y. Bioreactor Culture of Shoots and Somatic Embryos of Medicinal Plants for Production of Bioactive Compounds. Prod. Biomass Bioact. Compd. Using Bioreact. Technol. 2014, 3, 337–368.
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of Plant Secondary Metabolites: A Historical Perspective. Plant Sci. 2001, 161, 839–851.
- Bouyahya, A.; Abrini, J.; Bakri, Y.; Dakka, N. Essential Oils as Anticancer Agents: News on Mode of Action. Phytothérapie 2016, 146, 1–14.
- Bouyahya, A.; Guaouguaou, F.-E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2021, 12, 35–57.
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.-E.; Balahbib, A.; Belmehdi, O.; Salhi, N.; Imtara, H.; Mrabti, H.N.; El-Shazly, M. Moroccan Antidiabetic Medicinal Plants: Ethnobotanical Studies, Phytochemical Bioactive Compounds, Preclinical Investigations, Toxicological Validations and Clinical Evidences; Challenges, Guidance and Perspectives for Future Management of Diabetes Worldwide. Trends Food Sci. Technol. 2021, 115, 147–254.
- Bulugahapitiya, V.P. Plants Based Natural Products; University of Ruhuna: Fribourg, Switzerland, 2013.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20.
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary Metabolism of Pharmaceuticals in the Plant in Vitro Cultures: Strategies, Approaches, and Limitations to Achieving Higher Yield. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 239–265.
- Nalawade, S.M.; Tsay, H.-S. In Vitro Propagation of Some Important Chinese Medicinal Plants and Their Sustainable Usage. Vitr. Cell. Dev. Biol.-Plant 2004, 40, 143–154.
- Gaosheng, H.; Jingming, J. Production of Useful Secondary Metabolites through Regulation of Biosynthetic Pathway in Cell and Tissue Suspension Culture of Medicinal Plants. Recent Adv. Plant Vitr. Cult. 2012, 10, 53038.
- Gonçalves, S.; Romano, A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. In Secondary Metabolites—Sources and Applications; IntechOpen: London, UK, 2018; pp. 81–99.
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309.
- Mulabagal, V.; Tsay, H.-S. Plant Cell Cultures-an Alternative and Efficient Source for the Production of Biologically Important Secondary Metabolites. Int. J. Appl. Sci. Eng. 2004, 2, 29–48.
- Yue, W.; Ming, Q.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232.
- Yahya, N.A.; Attan, N.; Wahab, R.A. An Overview of Cosmeceutically Relevant Plant Extracts and Strategies for Extraction of Plant-Based Bioactive Compounds. Food Bioprod. Process. 2018, 112, 69–85.
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436.
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat Plants 2015, 4, 1000196.
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G. A Critical Analysis of Extraction Techniques Used for Botanicals: Trends, Priorities, Industrial Uses and Optimization Strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102.
- Jovanović, A.A.; DJordjević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Godjevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus serpyllum L. Herb Using Maceration, Heat-and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380.
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547.
- Haloui, I.; Meniai, A.-H. Supercritical CO2 Extraction of Essential Oil from Algerian Argan (Argania spinosa L.) Seeds and Yield Optimization. Int. J. Hydrog. Energy 2017, 42, 12912–12919.
- Meireles, M.A.A. Supercritical Extraction from Solid: Process Design Data (2001–2003). Curr. Opin. Solid State Mater. Sci. 2003, 7, 321–330.
- Souza, M.A.; Guzatti, J.G.; Martello, R.H.; Schindler, M.S.; Calisto, J.F.; Morgan, L.V.; Aguiar, G.P.; Locateli, G.; Scapinello, J.; Müller, L.G. Supercritical CO2 Extraction of Aloysia Gratissima Leaves and Evaluation of Anti-Inflammatory Activity. J. Supercrit. Fluids 2020, 159, 104753.
- Rahmalia, W.; Fabre, J.-F.; Mouloungui, Z. Effects of Cyclohexane/Acetone Ratio on Bixin Extraction Yield by Accelerated Solvent Extraction Method. Procedia Chem. 2015, 14, 455–464.
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A Biotechnological Tool for Enhanced Production of Secondary Metabolites in Hairy Root Cultures. Eng. Life Sci. 2019, 19, 880–895.
