
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Pérez-Campos Mayoral | -- | 2848 | 2023-01-10 14:19:45 | | | |
| 2 | Lindsay Dong | Meta information modification | 2848 | 2023-01-11 02:22:39 | | |
Video Upload Options
Glycosylation is a post-translational modification that affects the stability, structure, antigenicity and charge of proteins. In the immune system, glycosylation is involved in the regulation of ligand–receptor interactions, such as in B-cell and T-cell activating receptors. Alterations in glycosylation have been described in several autoimmune diseases, such as systemic lupus erythematosus (SLE), in which alterations have been found mainly in the glycosylation of B lymphocytes, T lymphocytes and immunoglobulins. In immunoglobulin G of lupus patients, a decrease in galactosylation, sialylation, and nucleotide fucose, as well as an increase in the N-acetylglucosamine bisector, are observed. These changes in glycoisolation affect the interactions of immunoglobulins with Fc receptors and are associated with pericarditis, proteinuria, nephritis, and the presence of antinuclear antibodies. In T cells, alterations have been described in the glycosylation of receptors involved in activation, such as the T cell receptor; these changes affect the affinity with their ligands and modulate the binding to endogenous lectins such as galectins. In T cells from lupus patients, a decrease in galectin 1 binding is observed, which could favor activation and reduce apoptosis. Furthermore, these alterations in glycosylation correlate with disease activity and clinical manifestations, and thus have potential use as biomarkers.
1. Introduction
2. Systemic Lupus Erythematosus
3. Glycosylation
3.1. O-Glycosylation
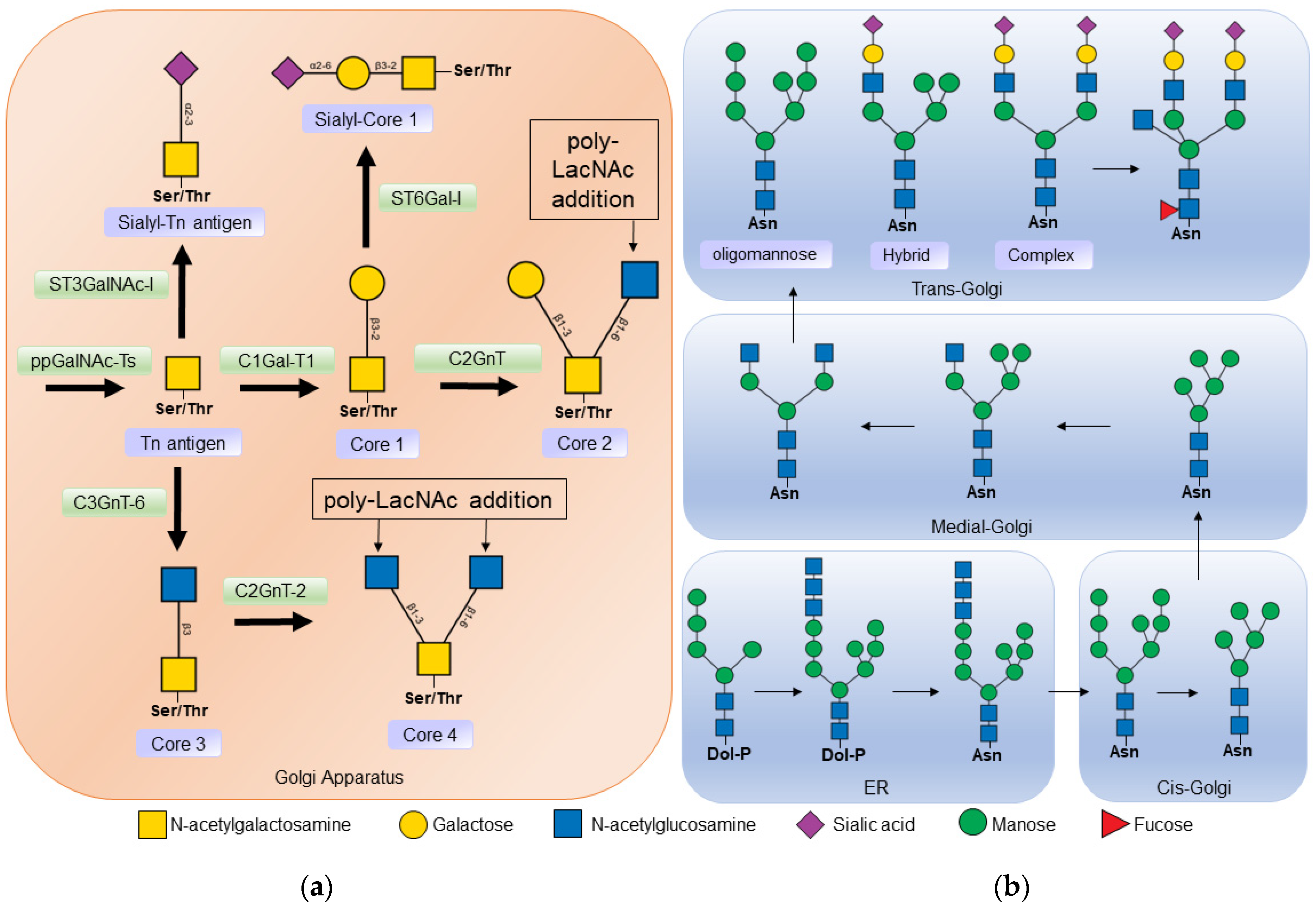
3.2. N-Glycosylation
4. Functions of Glycosylation in the Immune System
5. Alterations in Glycosylation in Systemic Lupus Erythematosus
5.1. Alterations in B Cell Glycosylation and Antibodies in SLE
5.1.1. Glycosylation of Constant Regions
5.1.2. Glycosylation of Variable Regions
5.2. Alterations in the Glycosylation of the T Cells in SLE
6. Alterations in Cytoplasmic O-GlcNAcylation
References
