Medicinal plants are rich sources of bioactive compounds widely used as medicaments, food additives, perfumes, and agrochemicals. These secondary compounds are produced under stress conditions to carry out physiological tasks in plants. Secondary metabolites have a complex chemical structure with pharmacological properties. The widespread use of these metabolites in a lot of industrial sectors has raised the need to increase the production of secondary metabolites. Biotechnological methods of cell culture allow the conservation of plants, as well as the improvement of metabolite biosynthesis and the possibility to modify the synthesis pathways.
1. Introduction
Plants can synthesize chemical compounds either as primary or secondary metabolites according to their biosynthetic pathways and their functions. The primary metabolites ensure the vital function of the plant. However, the process of secondary metabolites is not directly involved in plant growth and development. Even so, they have major roles in interactions with the environment as a means of defense and adaptation to environmental conditions [
1].
The biosynthesis of secondary metabolites is based on geographical area, genetics, climate, and environmental conditions [
2]. Under plant growth conditions, many secondary metabolites are amassed in distinct sites (vacuoles, specialized glands, trichomes, and sometimes only during certain developmental stages) to enable functional flexibility under the impact of environmental factors without influencing the cellular and physiological developmental pathways [
3]. Indeed, these substances have high values for humans as pharmaceuticals, nutraceuticals, and cosmetics, making them targets for metabolic engineering [
4]. Phytochemical investigations have identified an arsenal of secondary metabolites such as flavonoids, phenolic acids, nitrogen compounds, and terpenes [
5,
6].
The therapeutic effects of plants have been known since time immemorial [
7]. These molecules, which are made by plants, are now utilized by the pharmaceutical industry from used vegetable raw materials [
8,
9]. While secondary metabolites exhibit various biological properties [
10,
11,
12,
13], their distribution is very limited compared to primary metabolites. Many of these compounds occur in very low quantities in nature [
14,
15], necessitating massive harvesting. This over-harvesting can threaten the biodiversity of the plants from which these secondary metabolites originate.
Biotechnological approaches can be considered a key and powerful substitute in the production of secondary metabolites coming from medicinal plants to support industrial production and reduce the overexploitation of natural resources [
16]. However, cell, tissue, and plant organ culture techniques have been used for the production of these natural substances [
17]. In this regard, effort has been made towards optimizing the culture conditions for the production of secondary metabolites, as well as manipulating the synthesis of these phytoconstituents through the application of different technological approaches including cell line selection, elicitation, and precursor feeding [
18]. These efforts have been carried out to increase secondary metabolite production to meet the demand of the pharmaceutical industry and to conserve natural sources [
18,
19,
20,
21,
22].
Several extraction methods can be applied, depending on the physicochemical nature of these compounds of interest [
23]. These methods can be conventional or modern. Conventional methods are generally based on the extraction potential of the different solvents used before applying heat to them and/or mixing the solvents to obtain bioactive compounds, such as Soxhlet extraction, maceration, and hydrodistillation [
24,
25,
26], while modern extraction techniques allow for shorter extraction time and reduced solvent consumption [
27]. New extraction methods, including ultrasonic-assisted extraction [
28,
29,
30], supercritical fluid extraction [
29,
30,
31], and accelerated solvent extraction [
32], are fast and efficient for extracting chemicals from plant matrices. In addition, in situ extraction is considered an efficient method to recover secondary metabolites; moreover, it allows both to improve the yield of the product and to orient the secondary metabolite pathway’s in vitro culture system [
33,
34,
35]. As the results revealed, the use of perfluorodecalin in the in situ extraction system improved the performance of the cells’ culture as well as increased the production of targeted molecules [
36,
37]. The choice of an appropriate extraction method should be an essential consideration depending on the study objective, as the process of the extraction may fully influence the chemical composition and therefore the biological activity of the extract [
38].
Plant extracts constitute a mixture of bioactive or phytochemical compounds of several polarities, and their separation is an important challenge that leads to identification and characterization processes [
39]. In general, high-performance liquid chromatography (HPLC) and gas chromatography (GC) coupled with mass spectrometry (MS) or nuclear magnetic resonance spectroscopy (NMR) are widely used to characterize and quantify secondary metabolites in plant extracts.
For a long time, herbal treatments have been widely used for primary healthcare needs. Through time, and with progress in the field of pharmacopy, synthetic drugs have gradually started to be used instead of natural drugs, regardless of the side effects of the synthetic components [
40]. Moreover, these natural products have lower hydrophobicity and higher stereochemical content than synthetic products [
41]. Structural features of natural compounds can be effectively incorporated into synthetic drugs to increase chemical diversity, and molecular complicity is an important feature for drugs [
42], as molecular complexity has been correlated with biological activity [
43]. Indeed, in recent years, approval of synthetic drugs has declined substantially [
40,
43]. So far, many successes have been registered in the discovery of new active molecules in natural compounds. Some of these molecules have become medicines or new paths of inspiration in finding new ones [
44]. On the other hand, medicinal plants and their natural products are still the best pharmaceutical lead and offer an opportunity to discover new structures effective in a variety of human diseases [
38,
44]. However, such property may threaten the biodiversity of these medicinal plants due to overexploitation and unsustainable harvesting techniques [
45].