- Kawka, M.; Bubko, I.; Koronkiewicz, M.; Gruber-Bzura, B.; Graikou, K.; Chinou, I.; Jeziorek, M.; Pietrosiuk, A.; Syklowska-Baranek, K. Polyurethane Foam Rafts Supported in Vitro Cultures of Rindera Graeca Roots for Enhanced Production of Rinderol, Potent Proapoptotic Naphthoquinone Compound. Int. J. Mol. Sci. 2021, 23, 56.
- Nowak, B.; Kawka, M.; Wierzchowski, K.; Syklowska-Baranek, K.; Pilarek, M. MTMS-Based Aerogel Constructs for Immobilization of Plant Hairy Roots: Effects on Proliferation of Rindera Graeca Biomass and Extracellular Secretion of Naphthoquinones. J. Funct. Biomater. 2021, 12, 19.
- Syklowska-Baranek, K.; Rymaszewski, W.; Gawel, M.; Rokicki, P.; Pilarek, M.; Grech-Baran, M.; Hennig, J.; Pietrosiuk, A. Comparison of Elicitor-Based Effects on Metabolic Responses of Taxus× Media Hairy Roots in Perfluorodecalin-Supported Two-Phase Culture System. Plant Cell Rep. 2019, 38, 85–99.
- Syklowska-Baranek, K.; Pilarek, M.; Cichosz, M.; Pietrosiuk, A. Liquid Perfluorodecalin Application for in Situ Extraction and Enhanced Naphthoquinones Production in Arnebia Euchroma Cell Suspension Cultures. Appl. Biochem. Biotechnol. 2014, 172, 2618–2627.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Oladimeji, A.V.; Valan, M.F. HPLC Techniques for Phytochemistry. IJCS 2020, 8, 2590–2596.
- Nisar, B.; Sultan, A.; Rubab, S.L. Comparison of Medicinally Important Natural Products versus Synthetic Drugs-a Short Commentary. Nat. Prod. Chem. Res 2018, 6, 308.
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic Comparison of Approved Drugs from Natural Product versus Synthetic Origins. Bioorganic Med. Chem. Lett. 2015, 25, 4802–4807.
- Hann, M.M.; Leach, A.R.; Harper, G. Molecular Complexity and Its Impact on the Probability of Finding Leads for Drug Discovery. J. Chem. Inf. Comput. Sci. 2001, 41, 856–864.
- Selzer, P.; Roth, H.-J.; Ertl, P.; Schuffenhauer, A. Complex Molecules: Do They Add Value? Curr. Opin. Chem. Biol. 2005, 9, 310–316.
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661.
- Cordell, G.A. Sustainable Medicines and Global Health Care. Planta Med. 2011, 77, 1129–1138.
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59.
- Lautie, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020, 11, 397.
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Plant Cell Culture Strategies for the Production of Natural Products. BMB Rep. 2016, 49, 149.
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450.
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant Cell Culture Technology in the Cosmetics and Food Industries: Current State and Future Trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675.
- Alamgir, A.N.M. Cultivation of Herbal Drugs, Biotechnology, and in Vitro Production of Secondary Metabolites, High-Value Medicinal Plants, Herbal Wealth, and Herbal Trade. In Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1; Springer: Berlin/Heidelberg, Germany, 2017; pp. 379–452.
- Tasheva, K.; Kosturkova, G. Role of Biotechnology for Protection of Endangered Medicinal Plants. In Environmental Biotechnology—New Approaches and Prospective Applications; IntechOpen: London, UK, 2013; pp. 235–238.
- Vanisree, M.; Lee, C.-Y.; Lo, S.-F.; Nalawade, S.M.; Lin, C.Y.; Tsay, H.-S. Studies on the Production of Some Important Secondary Metabolites from Medicinal Plants by Plant Tissue Cultures. Bot. Bull. Acad. Sin 2004, 45, 1–22.
- Sood, H. Production of Medicinal Compounds from Endangered and Commercially Important Medicinal Plants through Cell and Tissue Culture Technology for Herbal Industry. In Bioactive Compounds in Nutraceutical and Functional Food for Good Human Health; IntechOpen: London, UK, 2020.
- Davoodi, A.; Khoshvishkaie, E.; Azadbakht, M. Plant Cells Technology as an Effective Biotechnological Approach for High Scale Production of Pharmaceutical Natural Compounds; A Meta-Analysis Study. Pharm. Biomed. Res. 2019, 5, 1–9.
- Karuppusamy, S. A Review on Trends in Production of Secondary Metabolites from Higher Plants by in Vitro Tissue, Organ and Cell Cultures. J. Med. Plants Res. 2009, 3, 1222–1239.