- Stanley, P.; Moremen, K.; Lewis, N.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R., Esko, J., Stanley, P., Hart, G., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N., Prestegard, J., et al., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022.
- Brockhausen, I.; Wandall, H.; Ten Hagen, K.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; ISBN 9780879697709.
- Sprovieri, P.; Martino, G. The role of the carbohydrates in plasmatic membrane. Physiol. Res. 2018, 67, 1–11.
- Maverakis, E.; Kim, K.; Shimoda, M.; Gershwin, M.E.; Patel, F.; Wilken, R.; Raychaudhuri, S.; Ruhaak, L.R.; Lebrilla, C.B. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: A critical review. J. Autoimmun. 2015, 57, 1–13.
- Zhou, R.W.; Mkhikian, H.; Grigorian, A.; Hong, A.; Chen, D.; Arakelyan, A.; Demetriou, M. N-glycosylation bidirectionally extends the boundaries of thymocyte positive selection by decoupling Lck from Ca2+ signaling. Nat. Immunol. 2014, 15, 1038–1045.
- Mihai, S.; Nimmerjahn, F. The role of Fc receptors and complement in autoimmunity. Autoimmun. Rev. 2013, 12, 657–660.
- Giovannone, N.; Smith, L.K.; Treanor, B.; Dimitroff, C.J. Galectin-Glycan Interactions as Regulators of B Cell Immunity. Front. Immunol. 2018, 9, 2839.
- Ząbczyńska, M.; Link-Lenczowski, P.; Pocheć, E. Glycosylation in Autoimmune Diseases BT—The Role of Glycosylation in Health and Disease; Lauc, G., Trbojević-Akmačić, I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 205–218. ISBN 978-3-030-70115-4.
- Li, X.; Xu, J.; Li, M.; Zeng, X.; Wang, J.; Hu, C. Aberrant glycosylation in autoimmune disease. Clin. Exp. Rheumatol. 2020, 38, 767–775.
- Lacki, J.K.; Porawska, W.; Mackiewicz, U.; Mackiewicz, S.; Müller, W. Changes in agalactosyl IgG levels correlate with radiological progression in early rheumatoid arthritis. Ann. Med. 1996, 28, 265–269.
- Akmačić, I.T.; Ventham, N.T.; Theodoratou, E.; Vučković, F.; Kennedy, N.A.; Krištić, J.; Nimmo, E.R.; Kalla, R.; Drummond, H.; Štambuk, J.; et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 2015, 21, 1237–1247.
- Wuhrer, M.; Selman, M.H.J.; McDonnell, L.A.; Kümpfel, T.; Derfuss, T.; Khademi, M.; Olsson, T.; Hohlfeld, R.; Meinl, E.; Krumbholz, M. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J. Neuroinflamm. 2015, 12, 235.