In addition, plant biotechnology has offered alternative ways to access and explore this chemical diversity through different in vitro culture techniques to produce natural products for the pharmaceutical industries [
46,
47,
48]. The cell culture technique can be used as a platform for the production of high-value secondary compounds [
46,
48,
49,
50]. Different biotechnology approaches represent a beneficial alternative for the production of secondary metabolites under highly controlled conditions [
51,
52]. Therefore, in vitro culture techniques such as plant organ culture provide plant material as a source of natural products [
38]. Multiple strategies using cell culture systems have been widely studied in the context of improving the production and manipulating the flow of the biosynthesis of desired secondary metabolites [
46,
53].
Plant cell and tissue culture offer an opportunity for the propagation of plants as well as the production of phytochemicals [
54]. Many plant species can be regenerated in vitro through several approaches started by explants. Any part of the plant, such as meristems, nodes, leaves, stems, roots, buds, embryos, etc., can be used for a limitless multiplication of a plant and the production of bioactive compounds under sterile conditions [
48,
55,
56,
57]. Due to its various advantages, in vitro culture has been used as a powerful strategy for the production of secondary metabolites [
22,
58].
2. Plant Secondary Metabolites
Plant Secondary metabolites (PSMs) are low-weight molecules synthesized by the plant to protect itself against potential enemies, including pathogens and herbivore attacks. Even abiotic factors can affect the biosynthesis of secondary metabolites [
59,
60].
Due to their excellent biological activity, PSMs have been broadly used for centuries as an important resource for traditional medicine, perfumes, and industrial raw materials [
61]. Subsequently, they have been widely applied as valuable compounds such as pharmaceuticals, cosmetics, and bio-pesticides [
4,
51,
61,
62]. PSMs have contributed greatly to the importance and commercial values of plants [
63].
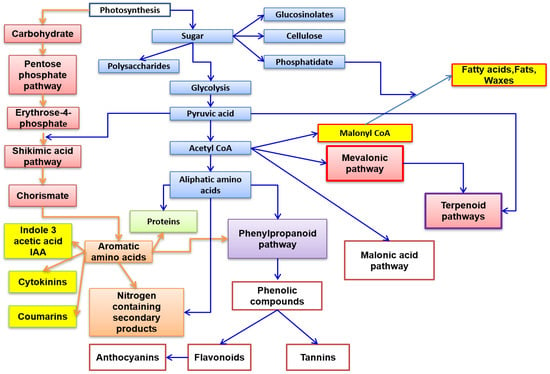
Phytochemical studies have identified an arsenal of secondary compounds such as flavonoids, phenols, nitrogen compounds, and terpenes [
5,
6]. Thus, a preview of the various biosynthetic pathways is represented in
Figure 1.
Figure 1. Principal biosynthetic pathways of major secondary metabolite plants’ classes.
3. Micropropagation as a Tool for the Production of Secondary Metabolites
Micropropagation is the reproduction of plants in vitro which leads to the multiplication of genetically identical copies of the parent plant asexually. Micropropagation offers the possibility of producing a limitless number of plants. Currently, this technique is applied for clone selection and rapid biomass production in several organizations or establishments for the large-scale production of higher plants.
In vitro propagation has become a crucial method for the mass production of medicinal plants and various protocols of the micropropagation of numerous medicinal species that have been successfully achieved either by organogenesis [
64,
65,
66,
67,
68] or by somatic embryogenesis [
69,
70,
71]. The micropropagation of many medicinal species has been revealed to be similar and with a little variation in their phytochemical content [
72].
Organogenesis is a micropropagation way that consists in the development of organs derived from cells or tissues. Plant regeneration through organogenesis involves specifically the induction and development of a shoot from an explant which is then transferred to a different medium for root induction [
73]. Several studies have demonstrated that a successful application of organogenesis on medicinal plants can be achieved by the correct establishment of the medium components and the selection of an adequate explant under highly controlled conditions (
Table 1).
Table 1. Micropropagation of medicinal plants by organogenesis methods.
Somatic cells can produce somatic embryos, which are similar to zygotic embryos, through a process called somatic embryogenesis. These somatic embryos can be developed into seedlings in an appropriate medium [
81]. Plant regeneration via embryogenesis occurs in two steps: the callus is grown on an auxin-rich embryogenic induction medium, sometimes combined with cytokinins, and is then transferred to an auxin-free medium, which results in the formation of mature embryos [
82]. The embryonic-like structure can be produced either directly on the explant or indirectly from the callus or cell suspension culture (
Table 2). This technique has also allowed genetic, morphological, and physiological manipulations to be performed [
83].
Table 2. Micropropagation of medicinal plants by somatic embryogenesis (SE).
Micropropagation could be an attractive commercial activity for the production of high-quality plants and offers advantages over conventional propagation practices [
88]. Thus, in vitro propagation is a sustainable alternative to the large-scale production of medicinal species with economic value. Castilho et al. [
80] allowed the use of an automated micropropagation system using bioreactors for industrial plant propagation as a possible way to reduce micropropagation costs [
89]. This can provide a means of supplying plant material capable of providing plant material that is able to produce phytocompounds [
19,
38,
48,
90] throughout the year without seasonal constraints [
16].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27228093