- Rao, S.R.; Ravishankar, G.A. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol. Adv. 2002, 20, 101–153.
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically Active Natural Product Synthesis and Supply via Plant Cell Culture Technology. Mol. Pharm. 2008, 5, 243–256.
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant Secondary Metabolites Synthesis and Their Regulations under Biotic and Abiotic Constraints. J. Plant Biol. 2020, 63, 203–216.
- Olivoto, T.; Nardino, M.; Carvalho, I.R.; Follmann, D.N.; Szareski, V.J.; Ferrari, M.; de Pelegrin, A.J.; de Souza, V.Q. Plant Secondary Metabolites and Its Dynamical Systems of Induction in Response to Environmental Factors: A Review. Afr. J. Agric. Res. 2017, 12, 71–84.
- Balandrin, M.F.; Klocke, J.A. Medicinal, Aromatic, and Industrial Materials from Plants. In Medicinal and Aromatic Plants I; Springer: Berlin/Heidelberg, Germany, 1988; pp. 3–36.
- Phillipson, J.D. Plants as source of valuable products. In Secondary Products from Plant Tissue Culture; Charlwood, B.V., Rhodes, M.J.C., Eds.; Clarendon Press: Oxford, UK, 1990; pp. 1–21.
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; p. 1.
- Arumugam, G.; Sinniah, U.R.; Swamy, M.K.; Lynch, P.T. Micropropagation and Essential Oil Characterization of Plectranthus Amboinicus (Lour.) Sprengel, an Aromatic Medicinal Plant. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 491–503.
- Galán-Ávila, A.; García-Fortea, E.; Prohens, J.; Herraiz, F.J. Development of a Direct in Vitro Plant Regeneration Protocol From Cannabis sativa L. Seedling Explants: Developmental Morphology of Shoot Regeneration and Ploidy Level of Regenerated Plants. Front. Plant Sci. 2020, 11, 645.
- Singh, D.K.; Nirwan, S.; Babbar, S.B. Micropropagation of Anacyclus Pyrethrum and Chemical Profiling of the Regenerated Plants for Pellitorine, the Active Principle. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 122, 249–255.
- Sottile, F.; Giuggioli, N.R.; Marinoni, D.T.; Peano, C.; Del Signore, M.B. Selection and Micropropagation of Valuable Caper Genotypes. Hortic. Sci. 2020, 47, 110–116.
- Wróbel, T.; Dreger, M.; Wielgus, K.; Slomski, R. Modified Nodal Cuttings and Shoot Tips Protocol for Rapid Regeneration of Cannabis sativa L. J. Nat. Fibers 2020, 19, 536–545.
- Bayarmaa, G.-A.; Lee, N.N.; Kang, H.D.; Oyuntsetseg, B.; Moon, H.K. Micropropagation of the Mongolian Medicinal Plant Zygophyllum Potaninii via Somatic Embryogenesis. Plant Biotechnol. Rep. 2018, 12, 187–194.
- Bertero, V.G.; Beznec, A.; Faccio, P.; Auteri, M.; Arteaga, M.; Bonafede, M.; Bossio, E. High-Efficiency Direct Somatic Embryogenesis and Plant Regeneration from Leaf Base Explants of “Peperina” (Minthostachys Verticillata). Vitr. Cell. Dev. Biol.-Plant 2020, 56, 915–919.
- Lema-Rumińska, J.; Kulus, D.; Tymoszuk, A.; Varejão, J.M.; Bahcevandziev, K. Profile of Secondary Metabolites and Genetic Stability Analysis in New Lines of Echinacea purpurea (L.) Moench Micropropagated via Somatic Embryogenesis. Ind. Crops Prod. 2019, 142, 111851.
- Yamada, Y.; Shoyama, Y.; Nishioka, I.; Kohda, H.; Namera, A.; Okamoto, T. Clonal Micropropagation of Gentiana Scabra Bunge Var. Buergeri Maxim. and Examination of the Homogeneity Concerning the Gentiopicroside Content. Chem. Pharm. Bull. 1991, 39, 204–206.
- Siahsar, B.; Rahimi, M.; Tavassoli, A.; Raissi, A. Application of Biotechnology in Production of Medicinal Plants. Am. Eurasian J. Agric Environ. Sci. 2011, 11, 439–444.