- Abida, R.; Yeoh, S.-A.; Isenberg, D.A. Advances in systemic lupus erythematosus. Medicine 2022, 50, 7–17.
- Boodhoo, K.D.; Liu, S.; Zuo, X. Impact of sex disparities on the clinical manifestations in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Medicine 2016, 95, e4272.
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13.
- Bacalao, M.A.; Satterthwaite, A.B. Recent Advances in Lupus B Cell Biology: PI3K, IFNγ, and Chromatin. Front. Immunol. 2020, 11, 615673.
- Rönnblom, L.; Elkon, K.B. Cytokines as therapeutic targets in SLE. Nat. Rev. Rheumatol. 2010, 6, 339–347.
- Cozzani, E.; Drosera, M.; Gasparini, G.; Parodi, A. Serology of Lupus Erythematosus: Correlation between Immunopathological Features and Clinical Aspects. Autoimmune Dis. 2014, 2014, 321359.
- Pisetsky, D.S. Evolving story of autoantibodies in systemic lupus erythematosus. J. Autoimmun. 2020, 110, 102356.
- Yazdany, J.; Davis, J. The role of CD40 ligand in systemic lupus erythematosus. Lupus 2004, 13, 377–380.
- Wofsy, D.; Seaman, W.E. Reversal of advanced murine lupus in NZB/NZW F1 mice by treatment with monoclonal antibody to L3T4. J. Immunol. 1987, 138, 3247–3253.
- Mihara, M.; Ohsugi, Y.; Saito, K.; Miyai, T.; Togashi, M.; Ono, S.; Murakami, S.; Dobashi, K.; Hirayama, F.; Hamaoka, T. Immunologic abnormality in NZB/NZW F1 mice. Thymus-independent occurrence of B cell abnormality and requirement for T cells in the development of autoimmune disease, as evidenced by an analysis of the athymic nude individuals. J. Immunol. 1988, 141, 85–90.
- Hassan, H.; Reis, C.A.; Bennett, E.P.; Mirgorodskaya, E.; Roepstorff, P.; Hollingsworth, M.A.; Burchell, J.; Taylor-Papadimitriou, J.; Clausen, H. The lectin domain of UDP-N-acetyl-D-galactosamine: Polypeptide N-acetylgalactosaminyltransferase-T4 directs its glycopeptide specificities. J. Biol. Chem. 2000, 275, 38197–38205.
- Wandall, H.H.; Irazoqui, F.; Tarp, M.A.; Bennett, E.P.; Mandel, U.; Takeuchi, H.; Kato, K.; Irimura, T.; Suryanarayanan, G.; Hollingsworth, M.A.; et al. The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: Lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology 2007, 17, 374–387.
- Magalhães, A.; Duarte, H.O.; Reis, C.A. The role of O-glycosylation in human disease. Mol. Asp. Med. 2021, 79, 100964.
- Brockhausen, I. Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1999, 1473, 67–95.
- Banerjee, D.K. N-glycans in cell survival and death: Cross-talk between glycosyltransferases. Biochim. Biophys. Acta 2012, 1820, 1338–1346.
- Trombetta, E.S. The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology 2003, 13, 77R–91R.
- Hernandez, J.D.; Klein, J.; Van Dyken, S.J.; Marth, J.D.; Baum, L.G. T-cell activation results in microheterogeneous changes in glycosylation of CD45. Int. Immunol. 2007, 19, 847–856.
- Chervenak, R.; Cohen, J.J. Peanut lectin binding as a marker for activated T-lineage lymphocytes. Thymus 1982, 4, 61–67.
- De Petris, S.; Takacs, B. Relationship between mouse lymphocyte receptors for peanut agglutinin (PNA) and Helix pomatia agglutinin (HPA). Eur. J. Immunol. 1983, 13, 831–840.
- van Vliet, S.J.; Vuist, I.M.; Lenos, K.; Tefsen, B.; Kalay, H.; García-Vallejo, J.J.; van Kooyk, Y. Human T cell activation results in extracellular signal-regulated kinase (ERK)-calcineurin-dependent exposure of Tn antigen on the cell surface and binding of the macrophage galactose-type lectin (MGL). J. Biol. Chem. 2013, 288, 27519–27532.