- Zahid, N.A.; Jaafar, H.Z.; Hakiman, M. Micropropagation of Ginger (Zingiber Officinale Roscoe)‘Bentong’and Evaluation of Its Secondary Metabolites and Antioxidant Activities Compared with the Conventionally Propagated Plant. Plants 2021, 10, 630.
- Al Khateeb, W.; Kanaan, R.; El-Elimat, T.; Alu’datt, M.; Lahham, J.; El-Oqlah, A. In Vitro Propagation, Genetic Stability, and Secondary Metabolite Analysis of Wild Lavender (Lavandula Coronopifolia Poir.). Hortic. Environ. Biotechnol. 2017, 58, 393–405.
- Devrnja, N.; Krstić-Milošević, D.; Janošević, D.; Tešević, V.; Vinterhalter, B.; Savić, J.; Ćalić, D. In Vitro Cultivation of Tansy (Tanacetum vulgare L.): A Tool for the Production of Potent Pharmaceutical Agents. Protoplasma 2021, 258, 587–599.
- Lata, H.; Chandra, S.; Techen, N.; Khan, I.A.; ElSohly, M.A. In Vitro Mass Propagation of Cannabis sativa L.: A Protocol Refinement Using Novel Aromatic Cytokinin Meta-Topolin and the Assessment of Eco-Physiological, Biochemical and Genetic Fidelity of Micropropagated Plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 18–26.
- Muraseva, D.S.; Kostikova, V.A. In Vitro Propagation of Spiraea Betulifolia Subsp. Aemiliana (Rosaceae) and Comparative Analysis of Phenolic Compounds of Microclones and Intact Plants. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 144, 493–504.
- Erişen, S.; Kurt-Gür, G.; Servi, H. In Vitro Propagation of Salvia sclarea L. by Meta-Topolin, and Assessment of Genetic Stability and Secondary Metabolite Profiling of Micropropagated Plants. Ind. Crops Prod. 2020, 157, 112892.
- Castilho, C.V.; Leitão, S.G.; Silva, V.D.; Miranda, C.d.O.; Santos, M.C.d.S.; Bizzo, H.R.; da Silva, N.C. In Vitro Propagation of a Carvacrol-Producing Type of Lippia Origanoides Kunth: A Promising Oregano-like Herb. Ind. Crops Prod. 2019, 130, 491–498.
- Zimmerman, J.L. Somatic Embryogenesis: A Model for Early Development in Higher Plants. Plant Cell 1993, 5, 1411.
- Razdan, M.K. Introduction To Plant Tissue Culture, 2/E; Oxford and IBH Publishing: Oxford, UK, 2002.
- Sharma, S.; Rathi, N.; Kamal, B.; Pundir, D.; Kaur, B.; Arya, S. Conservation of Biodiversity of Highly Important Medicinal Plants of India through Tissue Culture Technology-a Review. Agric. Biol. J. N. Am. 2010, 1, 827–833.
- Sharma, R.K.; Khajuria, A.K. Somatic Embryogenesis and Plant Regeneration in Ferula Jaeschkeana Vatke: A Threatened Medicinal Herb. Vegetos 2020, 33, 658–664.
- Soliman, H.I.; Abo-El-Hasan, F.M.; Ayman, S.; Mabrouk, Y.M. Influence of Plant Growth Regulators on Somatic Embryogenesis Induction in Seriphidium Herba-Album. Int. J. Environ. Agric. Biotechnol. 2018, 3, 264401.
- Kulus, D.; Tymoszuk, A. Induction of Callogenesis, Organogenesis, and Embryogenesis in Non-Meristematic Explants of Bleeding Heart and Evaluation of Chemical Diversity of Key Metabolites from Callus. Int. J. Mol. Sci. 2020, 21, 5826.
- Bhusare, B.P.; John, C.K.; Bhatt, V.P.; Nikam, T.D. Induction of Somatic Embryogenesis in Leaf and Root Explants of Digitalis Lanata Ehrh.: Direct and Indirect Method. S. Afr. J. Bot. 2020, 130, 356–365.
- Debnath, M.; Malik, C.P.; Bisen, P.S. Micropropagation: A Tool for the Production of High Quality Plant-Based Medicines. Curr. Pharm. Biotechnol. 2006, 7, 33–49.
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of Bioreactor Systems for Large Scale Production of Horticultural and Medicinal Plants. In Liquid Culture Systems for In Vitro Plant Propagation; Springer: Berlin/Heidelberg, Germany, 2005; pp. 95–116.
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
11 Jan 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