- Chien, M.-W.; Lin, M.-H.; Huang, S.-H.; Fu, S.-H.; Hsu, C.-Y.; Yen, B.L.-J.; Chen, J.-T.; Chang, D.-M.; Sytwu, H.-K. Glucosamine Modulates T Cell Differentiation through Down-regulating N-Linked Glycosylation of CD25. J. Biol. Chem. 2015, 290, 29329–29344.
- Lin, C.-R.; Wei, T.-Y.W.; Tsai, H.-Y.; Wu, Y.-T.; Wu, P.-Y.; Chen, S.-T. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 5006–5017.
- Chien, M.-W.; Fu, S.-H.; Hsu, C.-Y.; Liu, Y.-W.; Sytwu, H.-K. The Modulatory Roles of N-glycans in T-Cell-Mediated Autoimmune Diseases. Int. J. Mol. Sci. 2018, 19, 780.
- Giovannone, N.; Liang, J.; Antonopoulos, A.; Sweeney, J.G.; King, S.L.; Pochebit, S.M.; Bhattacharyya, N.; Lee, G.S.; Dell, A.; Widlund, H.R.; et al. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun. 2018, 9, 3287.
- Blidner, A.G.; Méndez-Huergo, S.P.; Cagnoni, A.J.; Rabinovich, G.A. Re-wiring regulatory cell networks in immunity by galectin-glycan interactions. FEBS Lett. 2015, 589, 3407–3418.
- Sweeney, J.G.; Liang, J.; Antonopoulos, A.; Giovannone, N.; Kang, S.; Mondala, T.S.; Head, S.R.; King, S.L.; Tani, Y.; Brackett, D.; et al. Loss of GCNT2/I-branched glycans enhances melanoma growth and survival. Nat. Commun. 2018, 9, 3368.
- Croci, D.O.; Morande, P.E.; Dergan-Dylon, S.; Borge, M.; Toscano, M.A.; Stupirski, J.C.; Bezares, R.F.; Avalos, J.S.; Narbaitz, M.; Gamberale, R.; et al. Nurse-like cells control the activity of chronic lymphocytic leukemia B cells via galectin-1. Leukemia 2013, 27, 1413–1416.
- van de Bovenkamp, F.S.; Derksen, N.I.L.; van Breemen, M.J.; de Taeye, S.W.; Ooijevaar-de Heer, P.; Sanders, R.W.; Rispens, T. Variable Domain N-Linked Glycans Acquired During Antigen-Specific Immune Responses Can Contribute to Immunoglobulin G Antibody Stability. Front. Immunol. 2018, 9, 740.
- van de Bovenkamp, F.S.; Derksen, N.I.L.; Heer, P.O.-D.; van Schie, K.A.; Kruithof, S.; Berkowska, M.A.; van der Schoot, C.E.; IJspeert, H.; van der Burg, M.; Gils, A.; et al. Adaptive antibody diversification through N-linked glycosylation of the immunoglobulin variable region. Proc. Natl. Acad. Sci. USA 2018, 115, 1901–1906.
- Sjöwall, C.; Zapf, J.; von Löhneysen, S.; Magorivska, I.; Biermann, M.; Janko, C.; Winkler, S.; Bilyy, R.; Schett, G.; Herrmann, M.; et al. Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus. Lupus 2014, 24, 569–581.
- Raju, T.S. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr. Opin. Immunol. 2008, 20, 471–478.
- Ferrara, C.; Stuart, F.; Sondermann, P.; Brünker, P.; Umaña, P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J. Biol. Chem. 2006, 281, 5032–5036.
- Albert, H.; Collin, M.; Dudziak, D.; Ravetch, J.V.; Nimmerjahn, F. In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc. Natl. Acad. Sci. USA 2008, 105, 15005–15009.
- Allhorn, M.; Olin, A.I.; Nimmerjahn, F.; Collin, M. Human IgG/Fc gamma R interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS ONE 2008, 3, e1413.
- Vučković, F.; Krištić, J.; Gudelj, I.; Teruel, M.; Keser, T.; Pezer, M.; Pučić-Baković, M.; Štambuk, J.; Trbojević-Akmačić, I.; Barrios, C.; et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015, 67, 2978–2989.
- Bondt, A.; Rombouts, Y.; Selman, M.H.J.; Hensbergen, P.J.; Reiding, K.R.; Hazes, J.M.W.; Dolhain, R.J.E.M.; Wuhrer, M. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol. Cell. Proteom. 2014, 13, 3029–3039.
- Koers, J.; Derksen, N.I.L.; Heer, P.O.-D.; Nota, B.; van de Bovenkamp, F.S.; Vidarsson, G.; Rispens, T. Biased N-Glycosylation Site Distribution and Acquisition across the Antibody V Region during B Cell Maturation. J. Immunol. 2019, 202, 2220–2228.
- Uhrík, L.; Hernychová, L.; Vojtěšek, B. Glycosylation as an Important Regulator of Antibody Function. Klin. Onkol. 2019, 32, 46–55.
- Ramos-Martínez, E.; Lascurain, R.; Tenorio, E.P.; Sánchez-González, A.; Chávez-Rueda, K.; Chávez-Sánchez, L.; Jara-Quezada, L.J.; Chávez-Sánchez, R.; Zenteno, E.; Blanco-Favela, F. Differential Expression of O-Glycans in CD4+ T Lymphocytes from Patients with Systemic Lupus Erythematosus. Tohoku J. Exp. Med. 2016, 240, 79–89.
- Urrea, F.; Zenteno, E.; Avila-Moreno, F.; Sanchez-Garcia, F.J.; Zuñiga, J.; Lascurain, R.; Ortiz-Quintero, B. Amaranthus leucocarpus lectin (ALL) enhances anti-CD3-dependent activation of murine T cells and promotes cell survival. Immunol. Invest. 2011, 40, 113–129.
- Dumont, F.; Habbersett, R.G. Unusual cell surface properties of the T lymphocyte population expanding in MRL/Mp-lpr/lpr mice. Immunology 1982, 47, 271–281.
- Sarkar, M.; Wu, A.M.; Kabat, E.A. Immunochemical studies on the carbohydrate specificity of Maclura pomifera lectin. Arch. Biochem. Biophys. 1981, 209, 204–218.
- Hernández, P.; Tetaert, D.; Vergoten, G.; Debray, H.; del Carmen Jimenez, M.; Fernández, G.; Agundis, C.; Degand, P.; Zenteno, E. Specificity of Amaranthus leucocarpus syn. hypocondriacus lectin for O-glycopeptides. Biochim. Biophys. Acta 2004, 1674, 282–290.
- Wu, A.M. Polyvalency of Tn (GalNAcα1→Ser/Thr) glycotope as a critical factor for Vicia villosa B4 and glycoprotein interactions. FEBS Lett. 2004, 562, 51–58.
- Chang, Y.-H.; Weng, C.-L.; Lin, K.-I. O-GlcNAcylation and its role in the immune system. J. Biomed. Sci. 2020, 27, 57.
- Song, N.; Qi, Q.; Cao, R.; Qin, B.; Wang, B.; Wang, Y.; Zhao, L.; Li, W.; Du, X.; Liu, F.; et al. MAVS O-GlcNAcylation Is Essential for Host Antiviral Immunity against Lethal RNA Viruses. Cell Rep. 2019, 28, 2386–2396.e5.
- Madsen-Bouterse, S.A.; Xu, Y.; Petty, H.R.; Romero, R. Quantification of O-GlcNAc protein modification in neutrophils by flow cytometry. Cytom. Part A J. Int. Soc. Anal. Cytol. 2008, 73, 667–672.
- Božič, J.; Stoka, V.; Dolenc, I. Glucosamine prevents polarization of cytotoxic granules in NK-92 cells by disturbing FOXO1/ERK/paxillin phosphorylation. PLoS ONE 2018, 13, e0200757.
- Tsokos, G.C.; Nambiar, M.P.; Juang, Y.-T. Activation of the Ets Transcription Factor Elf-1 Requires Phosphorylation and Glycosylation. Ann. N. Y. Acad. Sci. 2003, 987, 240–245.
- Enyedy, E.J.; Nambiar, M.P.; Liossis, S.N.; Dennis, G.; Kammer, G.M.; Tsokos, G.C. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 1114–1121.
- Nambiar, M.P.; Fisher, C.U.; Warke, V.G.; Krishnan, S.; Mitchell, J.P.; Delaney, N.; Tsokos, G.C. Reconstitution of deficient T cell receptor zeta chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 2003, 48, 1948–1955.




